Abstract
Salmonella enterica serovar Enteritidis (SE) is a causative agent for human food poisoning cases throughout the world. The ovaries and the oviducts of the laying hens are the major sites of SE colonization from which vertical transmission to eggs occurs. In this study, Salmonella-induced changes in T lymphocytes, B lymphocytes and macrophages in the ovaries and oviducts were assessed after primary and secondary experimental inoculations of laying hen with SE. Statistically significant increases in the numbers of T cells (both CD4+ and CD8+) and macrophages were observed 7 to 14 days after primary inoculation, followed by a peak in B-cell numbers from the 14th day post-primary inoculation onwards in the secretory areas of the oviducts. The peak in lymphocyte numbers immediately preceded a decline in the rate of SE recovery from the reproductive tract beginning at day 14. The correlation of decreased Salmonella recovery with elevated lymphocyte and macrophage numbers strongly suggests that local cell-mediated immunity is involved in controlling SE injection in the ovaries and oviducts.
1 Introduction
The incidence of Salmonella enterica serovar Enteritidis (SE) isolation associated with food poisoning has dramatically increased in many countries. Chickens appear to be the major reservoir of this organism, with eggs and poultry products being the most important sources of salmonellosis (Coyle et al., Citation1988). Contamination of eggshells with SE occurs as a result of intestinal infection, and there has been a correlation between faecal positivity and eggshell contamination (Gast & Beard, Citation1990). SE has been isolated from the contents of clean, intact eggs produced by both naturally and experimentally infected laying hens (Humphrey et al., Citation1989a; Timoney et al., Citation1989; Gast & Beard, Citation1990).
Another source of egg contamination of SE can be from the reproductive tissues such as ovaries and oviducts. SE organisms have been found from the reproductive tissues of infected hens that had no caecal colonization (Lister, Citation1988). Once the infection is established in laying hens in the reproductive organs, Salmonella persists for a long time with intermittent shedding (Lister, Citation1988; Humphrey et al., Citation1989b).
On the other hand, virgin hens experimentally infected with SE via infected semen had contaminated oviducts, but not ovaries, and laid eggs with contaminated contents (Reiber & Conner, Citation1995; Reiber et al., Citation1995). SE organisms were demonstrated on the mucosal surface of the oviduct and this study suggested a possibility of ascending infection from cloaca rather than hematogeneous infection (Hoop & Pospichil, Citation1993). The more frequent isolation of SE from the oviducts compared with the ovaries agrees with the isolation of SE more frequently from the egg white than the egg yolk of naturally infected hens (Humphrey et al., Citation1991; Hoop & Pospichil, Citation1993). The distribution patterns of SE in oviducts and the more frequent isolation of SE from the albumen than the yolk suggest that some eggs become infected in the peritoneum or oviducts prior to the production of shell (Timoney et al., Citation1989; Shivaprasad, et al., Citation1990).
Previous reports suggest that the reproductive organs of laying hens are important sites for SE colonization and for egg contamination. In order to develop a strategy to reduce SE contamination of the intact eggs, understanding the local immune responses in the reproductive organs in chickens is important. The nature host protective mechanisms involved against Salmonella is still not understood. Protection from SE by humoral defense mechanism alone seems unlikely because SE is an intracellular bacterium. For many intracellular organisms including Salmonella, cell-mediated immunity plays a major role (George et al., Citation1987; Hsu, Citation1989). The acquired resistance to the infection with intracellular bacteria such as Salmonella is mediated by various cytokines, which are secreted by activated CD4+ T lymphocytes (Kaufmann, Citation1993). Unlike the virgin hens, oviducts of the hens previously mated were free of SE after infection with contaminated semen (Reiber & Conner, Citation1995; Reiber et al., Citation1995) indicating a possible protective local immunity induced by the infected semen. Furthermore, it is possible that the mating will induce a non-antigen-specific immune mechanism in the oviduct against pathogens. The composition of various lymphoid tissues and lymphocytes in the oviducts of laying hens has been described in detail (Withanage et al., Citation1997). In the present study, the changes of the cell composition in the ovaries and oviducts have been investigated using immunohistochemical methods following primary and secondary inoculation with SE.
2 Materials and Methods
2.1 Experimental animals
White Leghorn TX DeKalb 190-day-old laying hens were purchased from a local farm (Chubu Kakin Sangyo, Gifu, Japan) and kept in wire-floored cages in an air-conditioned room with artificial illumination. All hens were acclimatized for at least 2 weeks prior to experimentation, and provided water and antibiotic-free layer-breeder ration (Nihonhaigo-shiryo, Aichi, Japan) ad libitum. Caecal swabs and faecal samples showed that they were free of Salmonella before the start of the experiments. The experimental procedures and animal management protocols were undertaken in accordance with the guideline on the Care and Management of Experimental Animals (Japan).
2.2 Bacteria
Salmonella enterica serovar Enteritidis phage type 4, originally isolated from a faecal sample of a patient with food poisoning, was grown in 10 ml tryptose soy broth (Nissui, Tokyo, Japan) at 37°C for 18 h. The cultured broth was diluted in sterile physiological saline in order to prepare the inoculum containing 6×107 colony forming units (CFU) SE/ml immediately before inoculation.
2.3 Experimental design
Thirty-five hens were used in the experiment (five per group, seven groups total) and 10 hens were kept as controls (five per group, two groups total). On day 0, all experimental hens were inoculated intravenously with 1 ml inoculum containing 6×107 CFU SE in physiological saline, whereas control groups were inoculated with physiological saline. The SE dose and the route of inoculation have been determined to give optimum infection of ovaries and oviducts with minimum physiological side effects (unpublished data). Five hens from the experimental groups 1 to 3 were euthanized by injecting 200 mg/bird sodium pentobarbital solution (Nembutal™; Abbott Laboratories, North Chicago, Illinois, USA) intravenously, at 7, 14, and 21 days post-primary inoculation (p.p.i.). Samples from the spleen, ovary, and upper (infundibulum and magnum) and lower (uterus and vagina) parts of the oviducts were collected aseptically for bacterial culture. Tissue samples for immunohistochemistry were snap-fozen in dry ice-cold acetone and stored at −80°C until use. On day 21 after the primary inoculation, the remaining 20 experimental birds were inoculated with 6×107 CFU SE in physiological saline, whereas control hens were injected with saline only. Similarly, five hens from the experimental groups 4 to 7 were euthanized and samples were collected on 1, 3, 7, and 14 days post-secondary inoculation (p.s.i.). Daily individual egg production and clinical signs were recorded throughout the experiment. Five control birds were euthanized and the samples were collected only at the start and at the end of the experiment due to the practical difficulties in having five birds each at every time point. The values of the cells in control birds were consistent at the start and at the end of the experiment.
2.4 Bacterial culture
Swabs collected aseptically from the homogenized spleen, ovaries, and upper (infundibulum and magnum) and lower oviducts (uterus and vagina) were cultured individually in Hajna tetrathionate broth (Eiken, Tokyo, Japan) at 37°C. After incubation for 24 h, a loop of broth culture was spread on mannitol lysine crystal violet brilliant green agar plates (Nissui) and incubated for 24 h at 37°C. Dark colonies with a diameter of 3 to 5 mm, a convex surface, and a black centre were presumptively identified as Salmonella and confirmed by slide agglutination using O-9 group-specific antiserum (Denkaseiken, Tokyo, Japan).
2.5 Immunohistochemistry
Three specimens each representing the upper, middle, and lower parts of the five oviductal regions (infundibulum, magnum, isthmus, uterus, and vagina) (Baumel, Citation1979) and the ovary were collected and fixed immediately in dry ice-acetone for 5 min. Frozen sections were embedded in TISSUE TEK™ (Miles, Elkhart. Indiana, USA) and serial 7 μm cross-sections were made by a cryostatic microtome (BRIGHT 5040; Bright Instruments, Huntington, UK). After drying in air, embedding medium was removed by dipping slides in 0.1 M phosphate-buffered saline (PBS) for 15 min at room temperature. The sections were incubated with 1% skimmed milk for 30 min at room temperature to minimize non-specific binding before incubation with specific monoclonal antibodies (mAbs). Mouse mAbs against chicken CD3, CD4, and CD8 (Southern Biotechnology Associates, Birmingham, Alabama, USA) and goat polyclonal antibodies against chicken immunoglobulin (Ig)A, IgG, and IgM (Bethyl Laboratories, Montgomery, Texas, USA) were used. Mouse mAb K1 was used for detecting chicken macrophages (Cheung & Lillehoj, Citation1991). The DAKO LSAB 2 kit™ (Dako Corporation, Carpinteria, California, USA) was used for detection of T lymphocytes and macrophages, and the specific colour reaction was developed with 3,3′-diaminobenzidine in Tris buffer according to the manufacturer's recommendations.
Fluorescein isothiocyanate-conjugated rabbit anti-goat IgG antibody (Sigma, St, Louis, Mussouri, USA) was used as the secondary antibody to detect B lymphocytes by the immunofluorescent method. All incubations were made at room temperature for 1 h, and the sections were thoroughly washed between incubations with 0.1 M PBS. For positive controls, spleen, jejunum, and cecum sections were used. For negative controls, 3% borne serum albumin was used instead of primary antibody. T cells and macrophages were observed and photographed by light microscopy (Model IIFX-DX; Nikon, Tokyo, Japan). B cells were visualized and photographed with a fluorescent microscope (model UFX IIA; Nikon). Cells were counted on at least five separate photographs taken at 100× magnification representing an area equivalent to 0.07 mm2. The photographs were taken across the whole width of the tissue to represent all parts of the relevant tissue (i.e., epithelium, lamina propria and the muscularis of the oviductal tissues).
2.6 Statistical analysis
The mean values of cell numbers for each experimental group were calculated and compared with the values of the respective control hens by Student's t-test. Statistically significant differences were considered at P<0.05. In most of the time points the standard deviation values for T-cell and macrophage numbers were very small. The numbers of B cells show considerable variations between birds in the same group. This was reflected by the higher P values in the Student's t-test.
3 Results
3.1 Clinical signs and the egg production
Four hens died within 24 h after the inoculation of SE. Initially, the hens were depressed and their water and feed intake were reduced significantly within the first week after primary and secondary inoculations. Furthermore, while the egg production rate was 0.866 eggs/hen/day (86%) before the primary inoculation, it declined and gradually recovered showing the egg production rates of 0.167, 0.201, and 0.382 eggs/hen/day on 7, 14 and 21 days p.p.i., respectively. One day after the secondary inoculation, the egg production dropped again to 0.238 and recovered gradually to 0.281, 0.181, and 0.523 egg/hen/day on 3, 7 and 14 days p.s.i. Some hens laid malformed, thin-shelled eggs in the early phase of infection. Their physiological condition such as feed and water intake as well as egg production improved within 1 week after each inoculation.
3.2 Recovery of SE
The spleen, ovary, upper and lower oviducts from control hens were free of SE throughout the study. The Salmonella-specific antibody levels were negligible in all hens before the start of the experiments, as reported earlier (Withanage et al., Citation1999). All samples examined from the infected hens necropsied at 7 and 14 days p.p.i. and at 1 and 3 days p.s.i. were infected with SE (). The recovery of SE from various organs started to decline from day 14 post primary and secondary inoculations. This decline correlated with the improved physiological condition of the hens.
Isolation of SE from the organs of infected laying hens
3.3 Numbers of macrophages, T cells and B cells in the ovaries and oviducts following SE inoculation
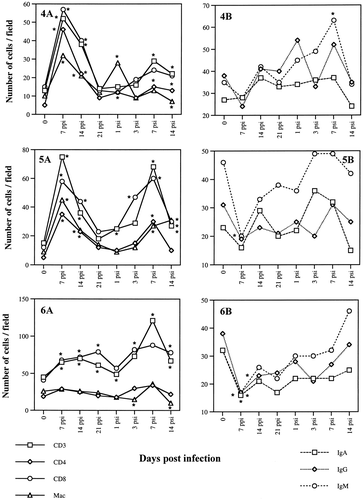
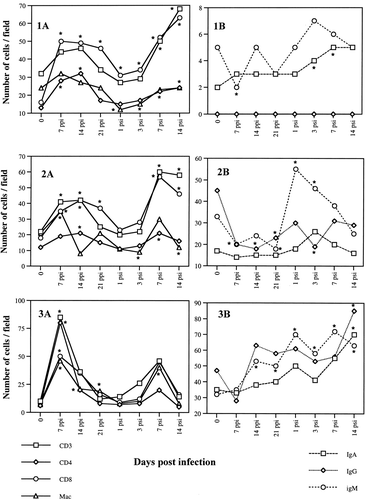
In general, the numbers of T-cell and B-cell subpopulations observed in the ovaries and oviducts fluctuated following SE inoculation (). The numbers of cells were greater in lower parts of the oviducts in both infected and uninfected hens. T cells increased and reached a peak by day 7, while B cells peaked at 14 days p.p.i. after the initial decline on the day 7 p.p.i. The peak in T cells was less distinguishable in the ovaries and in the vagina. The number of macrophages also declined initially but recovered to the pre-inoculation levels during the course of the infection. By day 21, the number of T-cell subsets returned to normal levels except for the ovary, infundibulum and vagina. In most parts of the oviducts examined, the numbers of both T cells and B cells started to increase significantly from the day 7 after the secondary inoculation. Numbers of IgM+ cells also increased rapidly after the secondary inoculation. However, the levels of the oviductal antibodies against SE did not correlate with the cellular response (Withanage et al., Citation1999). Numerous CD4+ and CD8+ T-cell aggregates of different sizes were observed in the SE-infected oviducts, mostly in the upper regions. Macrophages were also occasionally found within these areas. CD8+ and IgA+ cells were mainly found in the lamina propria just underneath the epithelial lining. Similar to the T cells, the number of macrophages were elevated in almost all the parts of the oviducts examined at 7 day p.p.i. The numbers of T cells and macrophages were higher in the ovary, infundibulum and vagina after the secondary infection. In general, a drastic decline of macrophage numbers was seen just after the secondary SE inoculation in all oviductal regions except for the isthmus. The fluctuations of the numbers of T cells, B cells and macrophages are summarized in .
Fig. 1 T cells and B cells and macrophages in the ovary (1), infundibulum (2), magnum (3), isthmus (4), uterus (5) and vagina (6) of laying hens following intravenous SE inoculations. 1A: T cells and macrophages. 1B: B cells. Samples were taken 7, 14, 21 days p.p.i. and 1,3,7, and 14 days p.s.i. of 6×107 CFU bacteria. T cells and macrophages were detected by the avidin–biotin–peroxidase immunohistochemical method. B cells were detected by indirect immunofluorescence test. Cells were counted on photographs taken at a magnification of 100× (counting area was equivalent to 0.07 mm2). * Significant difference (P<0.05) from the respective control values.
4 Discussion
Compared with the mammalian system, very little is known about the immune system of chickens. Although mammalian and avian immune systems are similar in many respects, distinct differences in the structure and function exist. For example, the thymus serves as the primary lymphoid organ for T-cell differentiation whereas differentiation of B cells takes place in the bursa of Fabricius located in the hindgut Chickens have well-developed secondary lymphoid structures such as the spleen, Harderian gland, bone marrow, conjunctiva-associated lymphoid tissue, gut-associated lymphoid tissue, bronchial-associated lymphoid tissue, and head-associated lymphoid tissue (Sharma, Citation1997). Although chickens lack functional lymph nodes, lymphatics with associated lymphoid nodules can be detected. In addition, many organs such as the liver, kidney, and pancreas have diffuse lymphoid cells scattered in the parenchyma (Sharma, Citation1997). However, the local immune system and its function associated with chicken's reproductive organs have not been well studied. The ovaries and oviducts are not different from other organs with respect to the presence of scattered lymphoid cells and macrophages in all anatomical regions (Withanage et al., Citation1997). In the chicken oviducts, T lymphocytes first appear at 5 weeks of age, their number peaked at 15 weeks in the magnum, isthmus and the uterus and at 19 weeks in the infundibulum and vagina (Khan et al., Citation1996). In chickens as in mammals, cells mediating different activities can be distinguished by cell surface antigens such as CD4 for helper T cells, CD8 for cytotoxic T cells, and CD3 as a common T-cell antigen (Vainio & Lassila, Citation1989).
Protection of mice against Salmonella infections depends on cellular and humoral immune responses (Collins, Citation1974). It is well known that T cells mediate immunity against intracellular bacteria by a number of different mechanisms mediated through different cell types and their soluble mediators (Mcgruder et al., Citation1993; Genovese et al., Citation2000; Babu et al., Citation2003). Although Salmonella infection mainly induces cell-mediated immunity through the activation of Th1-type cytokines, Salmonella typhimurium stimulates Th2 cells as well in mice (Vancott et al., Citation1997). SE infection induced secretion of all three subclasses of antibodies not only in serum, but also in the oviducts (Withanage et al., Citation1999). However, it is not clear whether these oviductal antibodies are directly secreted from the local B cells or diffused from the serum. Although it is well known that the ovaries secrete egg yolk containing a large amounts of IgG, we were unable to demonstrate any IgG-containing cells, suggesting that the yolk antibodies are directly diffused from the serum, It has been suggested that the immunoglobulins in the circulating blood are secreted into the maturing follicles and thus into the yolk, and immunoglobulins in the oviducts are incorporated into egg white with the secreted albumen as possible routes (Ling et al., Citation1998). In mammals, secretory immunoglobulins may be involved in keeping the reproductive tract aseptic by blocking bacteria from binding to the mucosal epithelium, agglutination of bacteria, and the opsonization of bacteria (Barr & Parr, Citation1985).
In this study, SE inoculation induced significant changes in the local cell populations, CD4+,and CD8+ cells peaking at 7 to 14 days after the primary and 7 days after the secondary SE inoculation followed by a B-cell peak from the 14th day onwards in the secretory areas of the oviducts, (i.e., magnum, isthmus and uterus). The peak in the lymphocyte numbers at 10 to 14 days p.p.i. and p.s.i. preceded a decline in the incidence rate of SE recovery from the reproductive tract, which began at day 14 p.p.i. day 7 p.s.i., respectively (). The recovery of SE decreased more rapidly p.s.i., especially in the upper oviducts. Clearance of SE from the spleen and from the lower oviduct seemed to be slower than that of ovaries and upper oviducts. That may be attributed to the large dose and the route of challenge. The relationship between the lymphocyte numbers and the bacterial recovery strongly suggests that both cell types are involved in controlling SE infection in the ovaries and oviducts. The results of this study extend the information concerning the role of local immune responses following SE infection. Elevated numbers of immune competent cells in the oviductal mucosa and the presence of SE-specific antibodies in the oviductal lumen (Withanage et al., Citation1999) suggest that complex interaction of cells and their soluble factors are closely involved in host defence against Salmonella infection. Future studies to identify the detailed protective immune mechanisms associated with other components of inflammatory responses (Oliphant et al., Citation1977, 1978, 1984Citation Citation; Butcher & Picker, Citation1996) in the oviduct will probably lead to the development of logical control strategy against SE and other mucosal pathogens.
Acknowledgments
Salmonella enterica serovar Enteritidis phage type 4 was kindly supplied by Dr T. Tsukamoto, Osaka Prefectural Institute of Public Health. This work was partially supported by Sasakawa Scientific Research Grant 9-251 from the Japan Science Society
References
- Babu , U. , Scott , M. , Myers , M.J. , Okamura , M. , Gaines , D. , Yanvy , H.F. , Lillehoj , H.S. , Heckert , R.A. and Raybourne , R.B. 2003 . Effects of live attenuated and killed Salmonella vaccine on T lymphocyte mediated immunity in laying hens . Veterinary Immunology and Immunopathology , 32 : 39 – 44 .
- Barr , E.L. and Parr , M.B. 1985 . Secretory immunoglobulin binding to bacteria in the mouse uterus after mating . Journal of Reproductive Immunnlogy , 32 : 71 – 82 .
- Baumel, J.J. (1979). Nomina Anatomica Avium. London: Academic Press
- Butcher , E.C. and Picker , L.J. 1996 . Lymphocyte homing and homeostasis . Science , 32 : 60 – 66 .
- Cheung , K.S. and Lillehoj , H.S. 1991 . Characterization of monoclonal antibodies detecting avian macrophages and NK cells . Veterinary Immunology and Immunopathology , 32 : 351 – 363 .
- Collins , F.M. 1974 . Vaccines and cell mediated immunity . Bacteriological Review , 32 : 371 – 402 .
- Coyle , E.F. , Plamer , S.R. and Riberio , C.D. 1988 . Salmonella phage type 4 infection: associated with hen's eggs . Lancet , 32 : 1295 – 1297 .
- Gast , R.K. and Beard , C.W. 1990 . Production of S. enteritidis contaminated eggs by experimentally infected hens . Avian Diseases , 32 : 438 – 446 .
- Genovese , L.L. , Lowry , V.K. , Genovese , K.J. and Kogut , M.H. 2000 . Longevity of augmented phagocytic activity of heterophils in neonatal chickens following administration of Salmonella enteritidis-immune lymphokines to chickens . Avian Pathology , 32 : 117 – 122 .
- George , A. , Nair , R. , Rath , S. , Ghosh , S.N. and Kamat , R.S. 1987 . Regulation of cell mediated immunity in mice immunized with Salmonella enteritidis . Journal of Medical Microbiology , 32 : 239 – 246 .
- Hoop , R.K. and Pospichil , A. 1993 . Bacteriological, serological, histological and immuno-histochemical findings in laying hens with naturally acquired S. enteritidis phage type 4 infection . Veterinary Record , 32 : 391 – 393 .
- Hsu , H.S. 1989 . Pathogenesis and immunity in murine salmonellosis . Microbiological Reviews , 32 : 390 – 409 .
- Humphrey , T.J. , Baskerville , A. , Chart , H. and Rowe , B. 1989a . Infection of egg laying hens with S. enteritidis PT 4 by oral inoculation . Veterinary Record , 32 : 531 – 532 .
- Humphrey , T.J. , Baskerville , A. , Mawer , S.L. , Rowe , B. and Hopper , S. 1989b . Salmonella enteritidis phage type 4 from the contents of intact eggs: a study involving naturally infected hens . Epidemiology and Infection , 32 : 415 – 423 .
- Humphrey , T.J. , Whitehead , A. , Gawler , A.H.L. , Henley , A. and Rowe , B. 1991 . Numbers of Salmonella enteritidis in the contents of naturally contaminated hen's eggs . Epidemiology and Infection , 32 : 489 – 496 .
- Kaufmann , S.H. 1993 . Immunity to intracellular bacteria . Annual Review of Immunology , 32 : 129 – 163 .
- Khan , M.Z.I. , Hashimoto , Y. , Konno , A. , Kon , Y. and Iwanaga , T. 1996 . Development of T-lymphocyte subpopulations in the postnatal chicken oviduct . Cell and Tissue Research , 32 : 317 – 325 .
- Ling , Y.S. , Guo , Y.J. , Li , J.D. , Yang , L.K. , Luo , Y.X. , Yu , S.X. , Zhen , L.Q. , Qiu , S.B. and Zhu , G.F. 1998 . Serum and egg yolk antibody titers from laying chickens vaccinated with Pasteurella multocida . Avian Diseases , 32 : 186 – 189 .
- Lister , S.A. 1988 . Salmonella enteritidis infection in broilers and broiler breeders . Veterinary Record , 32 : 351
- Mcgruder , E.D. , Ray , P.M.. , Tellez , G.I. , Kogut , M.H. , Corrier , D.E. , Deloach , J.R. and Hargis , B.M. 1993 . Salmonella enteritidis immune leukocyte stimulated soluble factors: effects on increased resistance to organ invasion in day old chickens . Poultry Science , 32 : 2264 – 2271 .
- Oliphant , G. , Randall , P.A. and Cabot , C.L. 1977 . Immunological components of rabbit Fallopian tube fluid . Biology of Reproduction , 32 : 463 – 539 .
- Oliphant , G. , Bowling , A. , Eng , L.A. , Keen , S. and Randall , P.A. 1978 . The permeability of the rabbit oviduct to proteins present in the serum . Biology of Reproduction , 32 : 516 – 520 .
- Oliphant , G. , Cabot , C. , Ross , P. and Marta , J. 1984 . Control of the humoral immune system within the rabbit oviduct . Biology of Reproduction , 32 : 205 – 212 .
- Reiber , M.A. and Conner , D.E. 1995 . Effects of mating activity on the ability of Salmonella enteritidis to persist in the ovary and oviducts of chickens . Avian Diseases , 32 : 323 – 327 .
- Reiber , M.A. , Conner , D.E. and Bilgili , S.F. 1995 . Salmonella colonization and shedding patterns of hens inoculated via semen . Avian Diseases , 32 : 317 – 322 .
- Sharma , J.M. 1997 . The structure and function of the avian immune system . Acta Veterinaria Hungarica , 32 : 229 – 238 .
- Shivaprasad , H.L. , Timoney , J.F. , Morales , S. , Lucio , B. and Baker , R.C. 1990 . Pathogenesis of Salmonella enteritidis infection in laying chickens. I. Studies on egg transmission, clinical signs, fecal shedding and serologic responses . Avian Diseases , 32 : 548 – 557 .
- Timoney , J.F. , Shivaprasad , H.L. , Baker , R.C. and Rowe , B. 1989 . Egg transmission after infection of hens with Salmonella enteritidis phage type 4 . Veterinary Record , 32 : 600 – 601 .
- Vainio , O. and Lassila , O. 1989 . Chicken T cell differentiation antigens and cell interactions . Critical Review of Poultry Biology , 32 : 97 – 102 .
- Vancott , J.L. , Kweon. , M. , Fujihashi , K. , Yamamoto , M. , Marinaro , M. , Kiyono , H. and McGhee , J.R. 1997 . Helper T subsets and cytokines for mucosal immunity and tolerance . Behring Institute Mitteilungen , 32 : 44 – 52 .
- Withanage , G.S.K. , Baba , E. , Sasai , K. , Fukata , T. , Kuwamura , M. , Miyamoto , T. and Arakawa , A. 1997 . Localization and enumeration of T and B lymphocytes in the reproductive tract of laying hens . Poultry Science , 32 : 671 – 676 .
- Withanage , G.S.K. , Sasai , K. , Fukata , T. , Miyamoto , T. and Baba , E. 1999 . Secretion of Salmonella-specific antibodies in the oviducts of hens experimentally infected with Salmonella enteritidis . Veterinary Immunology and Immunopathology , 32 : 185 – 193 .