Abstract
Urban gull populations have increased dramatically in the last 40 years, leading to widespread concerns about potential nuisance to humans, but little accompanying research into their ecology. This study aimed to provide the first long-term estimates of apparent adult survival rates for urban Herring Gulls Larus argentatus and Lesser Black-backed Gulls L. fuscus, based on colour ringing in Bristol, southwest England. Resightings of adult birds covering 18 years (1990–2007) were analysed using capture–mark–recapture methods, with candidate models testing for differences in survival and/or resighting rate through time and between the sexes. Both species showed high apparent annual survival rates (>0.90) in the early 1990s that declined to <0.70 by 2007. Male survival rates were higher than female rates in Lesser Black-backed Gulls, and male resighting rates were higher in both species. In the early 1990s, Bristol's urban gulls displayed similar adult survival rates to published estimates for rural colonies. Both species showed evidence of long-term declines in apparent survival, which may either reflect actual reductions in survival or increased permanent emigration from the Bristol colony. Anecdotal evidence supports the latter, linking emigration to urban redevelopment or human intervention.
Since first starting to colonise towns and cities around 50 years ago, urban gull populations have increased dramatically across Britain and Ireland, many other parts of Europe and beyond (eg Nankinov Citation1992, Benussi et al Citation1994, Dwyer et al Citation1996, Lilleør Citation2000, Temby Citation2000, François Citation2002, Martí & Del Moral Citation2003, Cadiou et al Citation2004, Soldatini et al Citation2008). In the UK, the main urban colonists are Herring Gulls Larus argentatus and Lesser Black-backed Gulls L. fuscus (Mitchell et al Citation2004, Rock Citation2005). Although initial colonisation was slow, by the 1980s urban populations of Herring and Lesser Black-backed Gulls were expanding rapidly throughout Britain and Ireland (Rock Citation2005). In the last 40 years, UK urban populations have increased by well over an order of magnitude, from estimates of around 1,300 pairs in 1969–70 (Cramp Citation1971) to 31,000 in 1999–2002 (Mitchell et al Citation2004). This is in sharp contrast to declines in breeding numbers of rural Lesser Black-backed Gulls and, particularly, of Herring Gulls which are now red listed (Eaton et al Citation2009). Quite apart from continuing growth over the last ten years, it is likely that most urban surveys underestimate population size, due to the difficulties of surveying in urban environments (eg access to superior vantage points and the complex topography of cityscapes; Madden & Newton Citation2004, Calladine et al Citation2006). The expansion of the urban population is such that there are now at least 400 towns and cities supporting colonies in Britain and Ireland, ranging in size from a few pairs to over 3,000 pairs (PR, pers obs).
The corollary of rapid population growth is that urban gulls have become increasingly unwanted and much complained about in many towns and cities because they present a plethora of problems (Belant Citation1997, Rock Citation2005). Local authorities receive a large number of complaints about noise, mess, damage and aggression (Rock Citation2005, Calladine et al Citation2006). The last two decades have seen the problems frequently being covered by the media and becoming a political issue for both local and national government (Hansard Citation2009, Citation2010, Citation2011). Whilst this has generated increased interest in controlling urban gull populations, reflected in increasing sums of money spent on interventions, very little is known about the ecology of urban gulls that could underpin possible management strategies (Rock Citation2005, Coulson & Coulson Citation2009). This contrasts markedly with the rural situation, where the ecology and behaviour of large gulls have been well studied (eg Tinbergen Citation1953, Spaans Citation1971, Cramp & Simmons Citation1983, Vercruijsse Citation1999). Towns and cities provide gulls with a range of potential benefits including extensive areas of roofing free from predators, a warmer climate and anthropogenic sources of food – notably waste or discards – that can be exploited at night thanks to street lighting (Rock Citation2005, Møller Citation2009). At the same time, urban colonies are also subject to widespread human disturbance, ranging from ongoing roof maintenance, through active measures to control gulls (eg replacement of eggs, removal of nests) or discouragement of breeding (eg netting, spikes or acoustic deterrents), to the demolition and redevelopment of key buildings used for nesting (Rock Citation2005, Calladine et al Citation2006). It is expected, therefore, that the ecology of urban gulls will differ from their rural counterparts, but there is limited empirical evidence. This balance needs to be redressed if sustainable solutions to human–gull conflicts are to be devised.
In long-lived species, such as many seabirds, adult survival may exert greater influence over population trends than factors such as fecundity or juvenile recruitment (Sæther & Bakke Citation2000, Sibly & Hone Citation2002). The longevity records of Herring and Lesser Black-backed Gulls are just under 35 years each (Fransson et al Citation2010). Several studies have estimated adult Herring or Lesser Black-backed Gull survival rates in rural colonies (eg Coulson & Butterfield Citation1986, Brown et al Citation2004, Breton et al Citation2008), but there is a lack of comparable work from urban colonies. In this paper we estimate apparent survival rates of adults of both species in the city of Bristol, southwest England. Our overall aim was to estimate adult survival rates over an 18-year period: this is, to our knowledge, the first long-term study on urban gulls. Within this, we aimed to evaluate two hypotheses: first, that adult survival rates were similar to published estimates for rural colonies and remained constant through time; and, second, that the sexes differed in their apparent survival and resighting rates. Bristol exemplifies the pattern and timescale of colonisation by gulls witnessed in many UK towns and cities, with its first roof-nesting Herring Gulls recorded in 1972 (P. Chadwick, pers comm), a breeding population of c 100 pairs established by 1980 (pers obs) and recent population estimates of 1,933 pairs in 2004 and at 2,495 pairs in 2010 (Rock Citation2004, Citation2010).
METHODS
Study area and data collection
Bristol (51.27°N 2.35°W) is the largest city in southwest England, with a human population of 430,000 (Bristol City Council Citation2012). At 2,495 breeding pairs, it is thought to be the third-largest gull colony in the Severn Estuary region, which as a whole supports over 25,000 pairs of urban gulls, spread across c 70 colonies (Rock Citation2005). The Bristol colony is 11 km from the coast and 38 km from the nearest major rural colonies on the islands of Flat Holm and Steep Holm. Gulls nest on a wide variety of roof types across the city.
Gulls have been colour ringed in Bristol since 1980 (the Bristol Scheme: Rock Citation1999). Birds were ringed as nestlings within 14 days of estimated fledging on accessible rooftops in the colony. A standard metal ring and a large (37 mm tall) Darvic/Vynalast colour ring showing a two-letter code were fitted: the latter may be read at distances of up to 400 m using a telescope (Rock Citation1999). In most years, 150–300 nestlings were ringed (mean = 230), at a ratio of approximately 3:1 Lesser Black-backed to Herring Gulls, reflecting the relative abundance of the two species. Three biometric measurements – total head length (ie head and bill), bill depth at gonys and wing length (maximum chord) – were made on all nestlings. In 1993 and 1994 blood samples were taken from 466 nestlings at the time of ringing to determine sexes using molecular methods (Griffiths Citation1991). A logistic regression developed from these nestlings (J. Cobby, pers comm) was used to predict the sex for all nestlings in the current study (see Appendix).
Resighting effort was similar throughout the study period, with two to four visits per week to the gull colony during the main breeding season (April–July inclusive). Observations of colour rings were made from sites offering commanding views over rooftops (with sessions lasting 30–60 minutes) and by driving standard routes through the city checking small groups of gulls seen from ground level.
Data analysis
The focus for this study was on adult survival in the Bristol breeding colony. We therefore limited the data to resightings of adults (ie fifth calendar year (5cy) and older) during the main breeding season (April–July) at the Bristol colony and removed resightings of immature birds (up to 4cy). Recruitment and survival rates of immatures will be investigated separately. The first resighting of a bird at the Bristol colony at an age of four or more years acted as the initial capture event (Lebreton et al Citation1992). Resightings during the 1980s were excluded as they were few in number, whilst records up to 2007 were included, giving a final sample size of 500 sightings of 191 individual Herring Gulls during 1991–2007 (129 males, 62 females) and 1,373 sightings of 505 individual Lesser Black-backed Gulls 1990–2007 (365 males, 140 females). The sex bias is consistent with the lower natal-colony fidelity of female gulls (Rock Citation2005). The Herring Gull data were sparse, limiting the power and goodness-of-fit testing (see below). To counter this, we compared only a small set of relatively simple models, but the results still need to be treated with some caution.
Ringing data were analysed with capture–mark–recapture (CMR) methods using MARK (version 5.1, White & Burnham Citation1999). Following convention, this involved fitting a very general model to the data, checking that it met the basic assumptions of CMR analysis, and then comparing this general model to a series of alternatives suggested a priori that were more parsimonious or represented alternative hypotheses about survival and resighting rates (Lebreton et al Citation1992). The relative strength of evidence for these different hypotheses was assessed using differences in the small-sample Akaike Information Criterion (AICc; Burnham & Anderson Citation2002).
The analysis started with the Cormack–Jolly–Seber (CJS) model generalised to two sexes (groups): where
represents survival, p the resighting probability, and subscripts s and t that survival and resighting probabilities vary between sexes and among years, respectively (Lebreton et al
Citation1992). The goodness-of-fit of this general model was examined using U-CARE software (version 2.3; Choquet et al
Citation2005). For Herring Gulls, the fit of the general model was adequate (TEST2 + TEST3,
= 55.5, p = 1.00). The power of these overall tests was probably limited by the sparse data, and so the component tests for individual years were also examined, to confirm that there were no indications of a systematic lack of fit (Cooch & White Citation2008).
For Lesser Black-backed Gulls, the general model did not fit the data (TEST2 + TEST3, = 142.0, p = 0.016), primarily due to component TEST2.CT (
= 53.2, p = 0.002). This was consistent with ‘immediate trap-dependence’: a violation of the basic CMR assumption of homogeneity in resighting probabilities, reflecting an elevated probability of resighting individuals seen in the previous year compared to the marked population as a whole. The model was reconfigured in a multi-state framework to model this heterogeneity (Lebreton & Pradel Citation2002). Following Giminez et al
Citation(2003), two states were defined – ‘resighted’ and ‘not resighted’ – and the resighting probabilities for these two states were set to 1 and 0 respectively. The transition probabilities to the resighted state represented the actual resighting probabilities, estimated separately for birds seen or not seen in the previous year (Giminez et al
Citation2003, Frederiksen et al
Citation2004). The resulting general model was
, where p
*
is the probability of resighting a bird that was seen in the previous year and p is the probability given that it was not resighted (Crespin et al
Citation2006). The new goodness-of-fit, calculated from the three components TEST3.SR, TEST2.Cl and TEST3.SM, indicated that model fit was acceptable (
= 88.9, p = 0.26).
By focusing upon the breeding season, where resighting effort was broadly constant, we aimed to eliminate time variation in resighting probability from the models, to produce more parsimonious models and to reduce the number of candidate model comparisons. In all instances, adding time dependence in resighting probability to the models presented here ( & 2) either increased the AICc by >4.6 (and often >10), or for four Lesser Black-backed Gull models where the AIC did decrease, those four models still received considerably less support (ΔAICc >6.6) than the preferred models with constant resighting probabilities and time-dependent survival, confirming the validity of this assumption. For brevity, we do not present these models, but start with the following general models: Herring Gull (ΔAICc relative to
= 53.2) and Lesser Black-backed Gull
(ΔAICc relative to
= 78.9).
Table 1. Comparison of the a priori models for Herring Gulls, based on the number of estimable parameters (np) and AICc. Basic model notation follows Lebreton et al
Citation(1992), with φ
 and p representing survival and resighting rates, respectively, and among the subscripts: t = full time-dependence, T = linear trend, Quad_T = quadratic trend, s = sex, no subscript = rate was constant over time.
and p representing survival and resighting rates, respectively, and among the subscripts: t = full time-dependence, T = linear trend, Quad_T = quadratic trend, s = sex, no subscript = rate was constant over time.
Modelling involved two stages. The first compared eight models to address our Hypotheses 1 and 2: that adult survival rates were constant through time and that adult survival and resighting rates differed between sexes. In the second stage, we retained the best resighting structure from stage one, and compared a further two models to evaluate the evidence for a trend in survival (
) through time (Hypothesis 1), rather than simply the presence of any time-dependent variation. Temporal survival trends were modelled as either a linear relationship (on a logit scale) or including a quadratic term, to assess evidence of simple non-linearity.
RESULTS
Herring Gulls
CMR modelling provided evidence that Herring Gull survival rates varied through time and resighting varied between the sexes (). Amongst the first eight models (), the four models containing had the lowest AICc values: addition of
decreased the ΔAICc by 2.6–2.9 compared to the equivalent models with constant survival rates (
or
; ). The evidence for differences in survival rates between males and females was equivocal, with the best-supported model
not modelling sex differences and the addition of sex to the survival term changing AICc by <1.5: increasing the AICc in two cases and decreasing it in the other two (). There was evidence of differences in the resighting rate between the sexes, with the two highest-ranked models including p
s
, and the addition of sex dependence always decreasing the AICc – by >2.25 in three of the four comparisons. The model with the greatest support from the first modelling stage (
) had time-, but not sex-dependent survival and sex-dependent resighting rates.
Table 2. Comparison of the a priori models for Lesser Black-backed Gulls. Notation as for , with the exception that p = probability of resighting individuals not seen in the previous year and p* = probability of resighting for birds resighted in the previous year.
In the second modelling stage there was strong support for a non-linear temporal trend in survival (
versus
ΔAICc = 5.5), with both trend models having greater support than general temporal variation
(ΔAICc = 12.9 and 7.4; ). The preferred model overall (
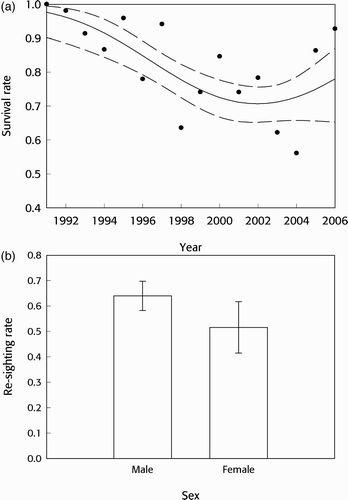
) revealed that estimated apparent survival rates were high (>0.95) at the start of the study period, but declined to 0.71 over the next ten years, before stabilising or even increasing slightly towards the end of the study (. The point survival estimates for 2005–06 and 2006–07 were both >0.85, suggesting a large increase in apparent survival, but need to be treated with caution due to wide confidence limits (0.52–0.97 and 0.15–1.00 respectively). This corresponded well to the survival estimates for individual years estimated for the equivalent general time model (
; . Male resighting rate (0.64) was higher than the female rate (0.52; .
Figure 1 Herring Gull survival (a) and resighting (b) rates from the preferred model (). In (a), estimated survival (solid line) is shown with 95% confidence limits (dashed lines) and point estimates for individual years (filled circles) based upon the equivalent fully time-dependent model (
): the latter are plotted at the first year in the pair between which survival was estimated eg at 1991 for survival between 1991 and 1992. In (b), estimates are shown with 95% confidence limits.
Lesser Black-backed Gulls
There was evidence of temporal variation in Lesser Black-backed Gull apparent survival rates and for differences among the sexes in both survival and resighting rates (). Amongst the first eight models () there was strong evidence for time dependence in survival, with the top three models including and the addition of
reducing AICc by >16 for all four possible comparisons with equivalent constant-survival models (eg
versus
). Sex dependence in survival rates was present in the top two models ranked by AICc and the addition of sex to the survival part of the model always decreased the AICc by at least 8.0. Similarly, adding sex dependence to the resighting part of the model always reduced the AICc (all ΔAICc >4.0). The preferred model from the first stage of modelling (
) had time and sex effects on survival, and sex-specific resighting rates. Sex was considered only as an additive term: hence the same temporal pattern was modelled for both sexes.
In the second stage of modelling there was evidence of a linear (on the logit scale) trend in survival rates, with the model including a quadratic effect of time on survival (
) marginally increasing AICc compared to a linear trend (
), but both types of model having substantially smaller AICc (ΔAICc >5) than the equivalent general time-dependent model (
; ). The preferred model for Lesser Black-backed Gulls (
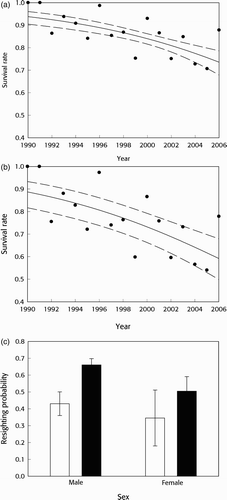
) indicated that males had greater apparent survival rates than females (by 0.05–0.14), with overall survival rates declining through time from 0.94 and 0.89 at the start of the study to 0.74 and 0.59 by the end ( & b). As for Herring Gulls, these rates agreed with individual year estimates made with the equivalent fully time-dependent model (
; & b). Resighting rates were higher for males than for females, and for birds seen in the previous year, revealing the apparent ‘trap dependence’ effect (.
Figure 2 Lesser Black-backed Gull survival and resighting rates based upon the preferred model (). The top panels show survival rates through time for (a) males and (b) females: solid lines are estimated survival rates, dashed lines represent 95% confidence limits and filled circles represent point estimates for individual years from the equivalent model with full time dependence (
): the latter plotted at the first year of the pair between which survival was estimated. Resighting rates (c) with 95% confidence limits are separated for males and females, and birds seen in the previous year (filled bars) or not seen in the previous year (open bars).
DISCUSSION
Long-term survival studies are rare and particularly important for long-lived species, but they bring additional challenges including the potential for ring loss over time, which could bias survival estimates (Nelson et al Citation1980, Kendall et al Citation2009). Whilst there has probably been some ring loss amongst Bristol Scheme gulls, we are confident for several reasons that this should have had a minimal impact upon our results. First, the very high survival rate estimates (>0.9) in the early 1990s would not be possible with a high rate of ring loss. Second, by including only birds aged four or above, the initial periods of elevated ring loss that can sometimes occur through damage to the ring during fitting were avoided (Ward Citation2000). Third, regular resightings of birds over ten years old made it clear that there was no excessive ring wear or colour fading, as rings were still clearly legible. Work with waders has revealed similarly high longevity of Darvic rings (≥17 years; Ward Citation2000). Finally, there was no evidence of ring-loss effects in the data analysis, as we refitted models: i) including age-dependent survival (which could indicate increasing ring loss with age) and the AICc increased >10 in every instance, and ii) just using birds in a narrow age range (four to eight years old), and the overall results and temporal pattern were the same. Although our apparent survival rates will be slight underestimates, the temporal trend should be unaffected.
Estimates of apparent survival rates were similar for Herring and Lesser Black-backed Gulls, and for both species, survival rates in the early to mid 1990s were similar to those reported in previous studies of rural colonies. Our estimates for Herring Gulls pre-1995 (0.88–0.98) compared with estimates of 0.87–0.94 from other colonies in Europe and North America (Chabrzyk & Coulson Citation1976, Coulson & Butterfield Citation1986, Allard et al Citation2006, Breton et al Citation2008). Similarly, our Lesser Black-backed Gull estimates over the same period (0.90–0.94) were similar to the upper end of previously estimated values in the range 0.78–0.98 (Wanless et al Citation1996, Brown et al Citation2004, Camphuysen & Gronert Citation2012).
The striking features about apparent survival rates in this study were the overall declines through time and the similar pattern in both species, suggesting a common cause. These declines occurred during a period of continuing growth in Bristol's breeding population, estimated at around 5% annually during 2004–10 (Rock Citation2010). Apparent survival combines mortality and permanent emigration and in species showing high breeding site fidelity, such as the large gulls (Tinbergen Citation1953), it may be expected to represent actual survival closely. By restricting the study to individuals of breeding age, the effects of changing natal dispersal through time were excluded. Mortality amongst the Bristol colony may have increased through time, perhaps through density-dependent competition, disease, or fatalities associated with the increased prevalence of control measures (eg roof netting; Rock Citation2005). However, although no data were collected on gull mortality, there was no evidence of increasing large-scale adult mortality over the study period that could account for the large falls in apparent survival rate.
A more likely driver of declining apparent survival was increased permanent emigration. This could be a density-dependent response to the increasing size of the Bristol colony, although many apparently suitable roofs remain unoccupied (pers obs). An alternative possibility is that emigration was being increased by anthropogenic disturbance. Attempts to control gulls in Bristol have increased as the gull population has expanded. These have included preventing access to roofs (eg with netting), forcing birds to relocate, and egg-oiling or replacement, which may also displace pairs (eg Moon Citation2009). At the same time, the decade from the late 1990s witnessed major redevelopment in Bristol city centre (Bristol City Council Citation2011), with demolition of many buildings that supported breeding gulls coinciding with the main decline in apparent survival rates. It is possible that, as with Great Skuas Stercorarius skua, breeding failure can lead to divorce (Catry et al Citation1997) followed by relocation. A study of urban Lesser Black-backed and Herring Gulls in Dumfries suggested that whilst some of the birds displaced from roofs by deterrent measures relocated within the colony, a proportion probably emigrated permanently (Coulson & Coulson Citation2009). Similarly, a mixture of local and longer-distance movements has been observed following the demolition of nesting buildings (Rock Citation2005).
If increased emigration from Bristol has underlain the decreases in survival rates, it raises the question of where these emigrant birds have gone. There have been about ten instances in recent years of an exchange of recruits between urban and rural colonies in the Severn Estuary region, similar to the number from rural to urban (pers obs), but many more recruits among urban colonies, leading to suggestions that rural and urban populations are largely discrete (Rock Citation2005, Citation2006). Within this, there are now more than 20 known instances in the Bristol Scheme data set of birds leaving the Bristol colony after documented disturbance and settling in other urban colonies in the Severn Estuary region. For example, two Lesser Black-backed Gulls displaced from Bristol in 1997 after the demolition of the building they had nested on for several years were found breeding several years later in urban colonies in Chippenham (32 km away) and Paulton (18 km away). Had they relocated in 1998, they may have been among the first colonists in these towns.
Sex differences in survival and resighting rates similar to those detected here have been reported previously amongst the Larus gulls. Male resighting rates were higher than for females in both species, consistent with previous work on Herring Gulls (Breton et al Citation2008). This may reflect more conspicuous behaviour such as taking up prominent positions within the breeding territory (Tinbergen Citation1953, Cramp & Simmons Citation1983). A notable feature of Lesser Black-backed Gull resighting rates was the greater resighting probability of individuals seen in the previous year compared to the average probability amongst the ringed birds. Several mechanisms may account for this observation, one of which is the possibility of birds missing one or more breeding seasons following disturbance, deterrence or demolition, compared to successful breeders, which are more likely to return the following year (Coulson & Coulson Citation2009). This kind of behaviour has been recorded several times in Bristol, with birds apparently absent from the breeding colony for one or more years following disturbance, but resighted subsequently, often in different parts of the city (pers obs).
The absence of a sex difference in Herring Gull survival rate was consistent with earlier studies (Pons & Migot Citation1995, Allard et al Citation2006, Breton et al Citation2008), although there have been hints that female survival may be slightly higher (Coulson & Butterfield Citation1986, Wanless et al Citation1996) or lower (Camphuysen & Gronert Citation2012). Our models including sex-specific survival rates were not rejected outright, but all had marginally greater AICc values. If the difference between the sexes was relatively small, the modest number of Herring Gulls in our study may not have provided sufficient power to detect an effect. Sex differences in Lesser Black-backed Gull survival have been little documented, but we found evidence that males had greater apparent survival than females. This could either reflect true differences in survival, with males faring better outside the breeding season than females or, perhaps, in spite of disturbance, their greater site fidelity (Rock Citation2005).
To relieve the problems increasingly being faced by those who live or work in close proximity to urban gull colonies, we need better information about the size, distribution and demography of those colonies, along with a clearer picture of their wider ecology (eg food sources). Colour ringing can be a powerful tool for investigating the lives of the large gulls (Rock Citation2002) and interrogating the Bristol Scheme data set further (more than 70,000 post-fledging records) will cast ever more light on the urban gull issue. In particular, the current study hints that attempts to control urban gull numbers and other forms of disturbance may generate short- and long-term behavioural and demographic responses in urban gull colonies, and may simply be moving the problems around. Understanding this kind of displacement is clearly a research priority.
ACKNOWLEDGEMENTS
Grateful thanks go to the owners and managers of the many buildings in Bristol used as vantage points or ringing locations, without whose help this study would not have been possible. Many thanks are due also to the birders who have contributed additional records of Bristol Scheme birds before, during and since the study period. Thanks, too, for helpful comments from the reviewers.
REFERENCES
- Allard , K. A. , Breton , A. R. , Gilchrist , H. G. and Diamond , A. W. 2006 . Adult survival of herring gulls breeding in the Canadian Arctic . Waterbirds , 29 : 163 – 168 . (doi:10.1675/1524-4695(2006)29[163:ASOHGB]2.0.CO;2)
- Belant , J. L. 1997 . Gulls in urban environments: landscape-level management to reduce conflict . Landscape and Urban Planning , 38 : 245 – 258 . (doi:10.1016/S0169-2046(97)00037-6)
- Benussi , E. , Flapp , F. and Mangani , U. 1994 . La popolazione di Larus cachinnans michahellis nidificante nella città di Trieste . Avocetta , 18 : 21 – 27 .
- Breton , A. R. , Fox , G. A. and Chardine , J. W. 2008 . Survival of adult Herring Gulls (Larus argentatus) from a Lake Ontario colony over two decades of environmental change . Waterbirds , 31 : 15 – 23 . (doi:10.1675/1524-4695(2008)31[15:SOAHGL]2.0.CO;2)
- Bristol City Council . 2011 . Bristol Local Economic Assessment, March 2011 , Bristol : Bristol City Council .
- Bristol City Council . 2012 . Briefing Note: Mid-2011 Population Estimates , Bristol : Bristol City Council .
- Brown , J. G. , Perrins , C. M. , Boyle , D. , Duffield , S. , Dunstan , A. , Easton , J. , Smith , S. and Parsons , M. 2004 . Seabird monitoring on Skomer Island in 1999–2002. JNCC Report 339 , Aberdeen : Joint Nature Conservation Committee .
- Burnham , K. P. and Anderson , D. R. 2002 . Model Selection and Multimodel Inference. Second edition edn , New York : Springer-Verlag .
- Cadiou , B. , Pons , J.-M. and Yésou , P. 2004 . Oiseaux marins nicheurs de France métropolitain (1960–2000) , Edited by: Cadiou , B. , Pons , J.-M. and Yésou , P. Meze : Éditions Biotope .
- Calladine , J. , Park , J. R. , Thompson , K. J. and Wernham , C. V. 2006 . Review of Urban Gulls and their Management in Scotland: a report to the Scottish Executive , Edinburgh : Scottish Executive .
- Camphuysen , C. J. and Gronert , A. 2012 . Apparent survival and fecundity of sympatric Lesser Black-backed Gulls and Herring Gulls with contrasting population trends . Ardea , 100 : 113 – 122 . (doi:10.5253/078.100.0202)
- Catry , P. , Ratcliffe , N. and Furness , R. W. 1997 . Partnerships and mechanisms of divorce in the Great Skua . Animal Behaviour , 54 : 1475 – 1482 . (doi:10.1006/anbe.1997.0552)
- Chabrzyk , G. and Coulson , J. C. 1976 . Survival and recruitment in Herring Gull Larus argentatus . Journal of Animal Ecology , 45 : 187 – 203 . (doi:10.2307/3774)
- Choquet , R. , Reboulet , A.-M. , Lebreton , J.-D. , Gimenez , O. and Pradel , R. 2005 . U-CARE 2.2 User's Manual , Montpelier : CEFE .
- Cooch , E. and White , G. 2008 . Program MARK: a gentle introduction Seventh edition www.phidot.org/software/mark/docs/book/
- Coulson , J. C. and Butterfield , J. 1986 . Studies on a colony of colour ringed Herring Gulls Larus argentatus. 1. Adult survival rates . Bird Study , 33 : 51 – 54 . (doi:10.1080/00063658609476892)
- Coulson , J. C. and Coulson , B. A. 2009 . Ecology and colonial structure of large gulls in an urban colony: investigations and management at Dumfries, SW Scotland . Waterbirds , 32 : 1 – 15 . (doi:10.1675/063.032.0101)
- Cramp , S. 1971 . Gulls nesting on buildings in Britain and Ireland . British Birds , 64 : 476 – 487 .
- Cramp , S. and Simmons , K. E.L. 1983 . Birds of the Western Palearctic. Volume III, Waders to Gulls , Edited by: Cramp , S. and Simmons , K. E.L. Oxford : Oxford University Press .
- Crespin , L. , Harris , M. P. , Lebreton , J.-D. and Wanless , S. 2006 . Increased adult mortality and reduced breeding success with age in a population of common guillemot Uria aalge using marked birds of unknown age . Journal of Avian Biology , 37 : 273 – 282 . (doi:10.1111/j.0908-8857.2006.03495.x)
- Dwyer , C. P. , Belant , J. L. and Dolbeer , R. A. 1996 . Distribution and abundance of roof-nesting gulls in the Great Lakes region of the United States . Ohio Journal of Science , 96 : 9 – 12 .
- Eaton , M. A. , Brown , A. F. , Noble , D. G. , Musgrove , A. J. , Hearn , R. D. , Aebischer , N. J. , Gibbons , D. W. , Evans , A. and Gregory , R. D. 2009 . Birds of Conservation Concern 3: the population status of birds in the United Kingdom, Channel Islands and Isle of Man . British Birds , 102 : 296 – 341 .
- François , R. 2002 . Numbers and behaviour of roof-nesting Herring Gulls Larus argentatus and Lesser Black-backed Gulls Larus fuscus in Belgium . Natuur.oriolus , 68 : 123 – 126 .
- Fransson , T. , Kolehmainen , T. , Kroon , C. , Jansson , L. and Wenninger , T. 2010 . EURING list of longevity records for European birds , www.euring.org/data_and_codes/longevity-voous.htm. Accessed: 28/2/2012
- Frederiksen , M. , Wanless , S. and Harris , M. P. 2004 . Estimating true age-dependence in survival when only adults can be observed: an example with Black-legged Kittiwakes . Animal Biodiversity and Conservation , 27 : 541 – 548 .
- Giminez , O. , Choquet , R. and Lebreton , J.-D. 2003 . Parameter redundancy in multistate capture–recapture models . Biometrical Journal , 45 : 704 – 722 . (doi:10.1002/bimj.200390043)
- Griffiths , R. 1991 . Sex-biased mortality in the Lesser Black-backed Gull at the nestling stage . Ibis , 134 : 237 – 244 . (doi:10.1111/j.1474-919X.1992.tb03805.x)
- Hansard . 2009 . House of Commons Debate: Controlling urban seagulls . Parliamentary Debates (Hansard) , 491 col. 469
- Hansard . 2010 . House of Commons Debate: Seagulls (Gloucester) . Parliamentary Debates (Hansard) , 534 col. 237WH
- Hansard . 2011 . House of Commons Debate: Seagulls (Coastal Towns) . Parliamentary Debates (Hansard) , 534 col. 142WH
- Kendall , W. L. , Converse , S. J. , Doherty , P. F. , Naughton , M. B. , Anders , A. , Hines , J. E. and Flint , E. 2009 . Sampling design considerations for demographic studies: a case of colonial seabirds . Ecological Applications , 19 : 55 – 68 . (doi:10.1890/07-1072.1)
- Lebreton , J.-D. and Pradel , R. 2002 . Multistate recapture models: modelling incomplete individual histories . Journal of Applied Statistics , 29 : 353 – 369 . (doi:10.1080/02664760120108638)
- Lebreton , J.-D. , Burnham , K. P. , Clobert , J. and Anderson , D. R. 1992 . Modeling survival and testing biological hypotheses using marked animals: a unified approach with case studies . Ecological Monographs , 62 : 67 – 118 . (doi:10.2307/2937171)
- Lilleør , O. 2000 . Roof-top breeding gulls in Århus city and Denmark . Dansk Ornithologisk Forenings Tidsskrift , 94 : 149 – 156 .
- Madden, B. & Newton, S.F. (2004) Herring Gull Larus argentatus. In Seabird Populations of Britain & Ireland, (Mitchell, P.I., Newton, S.F., Ratcliffe, N. & Dunn, T.E.) 242–262. T. & A.D. Poyser, London.
- Martí , R. and Del Moral , J. C. Dirección General de la Conservación de la Naturaleza – Sociedad Española de Ornitología . Madrid . Atlas de las Aves Reproductoras de España ,
- Mitchell , I. P. , Newton , S. F. , Ratcliffe , N. and Dunn , T. E. 2004 . Seabird Populations of Britain & Ireland , London : T. & A.D. Poyser .
- Møller , A. P. 2009 . Successful city dwellers: a comparative study of the ecological characteristics of urban birds in the Western Palearctic . Oecologia , 159 : 849 – 858 . (doi:10.1007/s00442-008-1259-8)
- Moon , S. 2009 . The Management of Nuisance Urban Gulls by Deployment of Egg Substitutes , Leicester : MSc dissertation, De Montfort University .
- Nankinov , D. N. 1992 . The nesting by the Herring Gull (Larus argentatus) in the towns and villages of Bulgaria . Avocetta , 16 : 93 – 94 .
- Nelson , L. J. , Anderson , D. R. and Burnham , K. P. 1980 . The effect of band loss on estimates of annual survival . Journal of Field Ornithology , 51 : 30 – 38 .
- Pons , J. M. and Migot , P. 1995 . Life-history strategy of the Herring Gull – changes in survival and fecundity in a population subjected to various feeding conditions . Journal of Animal Ecology , 64 : 592 – 599 . (doi:10.2307/5802)
- Rock , P. 1999 . The efficacy of the colour-ringing system used for Herring Larus argentatus and Lesser Black-backed Gulls L. fuscus in Bristol, 1980–1997 . Ringing & Migration , 19 : 306 – 310 . (doi:10.1080/03078698.1999.9674197)
- Rock, P. (2002) The Lesser Black-backed Gull. In The Migration Atlas: movements of the birds of Britain and Ireland, (eds Wernham, C.V., Toms, M.P., Marchant, J.H., Clark, J.A., Siriwardena, G.M. & Baillie, S.R.) 365–368. T. & A.D. Poyser, London.
- Rock , P. 2004 . Roof-Nesting Gulls in Bristol. Survey conducted in May & June 2004 , Bristol : Bristol City Council .
- Rock , P. 2005 . Urban gulls: problems and solutions . British Birds , 98 : 338 – 355 .
- Rock , P. 2006 . Roof-Nesting Gulls in Bath. Follow-up Survey conducted in April 2006 and summarising surveys from 1995 , Bath : Bath & North-East Somerset Council .
- Rock , P. 2010 . Roof-Nesting Gulls in Bristol. Survey conducted in May 2010 , Bristol : Bristol City Council .
- Sæther , B.-E. and Bakke , Ø. 2000 . Avian life history variation and contribution of demographic traits to the population growth rate . Ecology , 81 : 642 – 653 .
- Sibly , R. M. and Hone , J. 2002 . Population growth rate and its determinants: an overview . Philosophical Transactions of the Royal Society of London, Series B , 357 : 1153 – 1170 . (doi:10.1098/rstb.2002.1117)
- Soldatini , C. , Albores-Barajas , Y. V. , Mainardi , D. and Monaghan , P. 2008 . Roof nesting by gulls for better or worse? . Italian Journal of Zoology , 75 : 295 – 303 . (doi:10.1080/11250000701884805)
- Spaans , A. L. 1971 . On the feeding ecology of the Herring Gull Larus argentatus in the northern part of The Netherlands . Ardea , 59 : 73 – 188 .
- Temby , I. D. 2000 . “ Pieces of Silver: examples of the economic impact and management of the Silver Gull (Larus novaehollandiae) in Melbourne, Australia ” . In Human conflicts with wildlife: economic considerations Edited by: Clark , L. 154–162, Proceedings of the 3rd NWRC Special Symposium, August 1–3, 2000. Fort Collins, Colorado
- Tinbergen , N. 1953 . The Herring Gull's World , London : Collins .
- Vercruijsse , H. J.P. 1999 . Zilvermeeuwen uit de duinen van Schouwen. Verspreiding, sterfte en broedbiologie , Tilburg : IBN-DLO .
- Wanless , S. , Harris , M. P. , Calladine , J. and Rothery , P. 1996 . Modelling responses of herring gull and lesser black-backed gull populations to reduction of reproductive output: implications for control measures . Journal of Applied Ecology , 33 : 1420 – 1432 . (doi:10.2307/2404781)
- Ward , R. M. 2000 . Darvic colour-rings for shorebird studies: manufacture, application and durability . International Wader Study Group Bulletin , 91 : 30 – 34 .
- White , G. C. and Burnham , K. P. 1999 . Program MARK: survival estimation from populations of marked animals . Bird Study , 46 ( suppl. 1 ) : S120 – S139 . (doi:10.1080/00063659909477239)
APPENDIX Logistic regression for sexing Gulls
The probabilities of Herring Gull and Lesser Black-backed Gull nestlings being male (P male ) were predicted using logistic regressions that were calibrated on the 466 birds sexed using molecular methods. The function used three measurements: Total Head (TH) and Bill Depth (BD), to distinguish head morphology, and Wing Length (WL), which accounted for age/stage of development. The resulting equations were:
(1) for Herring Gull
(2) for Lesser Black-backed Gull
By rejecting the few birds for which the probability was in the range 0.45–0.55, >90% of both males and females were correctly sexed. Subsequent observations have confirmed the reliability of this approach, with very few contradictions in terms of behaviour or dimorphism.
It should be stated that this method, having been developed for the subspecies argenteus and graellsii in southwest England, may not be applicable to birds of the same taxa further north and will not apply to the subspecies argentatus or fuscus.