ABSTRACT
Three chromatographic procedures were investigated regarding their potential for the quantification of aniline and 19 of its methylated and chlorinated derivatives in groundwater. These methods were based on liquid-liquid-extraction in combination with gas chromatography and single quadrupole mass spectrometry (GC/MS) according to German standard DIN 38407–16:1999 and its extension using tandem mass spectrometry (GC/MS-MS), both following liquid–liquid extraction, and as third alternative the direct injection of the water sample into a liquid chromatograph coupled to tandem mass spectrometry (LC/MS-MS). Results were compared using fortified water and real-world contaminated groundwater used in an interlaboratory comparison. It could be shown that GC/MS and GC/MS-MS yielded results deviating less than 10% from each other, while all three procedures displayed quantification results deviating less than 15% from the intercomparison reference values in case of each analyte in the concentration range between 1 and 45 µg L−1. Though GC/MS-MS displays a ten-fold higher sensitivity than single quadrupole GC/MS, the precision of both methods in the concentration range was similar. LC/MS-MS has the advantage of no further sample preparation due to direct injection and leads for methylanilines and meta-, para-substituted chloroanilines to results sufficiently equivalent to the standardised GC/MS method. However, LC/MS-MS is not suitable for ortho-chloroaniline derivatives due to significantly lower ion yields than meta- and para-substituted chloroanilines.
1. Introduction
Due to their toxicity and persistence, aromatic amines constitute a significant class of water pollutants. Chlorinated anilines are widely used as chemical intermediates in the production of dyes, pesticides, and industrial compounds. They may be released into the environment directly as a result of industrial discharge from factories, or indirectly through the degradation of compounds such as azo-dyes [Citation1], pesticides [Citation2] and explosives [Citation3]. Aromatic amines are highly soluble in water and their potential as groundwater contaminants [Citation4] requires their monitoring in affected aqueous environmental compartments.
During the monitoring of contaminated groundwater remediation efforts performed by contract laboratories repeatedly severe discrepancies were observed regarding the quantification of certain aniline derivatives. Therefore, the responsible authorities required a specific proof of proficiency using real case groundwater from the monitoring area as part of a call for tender for further monitoring projects. Hereby, the anilines are to be determined by gas chromatography (GC/MS) after solid-phase extraction (SPE) or liquid-liquid-extraction (LLE) without derivatisation according to German standard DIN 38407–16:1999 [Citation5]. Although a variety of further techniques including solid-phase extraction with derivatisation [Citation6–10], solid phase micro extraction from the liquid phase [Citation11–15] or the headspace [Citation16,Citation17], liquid phase micro extraction [Citation18–22] and micro extraction by packed sorbent [Citation23,Citation24] have been reported they are not allowed in the monitoring because they have not been standardised and the laboratories have mostly no respective experience in routine usage. The monitoring is restricted to the use of standardised GC/MS, while non-standardised procedures including high performance liquid chromatography [Citation13,Citation18–21,Citation25] or capillary electrophoresis [Citation26] are not allowed because the equivalence of GC and LC methods have not been shown to the responsible authorities.
During long-term assistance of groundwater monitoring campaigns two aspects of quality assurance have been observed: (i) Chromatographic separation of aniline derivatives from ethylaniline and chloro-methyl-nitrobenzenes is not sufficient and qualifier ions of aniline derivatives display fragments similar to the target ions of chloronitrobenzenes. For instance, the qualifier ion m/z 121 of chloromethyl nitrobenzene and ethylaniline is identical to the target ion of dimethylaniline and the chromatographic separation (e.g. ethylaniline from 2,5-dimethylaniline) does not fulfil the requirements for peak quantification. ii) Further, manual liquid–liquid extraction is labour-intensive and another source of overall measurement uncertainty.
Solving problem (i) quantification by tandem mass spectrometry (MS–MS) after sample extraction and gas chromatography is an option due to unequivocal mass fragments’ higher sensitivity and consequently more precise analytical results. [Citation27] Liquid chromatography followed by MS–MS after direct injection of water sample may be regarded as an appropriate answer to problem (ii) in consideration of rapidness and high sensitivity [Citation28,Citation29].
Three procedures, GC/MS, GC/MS–MS, and LC/MS–MS, were compared with regard to aniline derivative quantification using industrially contaminated and spiked water samples in connection with an interlaboratory comparison. The pros and cons are discussed and the prospects to use LC/MS-MS as an alterative to GC/MS are evaluated.
2. Materials and Methods
2.1. Chemicals and solvents
Aniline and dichloroanilines (2,4-, 2,5-, 3,4-, 3,5-) were obtained as pestanal® quality, 2-methylaniline and 3-methylaniline as oekanal® quality from Riedel-de-Haen (Seelze, Germany). 4-methylaniline, 2,4-, 2,5-, 2,6- and 3,4-dimethylaniline were obtained from Aldrich/Fluka (Steinheim, Germany) and 2,3- and 3,5-dimethylaniline from Merck (Hohenbrunn, Germany). The chloroanilines (2-, 3-, 4-) were obtained from Dr. Ehrenstorfer (Augsburg, Germany). A mix of 19 aniline derivatives in methanol (each 1.000 ng µL−1) by Neochema (Bodenheim, Germany) was used for calibration. 3-Chloro-4-fluoroaniline (Promochem, Wesel, Germany) was used as an internal standard for gas chromatographical methods.
Multi-deuterated aniline derivatives were used as internal standards for LC/MS-MS. They were obtained from CDN Isotopes (Quebec, Canada): 2-, 3- and 4-methylanilines-d9, 2,4- and 2,6-dimethylanilines-d11, 3-chloroaniline-(2,4,6-d3), 4-chloroaniline-(2,3,5,6-d4), 3,4-dichloroaniline-(2,6-d2) and 3,5- dichloroaniline-(2,4,6-d3).
Methanol (MeOH) and toluene (picograde®) were obtained from Promochem and sodium azide (NaN3) was obtained from Merck (Darmstadt, Germany). Formic acid (HPLC-grade) from Sigma-Aldrich (Taufkirchen, Germany) and acetonitrile (ACN, Chemsolute®) from Th. Geyer (Renningen, Germany) were used for LC.
2.2. Gas chromatography-mass spectrometry (GC/MS and GC/MS-MS) after liquid-liquid extraction
Water samples of 200 mL were spiked with internal standard solution (3-chloro-4-fluoroaniline in water, 10 µg L−1) and extracted thrice with 10 mL of toluene. The unified extracts were concentrated to 1 mL on a rotavapor. GC/MS was carried out using a Hewlett Packard 6890 gas chromatograph (GC) equipped with a mass selective detector MSD 5973 N (Agilent, Waldbronn, Germany) and a CombiPAL autosampler (CTC Analytics, Zwingen, Switzerland). The capillary column was a PAHSelect (CP7462, Agilent), 30 m length x 0.25 mm ID x 0.15 µm film thickness. Tandem mass spectrometry (GC/MS-MS) was done on a Hewlett Packard 8890 gas chromatograph equipped with MSD 7000D and a MPS2 autosampler (Gerstel, Mülheim, Germany). The parameters for chromatography (retention time) and tandem mass spectrometry are given in . The capillary column was a ZB5ms, 30 m length x 0.25 mm ID x 0.25 µm film thickness (Phenomenex, Torrance, CA, USA). Oven and carrier gas conditions for both GC/MS and GC/MS-MS were identical: The oven program started at 70°C (held for 1 min), to 150°C at 3°C min−1 and further to 280°C at 10°C min−1. The injector temperature was 180°C, and transfer line was heated to 300°C. The flow of carrier gas hydrogen 5.0 (Air Liquide, Berlin, Germany) was 1 mL min−1. The method parameters for GC/MS-MS are given in and those for the standardised GC/MS method are given in the supplemental material in Table S1.
Table 1. Method parameters for GC/MS-MS.
2.3. Liquid chromatography-mass spectrometry (LC/MS-MS)
Water samples of 5 mL were fortified with 100 µL of a methanolic internal standard solution (1 µg mL−1), mixed and filtered through a syringe filter (regenerated cellulose, Whatman Spartan 13 mm, 0.2 µm, Cytiva Europe GmbH, Freiburg, Germany). Quantification was carried out using a 1290 Infinity II LC System (Agilent, Waldbronn, Germany) coupled to an API4000 QTRAP mass spectrometer (Sciex, Darmstadt, Germany). 10 µL of the filtrate were injected in LC using the integrated autosampler. The column was a Kinetex, 100 mm x 2.1 mm ID, 1.7 µm (Phenomenex, Aschaffenburg, Germany). The column oven was set at 45 °C, and the flow rate of the mobile phase was 500 µL min−1. The mobile phase was delivered according to a time-programmed gradient using channels A (H2O) and B (MeOH: ACN, 25:75, v:v, 0.1% formic acid). The mobile phase gradient consisted of 7% B (0–0.82 min) and was ramped to 35% B (0.82–3.27 min), then further to 95% B (3.27–5.31 min) ramp to 95% B, held (5.31–6.13 min) and ramped to initial conditions (7% B, 6.13–6.53 min) and equilibrated from 6.53 to 10.21 min. The MS–MS detection was performed by positive electrospray ionisation (ESI+) using the multiple-reaction monitoring (MRM) acquisition mode with a dwell time of 50 ms for each transition. The MRM parameters for each monitored transition were as follows: ion spray voltage: 4500 V; desolvation temperature: 450°C; ion source gas 1:55 arbitrary units (a.u.); ion source gas 2:45 a.u.; and curtain gas: 25 a.u. Liquid chromatography retention times, MRM transitions and internal standards used for quantification are given in . Further optimisation parameters are given in supplemental Table S6.
Table 2. Retention time, mass transitions and MS-MS-parameters for quantification of aniline and aniline derivatives with liquid chromatography (LC/MS-MS).
2.4. Calibration
All three analytical procedures were calibrated with the same set of ten calibration solutions that covered the concentration range between 0.1 and 100 µg L−1 for each compound equidistantly. Tap water was spiked with volume equivalents of a methanolic stock solution of the 19 aniline derivatives (each 100 µg mL−1) to arrive at the respective calibration solution. Respective internal standards were spiked from a methanolic stock solution containing 100 µg mL−1 of each compound to arrive at a concentration of 10 µg L−1. Calibration solutions were treated like a normal water sample according to the respective method protocol.
2.5. Preparation of the test batches and interlaboratory comparison
The groundwater was sampled on the site of a former dyes and explosives factory in Berlin, Germany. Until the shutdown of the factory in 1944 unknown amounts of aromatic compounds (mainly nitro- and chloronitrobenzene, aniline and monochlorobenzene) had been released into the subsoil and contaminated the groundwater with up to 3,000 µg L−1 of aniline and other organic compounds: monochlorbenzene (varying between 400 and 1.000 µg L−1), dichlorobenzenes (sum varying between 100 and 1.000 µg L−1), trichlorobenzenes (sum < 80 µg L−1), chloronitrobenzenes (sum < 1.000 µg L−1), dichloronitrobenzenes (sum < 40 µg L−1). The contaminant plume is more than 3 km long and 400 m wide. From the existing groundwater wells in a depth of screen (75–100 m below water table) one measuring point with a typical profile of aniline derivatives was selected to sample the test material. The groundwater as sampled (pH 7.4, conductivity 920 µS cm−1) and stabilised with NaN3 (0.1 g L−1) [Citation30] was used as test batch A while test batch B was obtained after fortification of tap water with selected aniline derivatives. This was achieved by addition of 250 µL of a stock solution containing the anilines in a range of 200–2,000 µg mL−1 in methanol. The blank value of the tap water (pH 7.5, conductivity 878 µS cm−1) used for preparation (batch B) was tested, and anilines were not detectable (< 0.05 µg L−1). summarises the concentrations in samples A and B.
Table 3. Overview of aniline derivative concentrations.
The organisation, structure and evaluation of the interlaboratory comparison for the detection of anilines and their derivatives was provided by BAM and described in a report of 2014, when the first of the three interlaboratory comparisons for anilines in groundwater was provided [Citation31]. Ten accredited routine laboratories and two laboratories from BAM took part in the most recent interlaboratory comparison, organised by BAM in October 2020. The assigned values for proficiency assessment were the averages X of all participants’ results in the case of sample A, and fortified values in the case of sample B in accordance with ISO 13528 [Citation32]. Returned intercomparison data were evaluated with the program ProLab Plus, Version 2019.1.22.0 (QuoData, Dresden, Germany).
3. Results and discussion
3.1. Co-elution of analytes and compound mapping
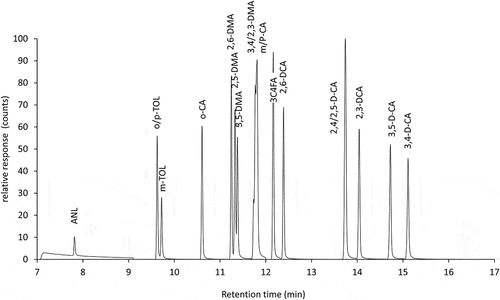
Gas chromatographic co-elutions of substituted anilines depend mainly on the separation conditions of the used columns. Here, the peak identity of all aniline derivatives in the calibration mix was verified using the authentic single compounds. show the typically reported co-elutions are 2-/4-methylaniline, 2,4-/2,6-dimethylaniline, 2,4-/2,5-dichloroaniline, while co-elutions of 2,3-/3,4-dimethylaniline and 3-/4-chloroaniline have rarely been reported [Citation31]. It is seen that the capability of GC to quantify co-eluting compounds depends on their concentration ratio: at dominance of one compound, the peak of the minor compound is situated on the tailing and quantification of the latter is impossible and both compounds are often quantified as sum. Since the detector response ratios of all coeluting pairs are close to 1:1 in single quadrupole and tandem-MS a summary quantification is possible. These co-elution difficulties are also reflected in the interlaboratory comparison participants’ results: Nine out of 10 participants in the interlaboratory comparison gave separate results for 2,5- and 3,5-dimethylaniline (ratio: 1:2) in sample B, whereas in sample A (ratio: 10:1), only the sum was reported by 10 participants. Liquid chromatography separation leads to baseline separation of all detectable compounds, co-elutions are not observed. It should be noted that there were no signals observed for ortho-chlorinated aniline derivatives (2-chloroaniline, 2,3-, 2,4-, 2,5-, 2,6-dichloroaniline) in the concentration range of interest (<1 mg L−1). This confirms the formerly observed limits of liquid chromatography in combination with electrospray tandem mass spectrometry [Citation33].
3.2. Method validation
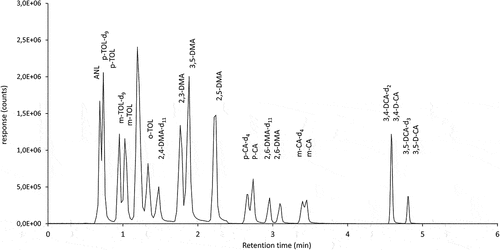
The standardised GC/MS procedure was verified, and the extension to GC/MS–MS and the LC/MS–MS procedure were validated. The sample extraction and gas chromatography followed the standard method [Citation5] in all three cases. The gas and liquid chromatograms of the standard mixture of aniline derivatives are shown in , and the chromatograms of test samples A and B are shown in .
Figure 3. GC/MS-MS chromatogram of groundwater sample A (above) and fortified tap water sample B (below).
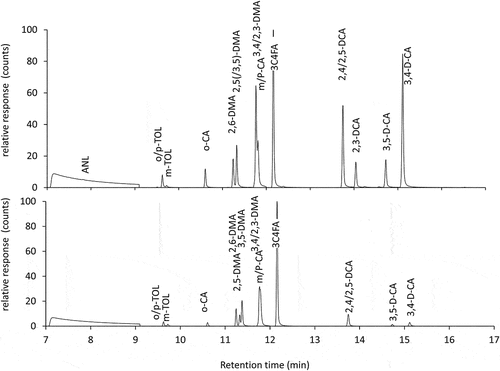
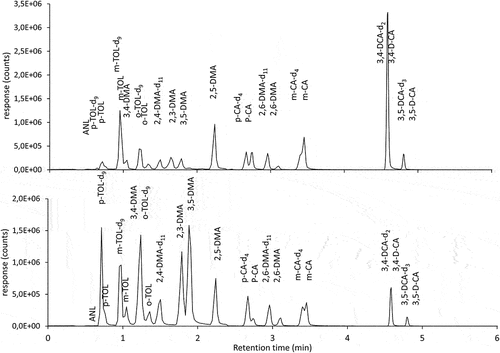
Figure 4. LC/MS-MS chromatogram of groundwater sample A (above) and fortified tap water sample B (lower).
The main analytical parameters of the GC/MS method are shown in Tables S1 and S2. Limits of detection (LOD) quantification (LOQ) are estimated based on calibration data according to DIN 32645 [Citation34]. The observed LOQ for GC/MS-MS is between 0.01 and 0.05 µg L−1 which is ten times lower than those for standardised GC/MS (see Tables S2 and S3). This expected higher sensitivity of the tandem mass spectrometry compared to single quadrupole mass spectrometry does not lead to a higher repeatability. One reason could be that the better matrix suppression by MS–MS has advantages near the LOQ but is not relevant for the relatively high concentration range present in these interlaboratory comparison samples. The recovery rate of four replicates on four days was observed between 91% and 111% at 2.5 µg L−1 for all individual compounds. The repeatability of each individual compound was observed between 5% and 10% for four repetitions of groundwater and fortified water analysis (extraction and gas chromatography).
The liquid chromatographic procedure was recently developed by BAM. The advantage of the direct injection of the water sample without LLE or further sample preconcentration produced a fast, simple, and specific method for quantification of aniline and its derivates for homogeneity and stability testing of test samples. shows a chromatogram of the standard mixture of aniline derivatives with LC/MS-MS.
The main analytical parameters of the LC/MS-MS method are shown in . The repeatability of the analysis from 10 replicate injections of a water solution spiked with 50 µg L−1 and close to the LOQ of 0.1 µg L−1 of each aniline derivative ranged from 2% to 7%. Water samples containing concentrations close to the LQQ 0.1 µg L−1 displayed repeatabilities between 8% and 15% (aniline: 23%). The linearity of instrumental response evaluated in the concentration range between 0.1 and 100 µg L−1 showed very good regression coefficients for all compounds (R2: 0.9988–0.9999). Linearity for the quantification of 3,4-dichloroaniline was observed in a smaller concentration range between 0.1 and 5 µg L−1. LOD and LOQ were estimated based on calibration data according to DIN 32645 [Citation34]. LODs were in the range of 0.01–0.05 µg L−1.
Table 4. Calibration curve equations for LC/MS-MS, coefficients of determination (R2), limits of detection (LOD) and quantification (LOQ), and repeatability (relative standard deviation, RSD, n = 10) at fortification levels of 0.1 and 50 µg L−1.
3.3. Results of GC/MS, GC/MS-MS, and LC/MS-MS and the interlaboratory comparison
summarise the quantification results obtained with the three procedures and the two water samples A and B along with the results of the interlaboratory comparison. Since the motivation for this interlaboratory comparison was the selection of proficient laboratories for the monitoring of a specific site with an unusual proportion of anilines partly present in medium concentration, it appeared reasonable to use real groundwater from that site. The intercomparison results were submitted to robust statistical analysis as laid down specifically for proficiency testing in DIN 38402 45 [Citation35]. The Hampel estimator was used as robust consensus mean X of all the laboratory means, and the q estimator was taken as an estimate for the reproducibility standard deviation sR. This robust procedure provided reasonable estimates for X and sR regardless of the distribution of laboratory values and without the need to identify outliers. In the case of sample A, the consensus values X and, in the case of sample B, the fortified values are used for the comparison of GC/MS-MS and LC/MS-MS with standardised GC/MS. display the GC and LC results as means and standard deviations of five (GC/MS-MS) or nine (GC/MS and LC/MS-MS) replicate determinations, respectively. Statistical parameters of the interlaboratory comparison are collected in supplemental Table S4.
Figure 5. Quantification results of methylated anilines – comparison of GC/MS, GC/MS-MS and LC/MS-MS (in-house, means and standard deviations) with the interlaboratory comparison results (robust means and expanded uncertainties U) on groundwater (sample A, above) and fortified water (sample B, below).
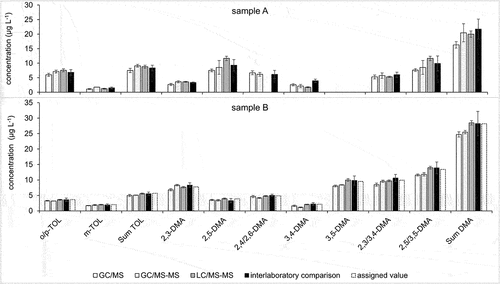
Figure 6. Quantification results of chloroanilines – comparison of GC/MS, GC/MS-MS and LC/MS-MS (in-house, means and standard deviations) with the interlaboratory comparison results (robust means and expanded uncertainties U) on groundwater (sample A, above) and fortified water (sample B, below).
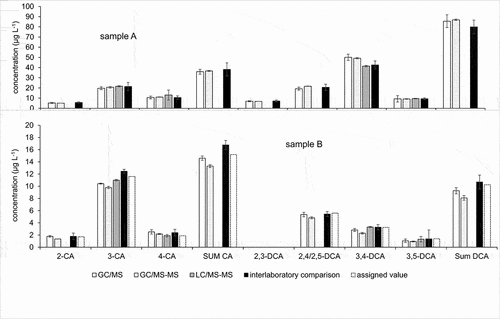
It is seen that GC/MS and GC/MS-MS produce results in good agreement within 10% deviation for all analytes in both samples. The LC/MS-MS results deviate in cases somewhat more; however, they are within the ±15% limit set as standard deviation for proficiency testing in accordance with the authorities responsible for groundwater monitoring.
While GC/MS–MS was not able to separate the pairs 2-/4-methylaniline, 2,5-/3,5-dimethylaniline 2,3-/3,4-dimethylaniline, and 2,4-/3,4-dichloroaniline (), the LC/MS-MS procedure displayed separation of all pairs and allowed for quantification of the respective individual compounds (). Specifically, the separate quantification of 2,4- and 2,6-dimethylaniline is not possible for gas chromatography. However, this method is limited in that ortho-chlorinated aniline derivates are not detectable and therefore missing in . This may be partly attributed to the pKa of the protonated forms MH+, which are between 0.46 and 2.00 for the dichlorinated anilines and 2.66 for 2-chloroaniline, while nearly all other chlorinated and methylated anilines display pKa close to or above 3 (see supplemental Table S-5). This is likely to shift the M/MH+ ratio towards the unprotonated form in case of the ortho-chloroanilines in the weakly acidic eluent. Labelled compounds for 2,3-, 2,5-, 3,4- and 3,5-dimethylaniline were not available as internal standards. The quantification of these compounds was related to 2,4- dimethylaniline-d11, which is the labelled compound most similar in structure and eluting closest to all of them. The statistical parameters and quantification results obtained this way do not suggest significantly different recovery rates or repeatabilities compared to 2,4-dimthylaniline. It can, however, not be ruled out that the proficiency of internal standards needs be studied on a case-by-case basis depending on the interaction of eluents and the respective matrix with the ionisation of analytes.
4. Conclusion
Though the quantification of ortho-chloroanilines is not possible, for the quantification of aniline, methylanilines and selected chloroanilines, the LC/MS-MS is a more selective and faster method that provides results comparable with the standardised GC method. Co-elutions from GC methods were not observed in the LC methods, which is why both methods may complement each other. It seems that the usage of deuterated internal standards for each single compound is not necessary, which gives LC/MS-MS a real chance as a cost-effective alternative to GC-methods in laboratory routine analysis.
Supplemental Material
Download PDF (326.8 KB)Acknowledgments
This work was supported by the Senate for Health, Environment and Consumer Protection, Berlin, Germany and the Federal Institute for Special Tasks Arising from Unification (BvS), Berlin, Germany.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed here.
References
- W.M.S. Alabdraba and M.B.A. Albayati, Int. J. Environ. Eng. Nat. Resour. 1, 179 (2014).
- V.E. Herrera-Gonzalez, N. Ruiz-Ordaz, J. Galindez-Mayer, C. Juarez-Ramirez, F. Santoyo-Tepole and E.M. Montiel, World J. Microbiol. Biotechnol. 29, 467 (2013). doi:10.1007/s11274-012-1200-5.
- H. Hofstetter, J.C. Spain, S.F. Nishino, J. Bolotin and R.P. Schwarzenbach, Environ. Sci. Technol. 42, 4764 (2008). doi:10.1021/es8001053.
- E.J. Weber, D. Colon and G.L. Baughman, Environ. Sci. Technol. 35, 2470 (2001). doi:10.1021/es001759d.
- DIN 38407-16:1999, German Standard Methods for the Examination of Water, Waste and Sludge – Jointly Determinable Substances (Group F) – Part 16: Determination of Aniline Derivates by Gas Chromatography (F16), Beuth Verlag GmbH, Berlin, Germany (1999).
- T.C. Schmidt, M. Less, R. Haas, E. von Löw, K. Steinbach and G. Stork, J. Chromatogr. A. 810, 161 (1998). doi:10.1016/S0021-9673(98)00233-7.
- M. Less, T.C. Schmidt, E. von Löw and G. Stork, J. Chromatogr. A. 810, 173 (1998). doi:10.1016/S0021-9673(98)00232-5.
- B. Jurado-Sánchez, E. Ballesteros and M. Gallego, Talanta 79, 613 (2009). doi:10.1016/j.talanta.2009.04.035.
- S. Mishra, V. Singh, A. Jain and K.K. Verma, Analyst 126, 1663 (2001). doi:10.1039/b103008f.
- M. Chen, G. Zhu, S. Wang, K. Jiang, J. Xu, J. Liu and J. Jiang, J. Sep. Sci. 41, 440 (2018). doi:10.1002/jssc.201700786.
- L. Müller, E. Fattore and E. Benfenati, J. Chromatogr. A. 791, 221 (1997). doi:10.1016/S0021-9673(97)00795-4.
- H. Van Doorn, C.B. Grabanski, D.J. Miller and S.B. Hawthorne, J. Chromatogr. A. 829, 223 (1998). doi:10.1016/S0021-9673(98)00760-2.
- W. Chang, Y. Sung and S. Huang, Anal. Chim. Acta 495, 109 (2003). doi:10.1016/j.aca.2003.08.021.
- T. Zimmermann, W.J. Ensinger and T.C. Schmidt, Anal. Chem. 76, 1028 (2004). doi:10.1021/ac035098p.
- N. Sharma, A. Jain and K.K. Verma, Anal. Methods 3, 970 (2011). doi:10.1039/c0ay00745e.
- C.T. Yan and J.F. Jen, Chromatographia 59, 517 (2004).
- C.T. Yan, T.S. Shih and J.F. Jen, Talanta 64, 650 (2004). doi:10.1016/j.talanta.2004.03.053.
- L. Zhu, C.B. Tay and H.K. Lee, J. Chromatogr. A. 963, 231 (2002). doi:10.1016/S0021-9673(02)00547-2.
- J. Peng, J. Liu, G. Jiang, C. Tai and M. Huang, J. Chromatogr. A. 1072, 3 (2005). doi:10.1016/j.chroma.2004.11.060.
- A. Sarafraz-Yazdi and Z. Eshaghi, J. Chromatogr. A. 1082, 136 (2005). doi:10.1016/j.chroma.2005.05.102.
- A. Sarafraz-Yazdi and Z. Eshaghi, Chromatographia 63, 563 (2006). doi:10.1365/s10337-006-0801-2.
- M. del Nogal Sanchez, C. Perez Sappo, J.L. Perez Pavon and B. Moreno Cordero, Anal. Bioanal. Chem. 404, 2007 (2012). doi:10.1007/s00216-012-6303-1.
- K. Reddy-Noone, A. Jain and K.K. Verma, Talanta 73, 684 (2007). doi:10.1016/j.talanta.2007.04.043.
- Z. Wang, Y. Liao, L. Chen and X. Huang, Talanta 220, 121423 (2020). doi:10.1016/j.talanta.2020.121423.
- X. Huang, N. Qiu, D. Yuan and Q. Lin, J. Chromatogr. A. 1216, 4354 (2009). doi:10.1016/j.chroma.2009.03.043.
- J. Zhang, X. Wu, W. Zhang, L. Xu and G. Chen, LCGC North Am. 39, 61 (2021).
- H.N. Snow, LCGC North America 39, 61–67 (2021).
- C. Zhou, C.Y. Luo, H.J. Yu, H.M. Zou, P.N. Xie, X.Y. Chen and Y.X. Li, Chin. J. Anal. Chem. 44, 935 (2016). doi:10.1016/S1872-2040(16)60900-3.
- K. Furukawa, M. Hashimoto and S. Kaneco, Anal. Sci. 33, 1189 (2017). doi:10.2116/analsci.33.1189.
- U. Dorgerloh, R. Becker, H. Theißen and I. Nehls, Environ. Sci. Processes Impacts 15, 2329 (2013). doi:10.1039/c3em00332a.
- R. Becker, U. Dorgerloh, A. Buchholz and A. Hofmann, Accred. Qual. Assur. 19, 371 (2014). doi:10.1007/s00769-014-1072-5.
- ISO 13528:2015, Statistical Methods for Use in Proficiency Testing by Interlaboratory Comparisons (ISO Geneva, Switzerland 2015).
- J. Schubert, O. Kappenstein, A. Luch and T. Schulz, J. Chromatogr. A. 1218, 5628 (2011). doi:10.1016/j.chroma.2011.06.072.
- DIN 32645:2008, Chemical Analysis - Decision Limit, Detection Limit and Determination Limit under Repeatability Conditions - Terms, Methods, Evaluation, Beuth Verlag GmbH, Berlin, Germany (2008)
- DIN 38402-45:2014, German Standard Methods for the Examination of Water, Waste Water and Sludge - General Information (Group A) - Part 45: Interlaboratory Comparisons for Proficiency Testing of Laboratories, Beuth Verlag GmbH, Berlin, Germany (2014)