Abstract
Whimbrels Numenius phaeopus are waders which exhibit no plumage difference between the sexes but some sexual size dimorphism exists, with females being on average larger than the males. During two breeding seasons in Iceland, 50 Whimbrels of the islandicus subspecies were caught on nests, measured and feather samples collected for DNA sexing. Generalised linear models were used to determine the utility of biometric data to sex the birds. Wing length and body mass were the only components that contributed significantly to the final model, which correctly predicted the sex of 76% of the birds sexed by DNA with at least 95% certainty.
Reliable sexing of individuals is of high importance in many studies of ecology, evolution and behaviour since the sexes can vary considerably in terms of many life-history variables (Durell et al Citation1993, Durell Citation2000, McCloskey & Thompson Citation2000, Both et al Citation2003, Bearhop et al Citation2006, Alves et al Citation2013). Many species of waders show reversed sexual size dimorphism, with the females being larger than the males. This dimorphism is, however, often weak and there can be a considerable overlap in biometrics between the sexes (Prater et al Citation1977), making sex determination in the field difficult. Molecular sexing of live birds has made it possible to find biometric methods to determine sex based on measurements for some species (Gunnarsson et al Citation2006, Hallgrimsson et al Citation2008).
The Whimbrel (Numenius phaeopus) is a large shorebird of the Scolopacidae family with a holarctic distribution that breeds in boreal, subarctic and arctic regions (Skeel & Mallory Citation1996). It is split into five subspecies, one of which, N.p. islandicus, breeds in Iceland, the Faeroes, the UK and presumably in Greenland, and winters mainly in West Africa (Delany et al Citation2009). It is on average larger than the nominate subspecies (Engelmoer & Roselaar Citation1998), which is found in Scandinavia, the Baltic States and northwestern Russia during the breeding season (Delany et al Citation2009). Whimbrels show no plumage difference between the sexes although the females are on average larger than the males in all biometric characteristics (Prater et al Citation1977). However, the extent to which biometrics can be used to correctly identify the sex of individual Whimbrels is poorly known. In this study, we investigate the reliability of biometric measurements in predicting the sex of N.p. islandicus using birds that were also sexed using DNA from feather samples.
In total, 50 Whimbrels were caught in south Iceland during the breeding season in 2009 and 2010. Birds were captured on nests using a tilting cage (RB60, www.moudry.cz), individually marked and weighed to the nearest 5 g with a Pesola balance. The length of the exposed culmen (bill length) and total head length (skull + culmen) were measured to the nearest 0.1 mm with vernier calipers, flattened (maximum) wing chord and tarsus–toe length to the nearest 0.1 mm with a stopped ruler and 8–10 feathers were plucked from the bird's chest and belly.
Birds were sexed from DNA extracted from their feathers. The basal part of the calamus was cut and placed in 250 µl of Chelex solution (6%) along with 2.5 µl of proteinase K (1%). Two feathers were used for each extraction. The sexing method used identifies gender-based variability in the introns of CHD1 genes (Fridolfsson & Ellegren Citation1999). Females display one (CHD1W) or two fragments (CHD1W and CHD1Z) while males display only one fragment (CHD1Z) that is clearly different in size from the female-specific CHD1W fragment.
All polymerase chain reactions (PCR) were conducted in a volume of 10 µl using 2.48 µl double-distilled water, 0.75 µl dinucleotide triphosphates (1 mM), 1 µl Tween 20 (1%), 1 µl Taq Buffer (10x), 1 µl bovine serum albumin (10 mg/ml), 0.34 µl 2550F primer, 0.34 µl 2718R primer (both listed by Fridolfsson & Ellegren Citation1999) and 0.09 µl UAmpliTaq enzyme. Then 3 µl (about 10-200 ng/µl) of DNA extraction was added to the PCR solution. The PCR conditions were as described by Fridolfsson & Ellegren Citation(1999). PCR products were separated by electrophoresis through 1.5% agarose gels and visualised under UV light after staining with ethidium bromide.
Generalised linear models with binomial errors and a logit link function were used to examine relationships between sexes based on DNA, body mass, length of wing, culmen, head and tarsus–toe, with sex as the binary response variable. Collinearity of the variables was estimated with the variance inflation factor (VIF). A VIF value smaller than 10 is generally not of concern (Quinn & Keough Citation2002).
Models predicting sex based on biometrics were compared to sexing results from DNA analysis. One male bird was excluded from part of the analysis because tarsus–toe measurements were not collected. Ideally, a conservative validation of the model would require two independent data sets, one for model building and one for testing but due to the small sample size this was not feasible. All statistical analysis was conducted using R (R Development Core Team Citation2011). Of 50 birds that were molecularly sexed, 24 were males and 26 were females. The averages of the different measures were all significantly higher for the females (). All measurements overlapped to some extent, with body mass and wing length having the least amount of overlap (. Analysis of collinearity indicated high dependence of the variables when head length was included (VIF for head length = 89.24). When total head length was excluded there was no sign of collinearity between the other variables, with the VIFs for body mass, wing, tarsus–toe and culmen being 1.43, 1.35, 1.77 and 1.73, respectively.
Table 1. Biometrics of N.p. islandicus breeding in south Iceland.
Figure 1. a) Measurements of mass and wing length of Whimbrels. Females are shown with solid circles and males with open circles. b) The probability of determining the sex of female Whimbrels using wing length and body mass according to a GLM. Females are shown with solid circles and males with open circles and the horizontal lines show the critical limit of 0.05 and the 0.5 midpoint.
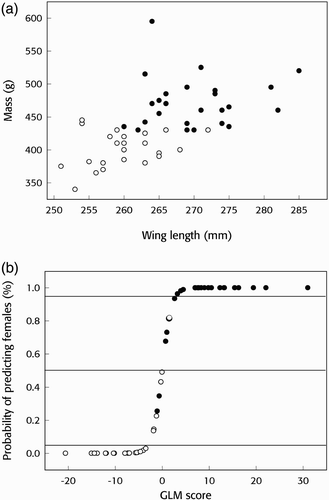
The full model (Eq 1) correctly sexed 95.9% (47/49) of the birds and 75.5% of those were sexed with ≥95% probability of being of the assigned sex (). Omitting culmen from the model (Eq 2) did not affect the results (). Wing length and body mass were the only components that contributed significantly to the model (Eq 3) and correctly predicted the sex of 46 of 49 birds (; .
Table 2. Comparison of full and reduced models. Deviance and probability are due to the last component added to the models with one degree of freedom. AIC is the Akaike Information Criterion, which reflects the overall fit of the model, with lower values indicating a better fit.
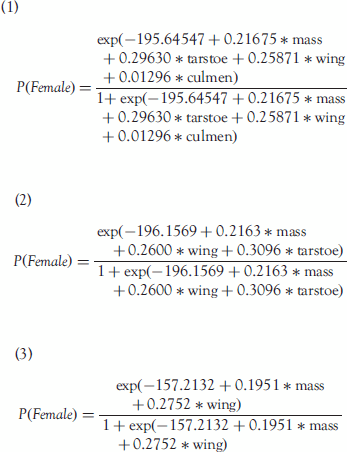
There was a negative relationship between body mass and capture date. As the breeding season progressed, the birds got lighter (linear regression: y = 514.657 - 1.520x; R2 = 0.154; P = 0.003). When tested separately for each sex, however, the relationship was significant only for females (females: y = 535.740 - 1.334x; R2 = 0.177; P = 0.018; males: 445.668 - 0.821x; R2 = 0.116; P = 0.058). Models with body mass corrected by capture date (ie with residual mass on capture date) were tested but predictions from those models were poorer than when body mass was unchanged.
The full model based on biometrics correctly predicted the sex of 96% of the birds when compared to DNA analysis. Wing length and body mass were the components that contributed the most to the model and, by including only these, the proportion of correctly sexed birds was only slightly lowered from the full model (by one bird out of 49). Body mass of Whimbrels is not constant over the breeding season and the birds get lighter as the season progresses. However, correcting for time of season did not improve the model, perhaps because of the high level of variability in mass with capture date. Using wing, culmen and tail length of skins of Hudsonian Curlew (N. p. hudsonicus), a discriminant function allowed 95% of birds to be correctly sexed (Skeel Citation1982). Tail length measurements might possibly improve the model presented here.
Discrimination models based on biometric data are a simple and inexpensive tool to predict the sex of birds. However, it has been shown that sexual dimorphism in waders can vary in time and between areas (Zwarts et al Citation1996, van de Pol et al Citation2009). The biometric measures reported here are slightly higher than measurements taken in an earlier study in Iceland (Gunnarsson Citation2000), although the difference is significant only for the wing length of males. Measurements in this study also differ from measurements of N.p. islandicus from Shetland (A. Perkins, pers comm), with Icelandic females being significantly heavier but tarsus–toe longer for birds from Shetland (significantly for females). Therefore caution is advised when using biometric measurements in predicting the sex of waders; models for sexing need be tested on a regular basis and updated as necessary.
Polymorphism in the Z chromosome has been reported for auklets (Aethia spp; Dawson et al Citation2001) and for Black-tailed Godwits (Schroeder et al Citation2008), causing some males to display two fragments instead of one, which might produce incorrect DNA sexing. In those cases though, other primers were used which amplify a different fragment of an intron of the same gene (Griffiths et al Citation1998). When the procedure was repeated with the 2550F and 2718R primers, no such polymorphism was detected (Dawson et al Citation2001, Schroeder et al Citation2008). The method developed by Fridolfsson & Ellegren Citation(1999) worked well for the Whimbrels and results from the agarose electrophoresis were conclusive. Our results show that wing length and body mass can be used for predicting the sex of Whimbrels N.p. islandicus during the breeding season with high levels of accuracy. These results are likely to have practical applications for sexing of the islandicus subspecies in the countries where they breed and during migration in other countries.
REFERENCES
- Alves , J. A. , Gunnarsson , T. G. , Potts , P. M. , Sutherland , W. J. and Gill , J. A. 2013 . Sex-biases in distribution and resource use at different spatial scales in a migratory shorebird . Ecology and Evolution , 3 : 1079 – 1090 . (doi:10.1002/ece3.503)
- Bearhop , S. , Phillips , R. A. , McGill , R. , Cherel , Y. , Dawson , D. A. and Croxall , J. P. 2006 . Stable isotopes indicate sex-specific and long-term individual foraging specialisation in diving seabirds . Marine Ecology Progress Series , 311 : 157 – 164 . (doi:10.3354/meps311157)
- Both , C. , Edelaar , P. and Renema , W. 2003 . Interference between the sexes in foraging Bar-tailed Godwits Limosa lapponica . Ardea , 91 : 268 – 272 .
- Dawson , D. A. , Darby , S. , Hunter , F. M. , Krupa , A. P. , Jones , I. L. and Burke , T. 2001 . A critique of avian CHD-based molecular sexing protocols illustrated by a Z-chromosome polymorphism detected in auklets . Molecular Ecology Notes , 1 : 201 – 204 . (doi:10.1046/j.1471-8278.2001.00060.x)
- Delany , S. , Scott , D. , Dodman , T. and Stroud , D. 2009 . An Atlas of Wader Populations in Africa and Western Eurasia , Edited by: Delany , S. , Scott , D. , Dodman , T. and Stroud , D. Wageningen , , the Netherlands : Wetlands International .
- Durell , S. E.A. and le , V. dit . 2000 . Individual feeding specialisation in shorebirds: population consequences and conservation implication . Biological Reviews , 75 : 503 – 518 . (doi:10.1111/j.1469-185X.2000.tb00053.x)
- Durell , S. E.A. , le , V. dit , Goss-Custard , J. D. and Caldow , R. W.G. 1993 . Sex-related differences in diet and feeding method in the oystercatcher Haematopus ostralegus . Journal of Animal Ecology , 62 : 205 – 215 . (doi:10.2307/5495)
- Engelmoer , M. and Roselaar , C. S. 1998 . Geographical Variation in Waders , Dordrecht , , the Netherlands : Kluwer Academic Publishers .
- Fridolfsson , A. K. and Ellegren , H. 1999 . A simple and universal method for molecular sexing of non-ratite birds . Journal of Avian Biology , 30 : 116 – 121 . (doi:10.2307/3677252)
- Griffiths , R. , Double , M. C. , Orr , K. and Dawson , R. J.G. 1998 . A DNA test to sex most birds . Molecular Ecology , 7 : 1071 – 1075 . (doi:10.1046/j.1365-294x.1998.00389.x)
- Gunnarsson , T. G. 2000 . Stofnvistfræði spóa á Suðurlandi. (Population ecology of the whimbrel Numenius phaeopus in S-Iceland) . Unpublished MSc thesis, University of Iceland
- Gunnarsson , T. G. , Gill , J. A. , Goodacre , S. L. , G'Elinaud , G. , Atkinson , P. W. , Hewitt , G. M. , Potts , P. M. and Sutherland , W. J. 2006 . Sexing of Black-tailed Godwits Limosa limosa islandica: a comparison of behavioural, molecular, biometric and field-based techniques . Bird Study , 53 : 193 – 198 . (doi:10.1080/00063650609461433)
- Hallgrimsson , G. T. , Palsson , S. and Summers , R. W. 2008 . Bill length: a reliable method for sexing Purple Sandpipers . Journal of Field Ornithology , 79 : 87 – 92 . (doi:10.1111/j.1557-9263.2008.00148.x)
- McCloskey , J. T. and Thompson , J. E. 2000 . Sex-related differences in migration chronology and winter habitat use of Common Snipe . Wilson Bulletin , 112 : 143 – 148 . (doi:10.1676/0043-5643(2000)112[0143:SRDIMC]2.0.CO;2)
- van de Pol , M. , Oosterbeek , K. , Rutten , A. L. , Ens , B. J. , Tinbergen , J. M. and Verhulst , S. 2009 . Biometric sex discrimination is unreliable when sexual dimorphism varies within and between years: an example in Eurasian Oystercatchers Haematopus ostralegus . Ibis , 151 : 171 – 180 . (doi:10.1111/j.1474-919X.2008.00891.x)
- Prater , A. J. , Marchant , J. H. and Vuorinen , J. 1977 . Guide to the Identification and Ageing of Holarctic Waders. BTO Guide 17 , Tring : British Trust for Ornithology .
- Quinn , G. P. and Keough , M. J. 2002 . Experimental design and data analysis for biologists , Cambridge : Cambridge University Press .
- R Development Core Team . 2011 . R: a language and environment for statistical computing , Vienna , , Austria : R Foundation for Statistical Computing . www.R-project.org/
- Schroeder , J. , Lourenço , P. M. , van der Velde , M. , Hooijmeijer , J. C.E.W. , Both , C. and Piersma , T. 2008 . Sexual dimorphism in plumage and size in Black-tailed Godwits Limosa limosa limosa . Ardea , 96 : 25 – 37 . (doi:10.5253/078.096.0104)
- Skeel , M. A. 1982 . Sex determination of adult Whimbrels . Journal of Field Ornithology , 53 : 414 – 416 .
- Skeel , M. A. and Mallory , E. P. 1996 . “ Whimbrel (Numenius phaeopus) ” . In The Birds of North America , Edited by: Poole , A. and Gill , F. Washington , , D.C : 219, Academy of Natural Sciences, Philadelphia, PA, and American Ornithologists' Union .
- Zwarts , L. , Hulscher , J. B. , Koopman , K. and Zegers , P. 1996 . Discriminating the sex of Oystercatchers Haematopus ostralegus . Ardea , 84 ( A ) : 1 – 12 .