Abstract
The genome of nearly all chickens contains various DNA proviral insertions of retroviruses of subgroup E avian leukosis virus (ALVE). However, the elimination or control of ALVE gene expression is desirable to improve productivity, to improve resistance to avian leukosis virus (ALV)-induced tumours, and to develop safer live virus vaccines in chick embryos and cultured chick cells. Restriction fragment length polymorphism and polymerase chain reaction methods are used to define the presence of ALVE genes; and the expression of ALVE in chicken plasma or on cells, and the susceptibility of cells to ALVE is determined by flow cytometry using a specific (R2) antibody. ADOL line 0 chickens have been selected to be free of ALVE genes, while being resistant (i.e. lack receptors to ALVE), but susceptible to exogenous ALV (i.e. ALVA, ALVB, ALVC and ALVJ). To develop improved line 0-type chickens, ADOL line 0 was outcrossed to a commercial line that had one ALVE gene and evidence for ALVE resistance. Rous sarcoma virus (RSV) challenge was used to confirm resistance of F1 chickens to ALVE, and susceptibility of F2 breeders to ALVA and ALVB using test chicks produced by matings to line 72. Selected F2 breeders were resistant to ALVE, but susceptible to exogenous ALVA, ALVB, ALVC and ALVJ, based on challenge tests of progeny chick cells using an enzyme-linked immunosorbent assay. The new line, 01, has evidence for improved egg size, productivity, fertility and hatchability. Similar procedures may be used for development of productive ALVE free chicken lines with preferred ALV susceptibility traits.
Résumé
Méthodes pour l’évaluation et le développement de souches de poulets de chair indemnes du virus endogène de la leucose aviaire sous groupe E (ALVE)
Le génome de presque tous les poulets contient différentes insertions provirales d'ADN de rétrovirus de la leucose aviaire du sous groupe E (ALVE). Cependant, l’élimination ou le contrôle de l'expression du gène de l’ALVE est souhaitable pour améliorer la productivité et la résistance à la leucose aviaire (ALV) induisant des tumeurs, et pour développer de manière plus sûre des vaccins vivants sur œufs embryonnés et sur cellules de poulet. Les méthodes RFLP et PCR sont utilisées pour mettre en évidence la présence des gènes d'ALVE . La cytométrie en flux utilisant un anticorps spécifique (R2) est employée pour déterminer l'expression d'ALVE dans le plasma ou les cellules de poulet et la sensibilité des cellules à l'ALVE. La lignée de poulet ADOL 0 a été sélectionnée comme étant indemne de gènes d’ALVE, cependant résistante, c'est-à-dire absence de recepteurs à l'ALVE, mais sensible aux ALV exogènes, c'est-à-dire ALVA, ALVB, ALVC, et ALVJ. Pour développer la lignée de poulet de type 0 améliorée, la lignée ADOL 0 a été croisée avec une lignée commerciale qui avait un gène d'ALVE et qui était résistante à l'ALVE. Le virus d’épreuve du sarcome de roux a été utilisé pour confirmer la résistance des poulets F1 à l'ALVE, et la sensibilité des reproducteurs F2 à l'ALVA et l'ALVB en utilisant le test des poulets issus après croisement avec la lignée 72. Les reproducteurs F2 sélectionnés étaient résistants à l'ALVE mais sensibles aux ALVA, ALVB, ALVC et ALVJ exogènes basé sur des tests d’épreuve des cellules des poulets de la descendance en utilisant un test ELISA. Les performances de la nouvelle lignée 01, ont été améliorées en terme de taille des œufs, productivité, fertilité et éclosabilité. Des procédures similaires peuvent être utilisées pour le développement de lignées de poulets indemnes d'ALVE avec des caractères sélectionnés de sensibilité à ALV.
Zusammenfassung
Methoden für die Evaluierung und Etablierung kommerzieller von endogenem aviärem Leukosevirus der Subgruppe E (ALVE) freien Hühnerlinien
Das Genom nahezu aller Hühner enthält verschiedene virale DNS-Insertionen von Retroviren der Subgruppe E des aviären Leukosevirus (ALVE). Die Eliminierung oder Kontrolle der ALVE-Genexpression ist jedoch wünschenswert, um die Produktivität und die Resistenz gegen ALV-induzierte Tumorbildung zu verbessern und um sicherere Lebendvirusvakzinen in Hühnerembryonen und Hühnerzellkulturen herstellen zu können. Zum Nachweis von ALVE-Genen wurden RFLP- und PCR-Methoden benutzt. Die Expression von ALVE im Hühnerplasma oder auf Zellen sowie die Empfänglichkeit von Zellen für ALVE wurde im Flowzytometer unter Verwendung eines spezifischen Antikörpers (R2) bestimmt. Hühner der ADOL-Linie 0 waren auf die Freiheit von ALVE-Genen selektiert worden. Gleichzeitig waren sie resistent gegenüber ALVE d.h. ihnen fehlten die Rezeptoren, jedoch empfänglich für exogenes ALV, d.h. für ALVA, ALVB, ALVC, und ALVJ. Zur Verbesserung von Hühnern des Linie-0-Typs wurde die ADOL-Linie 0 mit einer kommerziellen Linie, die ein ALVE-Gen und den Nachweis für die ALVE-Resistenz hatte, fremdgekreuzt. Belastungsinfektionen mit Rous-Sarkom-Virus wurden durchgeführt, um die Resistenz der F1-Hühner gegen ALVE zu bestätigen. Die Empfänglichkeit der F2-Zuchttiere für ALVA und ALVB wurde unter Verwendung von Testküken aus der Paarung mit Linie 72 ermittelt. Basierend auf Belastungstests mit Zellen der Nachkommen im ELISA wurden die selektierten F2-Zuchttiere als resistent gegen ALVE, aber empfänglich für ALVA, ALVB, ALVC, und ALVJ bezeichnet. Die neue Linie 01 wies eine höhere Legeleistung mit verbesserter Eigröße sowie Befruchtungs- und Schlupfrate auf. Ähnliche Methoden können für die Entwicklung von leistungsstarken ALVE-freien Hühnerlinien mit bestimmten ALV-Empfänglichkeits-Erbeigenschaften angewendet werden.
Resumen
Métodos para evaluar y desarrollar cepas comerciales de pollo libres de virus de la leucosis aviar endógeno subgrupo E (ALVE)
El genoma de casi todos los pollos contiene inserciones de ADN proviral de los retrovirus del subgrupo E del virus de la leucosis aviar (ALVE). Aun así, la eliminación o el control de la expresión del gen ALVE es deseable para mejorar la productividad, la resistencia a los tumores inducidos por el virus de leucosis aviar (ALV), y para desarrollar vacunas vivas más seguras en embriones de pollo y en cultivos celulares de pollo. Las técnicas de RFLP y PCR se usan para demostrar la presencia de genes de ALVE y la expresión de ALVE en plasma de pollo o en células, y la susceptibilidad de las células al ALVE se determina mediante citometría de flujo utilizando un anticuerpo especifico (R2). La línea 0 ADOL de pollos se ha seleccionado como una línea libre de genes ALVE, ya que es resistente, es decir, no tiene receptors para ALVE, pero es susceptible a los ALV exógenos, es decir, ALVA, ALVB, ALVC y ALVJ. Para desarrollar pollos mejorados de la línea 0, la línea 0 ADOL se cruzó con una línea comercial que únicamente tenía un gen de ALVE, y evidencias de resistencia a ALVE. El desafío experimental con el virus del sarcoma de Rous (RSV) confirmó la resistencia de los pollos F1 al ALVE, y la susceptibilidad de los reproductores F2 al ALVA y ALVB utilizando pollitos de prueba obtenidos mediante emparejamiento con la línea 72. Reproductores F2 seleccionados fueron resistentes al ALVE, pero susceptibles a los virus exógenos ALVA, ALVB, ALVC y ALVJ, en base a los desafíos experimentales de la progenie mediante un ELISA. La nueva línea, 01, evidenció una mejora en el tamaño de los huevos, productividad, fertilidad e incubabilidad. Se podrían utilizar procedimientos similares para desarrollar líneas productivas de pollos libres de ALVE con diferentes susceptibilidades a ALV.
Introduction
The genomes of most chickens contain DNA proviral insertions of retroviruses of subgroup E avian leukosis virus (ALVE) (Crittenden, Citation1991). Over 22 DNA proviral insertion sites, or ALVE genes, have been identified in White Leghorns, and individuals usually possess one to three or more ALVE genes. Each ALVE may code for a complete endogenous ALVE that can replicate and infect, or it may possess only some of the three genes coding for proteins; that is, envelope (env), capsid (the group-specific antigen, i.e. gag), and polymerase (pol). If an ALVE is incomplete or defective, the expressed products will be non-infectious. Individuals may have a combination of the ALVE genes present within a strain. Broiler strains contain additional ALVE genes, and individuals frequently contain six to 10 genes (Sabour et al., Citation1992). Thus, essentially all chickens possess some combination of ALVE genes, and many are now identifiable. Importantly, the identification of individual ALVE genes in the DNA of White Leghorns is now achievable using polymerase chain reaction (PCR) tests (Benkel, Citation1998). Although some ALVE genes appear to be harmless, those that express ALVE envelope can be detrimental as this results in decreased immune responses to tumour inducing exogenous subgroup A and B avian leukosis virus (ALV) (ALVA and ALVB) (Crittenden, Citation1991). The expression of the ALVE envelope in the embryo induces a degree of tolerance in the chick if it is infected with these viruses, particularly when infection occurs within a few days after hatch. Also, ALVE may recombine with exogenous ALV if they contaminate the environment and infect the chick. Fortunately, major poultry breeders have been testing for, and reducing or eradicating, exogenous ALV (Chase, Citation1991; McKay & Rosales, Citation2000). However, the expression of some ALVE genes, particularly ones that encode complete infectious ALVE, can lead to decreased production of eggs (Kuhnlein et al., Citation1989; Gavora et al., Citation1991), or meat (Sabour et al., Citation1992). For example, ALVE21 encoding infectious ALVE21 results in decreased growth in White Leghorns (Smith & Nelsen, Citation1993). ALVE21 is linked to the slow feather gene (K) and therefore is present in the female parent of feather sexed chickens (Bacon et al., Citation1988). Feather sexing is commonly used to identify gender of chicks at hatch by the chicken breeding industry.
Following the development of Southern blots to identify ALVE genes in DNA, an experimental chicken was identified that lacked ALVE genes (Astrin et al., Citation1979). This chicken and its relatives were used to establish a unique line termed line 0. The line 0 chickens were also selected for the presence or absence of specific ALV receptors by Crittenden at the Avian Disease and Oncology Laboratory (ADOL), enabling them to possess resistance to ALVE, but susceptibility to exogenous ALVA and ALVB retroviruses (Crittenden & Fadly, Citation1985). Line 0 is also susceptible to ALVC. The ADOL line 0 chick embryo fibroblasts (CEF) have been invaluable for the identification and eradication of exogenous ALVA from White Leghorn and broiler flocks, and more recently ALVJ from broiler breeder flocks (Fadly & Witter, Citation1998).
ALVE loci described here belong to one of several families of endogenous retroviruses (ERV) that have been chromosomally integrated into the genome and are inherited in a Mendelian fashion in chickens. In addition to the ALVE family of ERV loci, there are CR1, EAV and ART-CH families (Crittenden, Citation1991). Indeed, chickens of line 0 are likely to possess as many of these other ERV genes as other chickens. The ERV gene loci unrelated to ALVE have not been shown to have a direct deleterious affect on the health of chickens (Weiss, Citation2001). However, the recently identified J subgroup of ALV contains an envelope gene that is highly identical to the envelope gene of an ancient endogenous virus of the EAV family (Smith et al., Citation1999). Indeed, an EAV gene has been identified and sequenced in line 0 that is highly similar to the envelope of isolates of ALV J viruses (Silva et al., Citation2000). Thus, although ERV loci in addition to ALVE are not directly implicated in disease or susceptibility to disease, their existence may have detrimental influences (e.g. if they recombine with exogenous ALV).
It is envisioned that chickens lacking ALVE genes may be superior for certain industrial applications (e.g. the development of transgenic chickens; Zajchowski & Etches, Citation2000), or the production of vaccines in embryos or CEF (Hussain et al., Citation2001; Johnson & Heneine, Citation2001; Weiss, Citation2001). There is safety concern about the presence of ALVE gene products in human vaccines, especially live, attenuated vaccines (e.g. for influenza, measles, mumps and yellow fever). The contaminating ALVE particles may be of greatest concern for the very young, or immune-compromised patients. The current ADOL line 0 chickens have only fair production of medium size eggs, and egg-shell and hatchability traits are inferior compared with commercial egg production stocks (Bacon et al., Citation2000). Indeed, producers of specific pathogen free (SPF) eggs have not utilized line 0 because good egg production, fertility, and embryo survival are essential. Thus, it is critical to establish how current methods can be used to develop additional ALVE-free chicken lines with superior production characteristics. The current methods evaluated include detection of:
| 1. | ALVE genes using restriction fragment length polymorphism (RFLP) and PCR tests; | ||||
| 2. | ALVE envelope in the plasma, and susceptibility of cells to ALVE using R2 antibody that detects ALVE envelope expression on red blood cells; and | ||||
| 3. | resistance or susceptibility of pedigree chickens (or chick embryo fibroblasts) to ALVA, ALVB, ALVC and ALVE using appropriate Rous sarcoma virus (RSV). | ||||
Materials and Methods
Chicken lines
ADOL experimental lines
Chickens were progeny of SPF parents that were reared in isolation and thus free of many known avian pathogens (Bacon & Palmquist, Citation2002). However, some chickens had antibodies to chick anaemia virus, indicating chick anaemia virus infection had occurred. All chickens were vaccinated against Marek's disease virus using turkey herpesvirus (Witter et al., Citation1970). The ADOL lines 0 (susceptible to ALVA, ALVB, and ALVC but resistant to ALVE and lacking ALVE genes), semi-congenic line 0.44-VB*S1 (susceptible to ALVA, ALVB, ALVC and ALVE and lacking ALVE genes), 15B1 (susceptible to ALVA, ALVB, ALVC and ALVE and containing ALVE1 that is not normally expressed), and 72 (resistant to ALVA, ALVB and ALVE but susceptible to ALVC, and containing but not expressing ALVE1 and ALVE2) have been described previously (Bacon, Citation2000; Bacon et al., Citation2000). Line 0 is homozygous for the B*21 MHC haplotype.
Commercial egg layer strain
Two rapid feathering White Leghorn strains from Hy-Line International were initially evaluated, and one (termed strain 1) was utilized. It was among a number of pure-breeding commercial strains that has been screened for ALVE genes and infectious ALV for the past 15 years and was continuously selected for non-ALVE expression. DNA from four to eight chickens per strain had been tested by RFLP (see later) and this strain had evidence for only ALVE1 patterns (E. Smith, personal communication). More recently 30 chickens of this commercial strain 1 had been tested for ALVE envelope in their plasma and only 7% were positive (Bacon, Citation2000). Thus, preliminary data indicated strain 1 chickens possessed few ALVE genes with very limited expression. The strain 1 chickens were shown to be free of common pathogens, including infectious ALV, in tests conducted by Hy-Line International. They had been vaccinated for chick anaemia virus, reovirus, infectious bursal disease virus, and Newcastle bronchitis viruses, and had antibodies to these viruses.
Analysis of MHC haplotypes
Chicken MHC haplotypes were defined by hemagglutination of red blood cells (RBC) in microtitre plates using methods and antisera previously described (Fulton et al., Citation1996).
Analysis of DNA for ALVE genes
RFLP tests
Identification of ALVE genes was based on the size of characteristic DNA fragments following digestion with the endonuclease HindIII and electrophoresis. Southern blots were analysed using a 32P-labeled U3N probe for hybridization to the DNA fragments blotted onto nitrocellulose membranes (Crittenden, Citation1991).
PCR tests
Following the identification of ALVE genes using RFLP analyses, the chickens within the commercial strain 1 were analysed by PCR using primers for ALVE1 and ALVE15 (Benkel, Citation1998).
Analyses using R2 antibody
Haemagglutination
The production of R2 antibody and a simple test for agglutination of RBC in microtitre plates has been described (Bacon et al., Citation1996). R2 antiserum agglutinates RBC from a chicken that has receptors for (and is susceptible to) ALV-E and expresses an ALVE envelope gene.
Flow cytometric assessment of ALVE envelope in plasma
The details for detecting ALVE in plasma were previously published (Bacon, Citation2000). Briefly, the method utilizes RBC from line 0 chickens of two congenic types that lack ALVE; that is, RBC susceptible to ALVE infection (from line 0.44-VB*S1), and RBC resistant to ALVE infection (from line 0). RBC from the susceptible chickens will bind ALVE envelope glycoprotein 85 (gp85) if present in plasma. The gp85-bound RBC are then incubated with the R2 antibody. Using flow cytometry (FC), gp85 is detected indirectly with a fluorescein-tagged antibody to chicken immunoglobulin; plasmas lacking gp85 are non-reactive and only low background fluorescence is detected. Because RBC from resistant chickens are non-reactive regardless of the presence or absence of ALVE gp85 in plasma, a specific binding index (SBI) is used to compare relative binding of ALVE gp85 on susceptible and resistant RBC.
 If SBI≥1.4, the plasma sample is considered positive for the presence of ALVE envelope.
If SBI≥1.4, the plasma sample is considered positive for the presence of ALVE envelope.
FC assessment of susceptibility to ALVE
If a chicken does not express ALVE envelope then the FC assay already described can be used to predict the presence of receptors and susceptibility for ALVE, or the absence of receptors and resistance to ALVE. This is critical since line 0 chickens must have resistance to ALVE. Thus, if a chicken is identified with no ALVE genes, or has only ALVE-1 and/or ALVE15 that are not normally expressed, a FC test is conducted using its RBC and two types of plasma. In one well with RBC, plasma from a chicken known to express ALVE envelope (line 15I5) is added. In a second well, plasma from a bird that does not express ALVE envelope (line 0) is added to the RBC and allowed time to bind. R2 antibody is added to washed RBC, the FC test is completed, and the SBI is calculated. If SBI≥1.4 the chicken is predicted to be ALVE susceptible, whereas if SBI<1.4 the chicken is predicted to be resistant to ALVE (Bacon, Citation2000).
Viruses
ALV viruses utilized were subgroup A Rous associated viruses (RAV) RAV-1 and RSV RSV(RAV-1), subgroup B viruses RAV-2 and RSV(RAV-2), subgroup C virus RAV-49, subgroup J virus Hc1, and subgroup E viruses RAV-0 (also termed EV2), and RSV(RAV-60) (Crittenden et al., Citation1973, Citation1984; Smith & Crittenden, Citation1988; Fadly & Smith, Citation1999).
Tumour induction assay for assessment of ALV susceptibility
RSV tumours were induced by inoculating 0.1 ml subgroup A RSV(RAV-1), subgroup B RSV(RAV-2) or subgroup E RSV(RAV-0), into the wing web. Based on records the inoculum was expected to contain approximately 500 focus-forming units (FFU) each virus. Chicken wing webs were observed at 10, 14 and, if needed, 17 days post-inoculation to measure the thickness of the wing web tumour and to record the presence or absence of tumours (Bacon et al., Citation1983; Bates et al., Citation1998). Tumour development indicated the chicken was susceptible to that subgroup of ALV, and absence of a tumour indicated it was resistant to the inoculated virus. We elected to analyse ALV susceptibility using RSV tumour induction in hatched chicks rather than attempting to prepare chick embryo cells from each individual chick and then infecting with virus. It was essential to test many chicks from each breeder, and the use of cells from individual embryos would have required very extensive commitments in time and culture materials. ALV susceptibility may also have been defined by infecting the chorioallantoic membrane of individual fertile eggs with ALV. However, we had no recent expertise using this method.
Preparation of CEF and enzyme-linked immunosorbent assay tests for ALV susceptibility
Primary CEF cultures were prepared from three 11-day-old embryos per genotype group and frozen (Bacon et al., Citation2000). Two secondary plates from each culture were infected with a prototype ALV of subtype A (RAV-1), subtype B (RAV-2), subtype C (RAV-49), subtype J (Hc1) and subtype E (EV2). Eight days later, the supernatant fluids were tested for ALV capsid antigen (p27) by enzyme-linked immunosorbent assay (ELISA) (Smith et al., Citation1990). A positive ELISA test indicated the presence of capsid antigen and thus the CEF of that culture were susceptible to the utilized ALV. Conversely, a negative ELISA indicated the absence of capsid antigen and resistance to the ALV.
Results and Discussion
Analysis of parental strain chickens for ALVE
RBC from 55 breeding males of commercial strain 1 were incubated with R2 antibody. RBC from none of the males were agglutinated, indicating they all lacked ALVE envelope, or were resistant to subgroup ALVE. Plasma from 14 of the males was tested on RBC from line 0 versus 0-VB*S1 chickens. Following addition of R2 antiserum and flow cytometry the SBIs were<1.4, indicating ALVE was not present in the plasma. RFLP tests were made on DNA from the males and all 55 had bands corresponding to ALVE1, a non-expressed ALVE (Groudine et al., Citation1981; Crittenden, Citation1991). About two-thirds of the males also had a band corresponding to ALVE15. ALVE15 is known to consist of part of a LTR and is not expressed (Smith et al., Citation1984; Crittenden, Citation1991). Thus, the absence of ALVE expression was confirmed by RFLP analyses.
Importantly, 47 of the 55 males’ RBC were negative in FC following addition of 15I5 plasma with ALVE envelope, or line 0 plasma without ALVE envelope, and R2 antibody (i.e. they had SBI<1.4). This R2 plasma test result indicated 85% of the males lacked ALVE receptors. Those without receptors are resistant to ALVE, which is essential for line 0-type chickens. In addition, serological typing using MHC-specific alloantisera indicated that all strain 1 birds were homozygous for the B*2 haplotype.
Production of 0×1 F1 chickens
ALVE1 was identified in all of the commercial Strain 1 males, and therefore it was impossible to directly select for ALVE-free chickens within the pure commercial strain. Strain 1 commercial hens were selected that did not contain ALVE15 as indicated by an ALVE15-specific PCR test, and that lacked ALVE receptors based on R2 plasma tests. Eleven of these hens were artificially inseminated with pooled semen from five ADOL line 0 males to produce F1 chickens.
Eighty-nine F1 chicks were hatched at the ADOL. RFLP tests verified that all the chicks possessed only the ALVE1, inherited from commercial strain dam. As expected, all of the chicks were B*2/*21 based on blood-typing. The F1 hens and 18 males (two from each of nine productive dam families) were kept for mating to produce F2 chickens to demonstrate how one would select F2 breeders with line 0 characteristics.
Evaluation of F1 chickens for ALVE resistance
Twenty-eight F1 males not needed for breeding were challenged with RSV(RAV-60) in the wing web at approximately 24 weeks of age to verify resistance to ALVE. The injections were repeated three times due to unexpected results in control ADOL line 15B1 chickens that were selected for susceptibility to ALVE. In Trial 1 only one of three 15B1 chickens developed a tumour (), and therefore all the chickens were injected again. In Trial 2, 15B1 chicken I835A remained resistant. Trial 3 used 10 times the dose of virus injected in Trials 1 and 2. Based on analysis of inoculum following the third injection, each chicken had received 380 FFU RSV(RAV-60). Thus the inoculum did not have the number of FFU expected for Trials 1 and 2. In Trial 3 eight additional 15B1 chickens were injected and developed a tumour, but chicken I835A remained resistant. The RBC from chicken I835A were also resistant to ALVE based on the plasma R2 antibody test, whereas this test was positive for the eight 15B1 chickens that developed a tumour (data not tabulated). We conclude that the RSV(RAV-60) virus was effective, but chicken I835A was resistant to ALVE (i.e. TVB*S3/*S3). In Trials 1 and 2 none of three line 0 controls developed a tumour as expected. However, in Trial 3 line 0 chicken I938C developed a tumour. This tumour was unexpected since line 0 is resistant to ALVE. The tumour may have resulted from receipt of too much virus after three injections, or perhaps it occurred in the absence of RSV(RAV-60) infection but because of the direct transcription of viral RNA into v-src DNA, which is known to induce sarcomas (Halpern et al., Citation1990), or the RSV(RAV-60) inoculum may have contained a few viral particles of another subgroup. Most importantly, none of the 28 0×1 F1 males developed tumours following three successive injections with RSV(RAV-60) (). This indicates that all of the F1 chickens were resistant to ALVE, and confirms that R2 antibody tests on strain 1 breeders had detected resistance to ALVE.
Table 1. Tumour induction by RSV(RAV-60) in lines 15B1, 0 and 0×1 F1 chickens
Evaluation of 0×1 F1 chickens for ALVA and ALVB resistance
To be comparable with line 0, the prototype line 01 must be homozygous susceptible to ALVA (TVA*S/*S) and ALVB while also being resistant to ALVE (TVB*S3/*S3). If each F1 breeder were homozygous susceptible to ALVA and ALVB, then all of the F2 and subsequent generations of chickens would also possess this susceptibility. Therefore, the F1 breeders were analysed for susceptibility to ALVA and ALVB by conducting challenge tests on pedigreed offspring. Each F1 hen and male breeder was mated to line 72 to produce 10 or more chicks per breeder. Line 72 is resistant to ALVA (TVA*R/*R), as well as ALVB and ALVE (both determined by the TVB*R/*R genotype). At 4 to 6 weeks of age the test chicks were inoculated with subgroup A RSV(RAV-1) in the right wing web and subgroup B RSV(RAV-2) in the left wing web. If the F1 breeder was homozygous susceptible to ALVA (TVA*S/*S) then all of the test chicks from that breeder should be susceptible to ALVA and develop tumours. However, if a breeder was heterozygous TVA*S/*R for susceptibility then approximately 50% of the test chicks should be TVA*R/*R and should not develop a tumour. An F1 breeder could not be homozygous resistant since its line 0 parent was homozygous susceptible (TVA*S/*S) for ALVA. All 40 female and nine male F1 breeders mated to line 72 produced chicks that developed tumours after injection RSV(RAV-1) (, column 3), demonstrating that all of the F1 breeders were TVA*S/*S.
Table 2. Use of 0×1 F1 chickens to test RSV resistance and to produce F2 chickens
Following injection of RSV(RAV-2), 35 of 40 female and eight of nine male F1 breeder's chicks consistently developed tumours, indicating these breeders were TVB*S3/*S3 (, column 4). However, one male (M #9) and five female F1 breeders (F #2, F #4, F #5, F #8, and F #9) had about one-half of their offspring develop no tumour, indicating these breeders were heterozygous for the ALVB receptor gene (i.e. TVB*S3/*R). All of these F1 breeders were offspring of commercial hen A, indicating that hen A was heterozygous for TVB*S3/*R. Out of five other hen families there was only one chick that did not develop a tumour by 2 weeks post-injection of RSVB. However, these failures to develop a tumour never approached 50% of the chicks from an original line 1 parental hen breeder, and therefore this absence of a tumour was not attributable to resistance at the viral receptor level. Failure of tumour development in these five chicks after RSVB injection may have been due to a missed injection, but this is unlikely. It was more likely due to the slow growth of RSV-B. The RSV-B tumours developed later than the RSV-A tumours and only grew to about one-half the size at 2 weeks post-injection (i.e. an average of about 0.5 cm versus 1 cm). The chicks were not retained beyond 17 days post-injection due to the rapid growth of the RSV-A tumours. We conclude that the 0×1 F2 chicks from 35 female and eight male F1 breeders are fully susceptible to ALVB and are useful for the development of line 01.
Selection of 0×1 F2 chickens to produce prototype ALVE free line 01 chickens
When the F1 chickens were 25 to 31 weeks of age the semen of one male from each of nine dam families was used to artificially inseminate hens twice weekly using non-sib pedigree matings (, columns 5 and 6). Eggs were collected and set weekly for 33 days to produce 0×1 F2 chicks. At 3 to 4 weeks of age, 1041 F2 chicks were blood-typed and 262 (25%) that were B*21/*21 were retained. The residual B*2/*21 and B*2/*2 chicks were terminated. When RSV tests were completed, 30 F1 families were identified in which both parents were homozygous susceptible to ALVA and ALVB. DNA from 129 F2 chicks (62 male and 67 female) from these families was tested for the presence of the ALVE1 gene by Southern blots. Forty-six F2 chickens (25 male and 21 female) lacked ALVE genes (, columns 8 and 9). Thus, 46/129 (35.6%) of the F2 chickens from these matings lacked ALVE genes, somewhat more than the 25% anticipated. These selected F2 chickens were derived from eight of the original line 1 grand-dam families and were used in non-sib pedigree matings to produce generation 1 of the new line 01.
Susceptibility of CEF from lines 0, 0×1 F2 and 01 to ALVA, ALVB, ALVC, ALVJ and ALVE
Initial selection of line 01 breeders was done only for ALVA, ALVB and ALVE, but final susceptibility tests were also conducted for ALVC and ALVJ. An infection assay was conducted using CEF obtained from embryos of control lines 15B1 and 0, from eight matings of 0×1 F1 chickens to provide F2 embryos, and from six matings of 0×1 F2 chickens to provide 01 embryos. The line 0, 0×1 F2 and 01 CEF were all susceptible to ALVA, ALVB, ALVC and ALVJ, but resistant to ALVE. Although the commercial strain 1 had not been tested for ALVC and ALVJ susceptibility, these results demonstrate that strain 1 and the new line 01 are like the original line 0 and possess susceptibility to ALVC and ALVJ. In contrast to lines 0 and 01, line 15B1 was susceptible to ALVE as expected (). These results confirm that lines 0 and 01 are both resistant to ALVE but susceptible to common exogenous ALV.
Table 3. Comparison of susceptibility of CEF from ADOL lines 0×1 F2, 01, 0, and15B1 to ALV A, ALV B, ALV C, ALV J and ALV E
In our current selection no attempt was made to define susceptibility of receptor genes using PCR or RFLP analyses. However, the receptor genes for subgroups A, B, and E ALV are becoming defined at the DNA level. Recently, a RFLP method has been defined to distinguish TVB*R from TVB*S1 and TVB*S3 (Klucking et al., Citation2002). However, ways to distinguish TVB*S3 from TVB*S1 by RFLP or PCR are not defined. If DNA analyses are eventually developed, then selection for ALV receptors at the DNA level may decrease the time and effort needed to develop line 0-type chickens.
Reproductive characteristics of line 0, 0×1 F1 and 0×1 F2 chickens
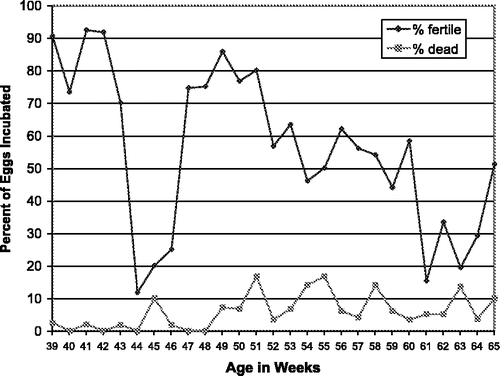
In 2000/2001 a fertility test of ADOL line 0 males was conducted. Pooled semen from six males was used to inseminate 21 hens once weekly to provide about 60 eggs weekly for fertility data (). At 41 weeks of age fertility was at 92%, by 52 weeks of age fertility had rapidly dropped to less that 60%, and after 60 weeks fertility was below 40%. The drastic drop in fertility at 44 to 46 weeks of age is attributed to no artificial insemination (AI) during holidays. At 64 weeks of age artificial insemination was performed twice a week and fertility increased from 30% to 50%. Embryo mortality increased as the hens aged, and after 49 weeks 5% to 16% of the 11-day-old embryos were dead.
In 2001/2002, additional eggs were obtained from line 0, 0×1 F1 and 0×1 F2 chickens following twice-weekly AI for analyses of egg weight, size, production and fertility (). Chickens of these different lines were hatched, reared and caged in different facilities, and fed and illuminated under different schedules, so the data were not analysed statistically for comparisons. However, summaries indicate that at 30 to 40 weeks of age the F1 hens laid 13% more eggs than line 0 hens. The F2 hens also produced 10% more eggs than line 0 hens (excluding two of 21 hens with poor production). By 50 weeks of age egg production was only 41% in line 0, and the F1 and F2 chickens laid 34% and 25% more eggs (excluding three F2 non-layers). It is expected that egg production by the F2 hens could be more variable and result in a lower mean rate than in the F1 hens, but with selection in future generations we anticipate that hens of the new 01 line should equal or exceed the egg production of the F1 hens. We conclude outcrossing to the commercial strain has considerably improved egg productivity compared with the original line 0.
Table 4. Reproduction and egg characteristics of line 0, 0×1 F1 and 0×1 F2 chickens
Egg fertility at 30 to 40 weeks was 92 to 96% for lines 0 and 0×1 F1 (). The 0×1 F2 chickens averaged only 83% fertility due to low fertility of two hens mated to one of 11 sires, and a hen with poor egg production and two hens with small egg size. However, 17 hens averaged 91% fertility. The lines attained similar percentages of fertility of laying hens at 50 weeks of age. Thus, fertility following twice-weekly AI was relatively high in line 0, F1 and F2 chickens, but in 2001 we noted line 0 fertility can drop off dramatically at 50 weeks of age when hens receive AI once weekly (see earlier).
At 40 weeks of age hatchability was not analysed in line 0; however, when eggs were candled at 11 days, 9% of the fertile eggs were dead (). At 30 weeks 93% of eggs set from F1 hens hatched, and at 40 weeks 73% of eggs set from F2 hens hatched. At 50 weeks 79% of eggs set from line 0 hatched, but hatchability was not defined in eggs from F1 hens. Hatchabililty of F2 eggs was 81%. Thus, the hatchability was similar for fertile eggs from line 0 and 0×1 F2 chickens.
Egg weight increased about 6.3 g in line 0×1 F2 compared with line 0 hens about 40 weeks of age (). At 30 weeks the F1 eggs weighed less, but this was attributed to the hens being about 10 weeks younger in age. At 50 weeks of age the average egg weight was 55.9 g in line 0, and this increased by about 2 g in eggs produced by the 0×1 F1 and F2 hens. Egg breadth and length also increased in eggs produced by F1 and F2 hens, but the shape indices were similar.
The existing line 0 continues to be maintained in a SPF status at the ADOL. The F3 and F4 generation of the new line 01 will be reproduced by limited numbers of breeders maintained in isolators until the line is certified free of pathogens and can be moved to the house with breeders of other SPF lines of chickens. In 2005 it will be possible to design experiments to compare reproductive characteristics of breeders in lines 0 and 01 that are grown and caged in the same environment. These lines are reproduced using non-sib breeders (line 0 has 17 males and 120 hens per generation; Bacon et al., Citation2000), and breeders are selected from families with good productivity and egg characteristics. Fertile eggs from lines of chickens at the ADOL are provided as available upon request for a nominal fee.
Utility of current methods for development of chicken lines with other desirable ALV traits
The objective in this paper was to replicate a line with the exact virological features of line 0, but with improved reproductive characteristics. However, these procedures may be adapted to develop additional lines with unique beneficial ALV characteristics. For example, producers of vaccines, or of transgenic chickens, particularly ones developed by ALV vectors, may desire a line that in addition to being free of all ALVE genes and possessing resistance to ALVE (as lines 0 and 01), also has resistance to common exogenous ALV (i.e. ALVA and ALVB). The development of transgenic chickens with an ALV vector requires the chicken to be susceptible to the envelope subgroup of the vector, but resistance to other subgroups may be preferred. No chicken has been shown to have resistance to ALVJ, so susceptibility to ALVJ would remain. The development of chickens lacking ALVE could be achieved by the crossing of a commercial strain to line 0 as described here, followed by selection for absence of the provirus by PCR or dot blot with a complete ALVE, or U3N LTR probe; or PCR designed to detect the U3N region of the LTR regardless of its position in the genome (Crittenden et al., Citation1989; Crittenden & Salter, Citation1989). Then resistance to ALVA and ALVB could be defined using PCR (see Klucking et al. [Citation2002] for references) or progeny tests of breeders to line 72 where chicks are challenged with RSV as described here. A (commercial strain×line 0) F2 cross-mating would be needed to obtain TVA*R/TVA*R and TVB*R/*R homozygous chicks with resistance to ALVA and ALVB. If a commercial strain did not have some chickens with the TVA*R and TVB*R resistance genes they would have to be introduced from another line—e.g. line 72 or RH-C maintained at the ADOL (Bacon et al., Citation2000).
Another breeders’ objective may be to eliminate only expression of ALVE envelope without foregoing the costly elimination of all ALVE genes from a strain. This could be accomplished by identifying and culling breeders with plasma containing ALVE envelope using the R2 plasma assay. Alternatively, with some development of PCR primers the breeder may evaluate whether a strain has the TVB*R gene using PCR (Klucking et al., Citation2002) and eliminate chickens with the TVB*S1 or TVB*S3 genes that permit infection and expression of ALVE. However, it is uncertain whether TVB*R/*R homozygotes will curtail expression of envelope by incomplete ALVE genes that code for envelope (e.g. ALVE6 or ALVE9) or envelope and the group-specific (gag) antigen (ALVE3) that often exist in commercial strains (Crittenden, Citation1991; Bacon, Citation2000). If ALVE6, ALVE9 or ALVE3 genes did express envelope in TVB*R/*R chickens, but not all chickens were homozygous for any of the ALVE genes, then their presence could be detected by PCR (Benkel, Citation1998) and chickens lacking these genes could be selected for breeding.
Conclusions
Based on the development of line 01 we conclude that it is possible to:
| 1. | screen existing commercial line chickens’ RBC and plasma with R2 antibody tests to identify chickens that are susceptible to ALVE and/or express ALVE; | ||||
| 2. | use RFLP and/or PCR to identify which ALVE genes exist in a commercial strain and select birds from an F2 cross that lack ALVE; | ||||
| 3. | challenge excess F1 males with RSV(RAV60) to confirm resistance to ALVE; | ||||
| 4. | challenge test chicks resulting from selected F1 breeders mated to line 72 with ALVA and ALVB to determine susceptibility to exogenous ALV; | ||||
| 5. | assure susceptibility of selected F2 breeders to exogenous ALV and resistance to endogenous ALV by doing ALV challenge tests on progeny CEF using ELISA; and | ||||
| 6. | improve egg size, productivity, fertility and hatchability in the new strain of F2 chickens. | ||||
Acknowledgments
The authors are grateful to Evelyn Young for excellent technical assistance in flow cytometric analyses, DNA purification and performance of RFLP analyses, avian leukosis virus assays, and Rous sarcoma virus challenge; to Matt Morse and Deborah Ferguson for compiling egg production data; to Pamela Campbell for preparing CEFs; and to Lyman Crittenden for instructive suggestions during review of the manuscript. This research was funded in part by a Cooperative Research and Development Agreement between Hy-Line International and the USDA, No. 58-3K95-0-850.
References
- Astrin , SM , Buss , EG and Hayward , SW . 1979 . Endogenous viral genes are nonessential in the chicken . Nature , 281 : 339 – 341 .
- Bacon , LD . 2000 . Detection of endogenous avian leukosis virus envelope in chicken plasma using R2 antiserum . Avian Pathology , 29 : 153 – 164 .
- Bacon , LD and Palmquist , D . 2002 . Chicken lines differ in production of interferon-like factors by peripheral white blood cells stimulated with phytohemagglutinin . Poultry Science , 81 : 1629 – 1636 .
- Bacon , LD , Crittenden , LB , Witter , RL , Fadly , A and Motta , J . 1983 . B5 and B15 associated with progressive Marek's disease, Rous sacoma, and avian leukosis virus-induced tumours in inbred 15I4 chickens . Poultry Science , 62 : 253 – 257 .
- Bacon , LD , Smith , EJ , Crittenden , LB and Havenstein , GB . 1988 . Association of the slow-feathering (K) and endogenous viral (ev21) gene on the Z chromosome of chickens . Poultry Science , 67 : 191 – 197 .
- Bacon , LD , Smith , EJ , Fadly , AM and Crittenden , LB . 1996 . Development of an alloantiserum (R2) that detects susceptibility of chickens to subgroup E endogenous avian leukosis virus . Avian Pathology , 25 : 551 – 568 .
- Bacon , LD , Hunt , HD and Cheng , HH . 2000 . A review of the development of chicken lines to resolve genes determining resistance to diseases . Poultry Science , 79 : 1082 – 1093 .
- Bates , P , Rong , L , Varmus , HE , Young , JA and Crittenden , LB . 1998 . Genetic mapping of the cloned subgroup A avian sarcoma and leukosis virus receptor gene to the TVA locus . Journal of Virology , 72 : 2505 – 2508 .
- Benkel , BF . 1998 . Locus-specific diagnostic tests for endogenous avian leukosis-type viral loci in chickens . Poultry Science , 77 : 1027 – 1035 .
- Chase WB 1991 Eradication of avian leukosis virus by breeder companies: results, pitfalls and cost benefit analysis Avian Tumour Virus Symposium Seattle, WA, 28 July pp. 5–7 Kennett Square PA American Association of Avian Pathologists
- Crittenden , LB . 1991 . Retroviral elements in the genome of the chicken: implications for poultry genetics and breeding . Critical Reviews of Poultry Biology , 3 : 73 – 109 .
- Crittenden , LB and Fadly , AM . 1985 . Responses of chickens lacking or expressing endogenous avian leukosis virus genes to infection with exogenous virus . Poultry Science , 64 : 454 – 463 .
- Crittenden , LB and Salter , DW . 1989 . Spontaneous mobility of subgroup A avian leukosis virus proviruses in the genome of the chicken . Avian Pathology , 27 : S26 – S35 .
- Crittenden , LB , Wendel , EJ and Motta , JV . 1973 . Interaction of genes controlling resistance to RSV(RAV-0) . Virology , 52 : 573 – 584 .
- Crittenden , LB , Smith , EJ and Fadly , AM . 1984 . Influence of endogenous viral (ev) gene expression and strain of exogenous avian leukosis virus (ALV) on mortality and ALV infection and shedding in chickens . Avian Diseases , 28 : 1037 – 1056 .
- Crittenden , LB , Salter , DW and Federspiel , MJ . 1989 . Segregation, viral phenotype, and proviral structure of 23 avian lelukosis virus inserts in the germ line of chickens . Theoretical and Applied Genetics , 77 : 505 – 515 .
- Fadly , AM and Smith , EJ . 1999 . Isolation and some characteristics of a subgroup J-like avian leukosis virus associated with myeloid leukosis in meat-type chickens in the United States . Avian Diseases , 43 : 391 – 400 .
- Fadly AM Witter RL 1998 Oncornaviruses: leukosis/sarcoma and reticuloendotheliosis In Swayne E.E., Glisson J.R., Jackwood D.J., Pearson J.E., Reed W.M. (Eds) A Laboratory Manual for the Isolation and Identification of Avian Pathogens 4th edn pp. 185–196 Kennett Square PA American Association of Avian Pathologists
- Fulton , JE , Young , EE and Bacon , LD . 1996 . Chicken Mhc alloantiserum cross-reactivity analysis by hemagglutination and flow cytometry . Immunogenetics , 43 : 277 – 288 .
- Gavora , JS , Kuhnlein , U , Crittenden , LB , Spencer , JL and Sabour , MP . 1991 . Endogenous viral genes: association with reduced egg production rate and egg size in White Leghorns . Poultry Science , 70 : 618 – 623 .
- Groudine , M , Eisenman , R and Weintraub , H . 1981 . Chromatin structure of endogenous retroviral genes and activation by an inhibitor of DNA methylation . Nature , 292 : 311 – 317 .
- Halpern , MS , Ewert , DL and England , JM . 1990 . Wing web or intravenous inoculation of chickens with v-src DNA induces visceral sarcomas . Virology , 175 : 328 – 331 .
- Hussain , AI , Shanmugam , V , Switzer , WM , Tsang , SX , Fadly , A , Thea , D , Helfand , R , Bellini , WJ , Folks , TM and Heneine , W . 2001 . Lack of evidence of endogenous avian leukosis virus and endogenous avian retrovirus transmission to measles, mumps and rubella vaccine recipients . Emerging Infectious Diseases , 7 : 66 – 72 .
- Johnson , JA and Heneine , W . 2001 . Characterization of endogenous avian leukosis viruses in chicken embryonic fibroblast substrates used in production of measles and mumps vaccines . Journal of Virology , 75 : 3605 – 3612 .
- Klucking , S , Adkins , HB and Young , JAT . 2002 . Resistance to infection by subgroups B, D, and E avian sarcoma and leukosis viruses is explained by a premature stop codon within a resistance allele of the tvb receptor gene . Journal of Virology , 76 : 7918 – 7921 .
- Kuhnlein , U , Sabour , M , Gavora , JS , Fairfull , RW and Bernon , DE . 1989 . Influence of selection for egg production and Marek's disease resistance on the incidence of endogenous viral genes in White Leghorns . Poultry Science , 68 : 1161 – 1167 .
- McKay JC Rosales AG 2000 Control and Eradication of ALV-J International Symposium on ALV-J and Other Avian Retroviruses Rauischholzhausen, Germany, 5–8 June pp. 248–255 Giessen World Veterinary Poultry Association and Institut fur Geflugelkrankheiten, Justus Liebig University
- Sabour , MP , Chambers , JR , Grunder , AA , Kuhnlein , U and Gavora , JS . 1992 . Endogenous viral gene distribution in populations of meat-type chickens . Poultry Science , 71 : 1259 – 1270 .
- Silva , RF , Fadly , AM and Hunt , HD . 2000 . Hypervariability in the envelope genes of subgroup J avian leukosis viruses obtained from different farms in the United States . Virology , 272 : 106 – 111 .
- Smith , EJ and Crittenden , LB . 1988 . Endogenous viral genes in a slow-feathering line of White leghorn chickens . Avian Pathology , 15 : 395 – 406 .
- Smith , EJ and Nelsen , TC . 1993 . Effect of tumour virus susceptibility alleles on the late-feathering gene K on early growth of White Leghorns . Poultry Science , 72 : 1400 – 1404 .
- Smith , EJ , Bizub , D , Scholl , D and Skalka , AM . 1984 . Characterization of a solitary long terminal repeat of avian endogenous virus origin . Virology , 134 : 493 – 496 .
- Smith , EJ , Fadly , AM and Crittenden , LB . 1990 . Interactions between endogenous virus loci ev 6 and ev 21.1. Immune response to exogenous avian leukosis virus infection . Poultry Science , 69 : 1244 – 1250 .
- Smith , LM , Toye , AA , Howes , K , Bumstead , N , Payne , LN and Venugopal , K . 1999 . Novel endogenous retroviral sequences in the chicken genome closely related to HPRS-103 (subgroup J) avian leukosis virus . Journal of General Virology , 80 : 261 – 268 .
- Weiss , RA . 2001 . Commentary: adventitious viral genomes in vaccines but not in vaccinees . Emerging Infectious Diseases , 7 : 153 – 154 .
- Witter , RL , Nazerian , K , Purchase , HG and Burgoyne , GH . 1970 . Isolation from turkeys of a cell-associated herpesvirus antigenically related to Marek's disease virus . American Journal of Veterinary Research , 31 : 525 – 538 .
- Zajchowski , LD and Etches , RJ . 2000 . Transgenic chickens: past, present and future . Avian and Poultry Biology Reviews , 11 : 63 – 80 .