Abstract
After the discovery of poultry infected with highly pathogenic avian influenza (HPAI) virus of subtype H7N7 in the central area of the Netherlands on 28 February 2003, the hypothesis was put forward that an outbreak of the low pathogenic (LP) variant of H7N7 had preceded, unnoticed, the occurrence of the HPAI virus. Consequently, a cross-sectional serological survey of the Dutch poultry population was executed in the second week of March 2003. The basic requirements set were detection of a 5% prevalence of flocks exposed to LPAI virus with 95% confidence within the production type stratification level within each province in the Netherlands. Because of supposed higher risk of avian influenza infections in ducks, turkeys and free-range poultry, all the commercial flocks of these production types present in the Netherlands were sampled.
The serological screening of 28018 sera from 1193 randomly selected poultry farms, located outside surveillance zones showed that LPAI H7 virus infections had occurred on three neighbouring farms all located in the southwest of the Netherlands. No antibodies against the neuraminidase N7 subtype were detected in the sera of these farms, indicating that the subtype was different from the HPAI H7N7 subtype that caused the avian influenza epidemic in 2003. In addition, evidence of infections with non-H5 or non-H7 subtypes of influenza A virus were obtained in two other farms located in the northeast and the southeast of the Netherlands. It was concluded that the HPAI subtype H7N7 outbreak was most likely not preceded by a significant circulation of a LPAI subtype H7N7 virus. Based on the Dutch experience, recommendations are made to detect avian influenza infections faster in the future.
Introduction
Influenza A viruses infecting poultry can be divided into two distinct groups on the basis of their ability to cause disease in chickens (Alexander, Citation2002). The highly pathogenic avian influenza (HPAI) viruses cause mortality rates up to 100% in several days. Low pathogenic avian influenza (LPAI) viruses cause subclinical to mild, primarily respiratory disease, which however can result in a serious disease when being complicated by concurrent infections and/or suboptimal environmental conditions. Strains of the H5 and H7 subtype with multiple basic amino acids at the cleavage site of the HA protein that do cause low mortality in experimental infections are also classified as HPAI viruses because the virulence can easily increase by a single mutation. Moreover, LPAI viruses of subtype H5 or H7 can easily acquire multiple basic amino acids at the cleavage site and thus exhibit highly pathogenic characteristics following a few mutations (Swayne & Halvorson, Citation2003). So, circulation of these LPAI viruses in poultry is undesirable as they may result in an HPAI outbreak. Examples of HPAI outbreaks that developed from already circulating LPAI strains have been reported in the US (Bean et al., Citation1985), Mexico (Garcia et al., Citation1996), Italy (Capua & Marangon, Citation2000) and Chili (Rojas et al., Citation2002)
After the discovery of HPAI virus of subtype H7N7 in the central area of the Netherlands on 28 February 2003 (Elbers et al., Citation2004a), a discussion was immediately started with respect to the origin of the virus. The hypothesis was put forward that an outbreak of the low pathogenic variant of H7N7 had preceded the occurrence of highly pathogenic virus and had circulated and remained unnoticed in commercial poultry for some time. To investigate this hypothesis, a cross-sectional serological survey of the Dutch poultry population was executed in the second week of March 2003.
Materials and Methods
Study design
A stratified random sampling design was set up, with the 12 provinces in the Netherlands outside the restriction zones of the HPAI epidemic as a first stratification level, and the production type (ducks, turkeys, broilers, parent stock, layers, and poultry with a free-range housing system) as a second stratification level. The basic requirements set were detection of a 5% prevalence of flocks with exposure to LPAI virus with 95% confidence within the production type stratification level within a province. Because of a historical risk of avian influenza (AI) infections of ducks, turkeys and free-range poultry, all the commercial flocks of these production types present in the Netherlands were sampled. The minimum size of a poultry farm according to the Dutch rules is 250 birds. Within each of the randomly selected farms, 10 blood samples were randomly collected in each of the poultry houses to obtain a reliable impression on the prevalence of AI virus. Basic demographic data on all poultry flocks in the Netherlands from a database of the Dutch Product Board for Poultry and Eggs were used as the sampling frame to calculate the number of farms to be sampled from a specific production type per province (). Farms located within the surveillance and protection zones were excluded because blood samples of these farms were already collected and tested because of the control measures.
Table 1. Numbers of farms for each type of production per province that were sampled in the cross-sectional serological survey for the presence of LPAI in the Netherlands in 2003
As can be seen in , there were differences in the required sample size and actually sampled farms. This difference was partly caused by the fact that a portion of the randomly selected poultry farms housed no poultry at the actual period of sampling. Furthermore, in the days between designing the study and the actual sampling, the epidemic was spreading resulting in a larger restriction area (in a densely populated area) and the target population to be sampled became smaller. Furthermore, it was possible that the database was not fully updated, because poultry farmers had changed the production type of their operation (very) recently. This recent change of production type was especially relevant within the layer industry, whereas many layer farms had changed from in-house to the free-range system ().
Serological testing
Five thousand and fifty samples that were present in the refrigerators of the Dutch Animal Health Service (AHS) at time (start) of the HPAI subtype H7N7 outbreak were suitable for testing for presence of antibodies against LPAI. The oldest sera were taken on 17 February 2003. An additional 22,968 blood samples were collected from randomly selected farms between 10 and 15 March 2003 and sent to the AHS. Samples were taken randomly within poultry houses and collected in serum gel tubes (Sarstedt). The AHS registered the farm, the province and the type of poultry. In , the number of farms in the survey is given per province and per type of poultry. The survey comprised in total poultry from 1115 chicken and turkey farms, from which 26,898 blood samples were tested in an enzyme-linked immunosorbent assay (ELISA) (FlockChek AIV antibody Test Kit; IDEXX Corporation, Westbrook, ME, USA) for the presence of antibodies against AI virus. Samples that scored positive in the ELISA were subsequently tested at the national reference laboratory at the Central Institute for Animal Disease Control in Lelystad (CIDC-Lelystad), in the haemagglutination inhibition (HI) test according to Annex III of the EU Council directive 92/40 (1992). Six to nine haemagglutination units of A/parrot/Northern Ireland/H7N1/vr7367/73 H7N1 were used as the antigen in the test. A specific pathogen free serum and chicken sera against A/parrot/Northern Ireland./vr7367/73 H7N1 and against A/tern/S. Africa/61 H5N2 were used as controls. Sera from houses with more than one positive sample in the ELISA and a negative result in the HI test for H7 were subsequently tested in the HI test using A/chicken/Netherlands/1/85 H1N1, A/duck/Ukraine/63 H3N8, A/duck/Czechoslovakia/56 H4N6, A/chicken/Belgium/150/99 H5N2, A/turkey/Massachussetts/65 H6N2, A/turkey/Ontario/6118/67 H8N4, A/chicken/Netherlands/4271/80 H9N2, A/chicken/Germany/N/49 H10N7, A/duck/England/56 H11N6 and A/duck/Alberta/60/76 H12N5 antigen and appropriate positive control sera produced in experimentally inoculated specific pathogen free chickens.
All 1120 serum samples derived from ducks (78 farms) were directly tested with the HI test (CIDC-Lelystad).
Antibodies against N7, N1 and N3 were detected using an indirect immunofluorescence assay (iIFAT) on insect cells expressing the appropriated neuraminidase type. Cells expressing N1 and N3 were kindly provided by Dr G. Cattoli (Istituto Zooprofilattico Sperimentale delle Venezie, Italy) and the N7 protein was produced at CICD-Lelystad as described by Capua et al. (Citation2002).
Results
General
In total, 28,018 sera from 1193 farms were tested in the ELISA (26,898 sera from 1115 chicken or turkey farms) or the HI test (1120 duck sera) for antibodies against AI virus. A total 250 sera (from 203 different poultry houses) were positive in the ELISA but negative in the HI test (all sera were tested using H7 antigen; sera from houses with more than one positive sample were also tested using H1, H3, H4, H5, H6, H8, H9, H10, H11 and H12 antigen). In the sera of five farms a seroprevalence of 90% to 100% was detected for antibodies against AI virus (). From farm 1 (), a turkey farm in the southeast of the Netherlands (), nine out of 10 sera were positive in the ELISA and all 10 samples were positive to H1 in the HI test. In addition to turkeys, swine were raised at this farm.
Figure 1. Location of five poultry flocks within the Netherlands with antibodies against AI virus found during the cross-sectional serological survey for the presence of LPAI in 2003.
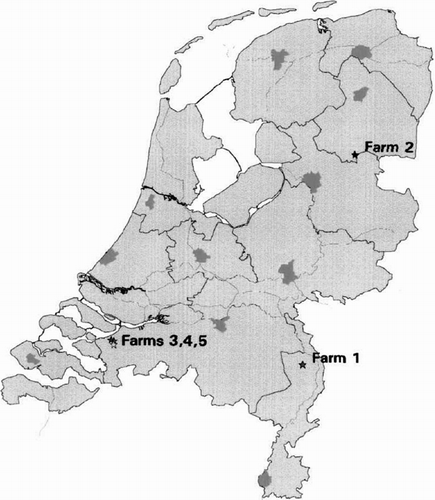
Table 2. LPAI-infected farms, results of ELISA and HI test
From farm 2 (), a free range layer farm in the northeast of the Netherlands (), 29 out of 30 sera were (strong) positive in the ELISA. All sera were negative in the HI test using influenza virus of the H1, H3, H4, H5, H6, H7, H8, H9, H10, H11 or H12 subtypes. Due to the limited amount of serum, further testing against H2 and H13 to H15 or using the of agar gel precipitation test was not possible.
Sera from farms 3, 4 and 5 were 100% positive in the ELISA and 93% to 100% positive in the HI test using H7 virus as antigen (). These three farms were located within 3 km of each other in the southwestern part of the Netherlands near important waterways with abundant wild waterfowl (). There were no detectable antibodies against N1 and N7 in the sera of these three farms. However, 17 of 20 turkey sera and 19 of 24 chicken sera scored positive in the iIFAT on insect cells expressing the N3 neuraminidase subtype.
History of flocks (farms 3, 4 and 5) with antibodies against subtype H7
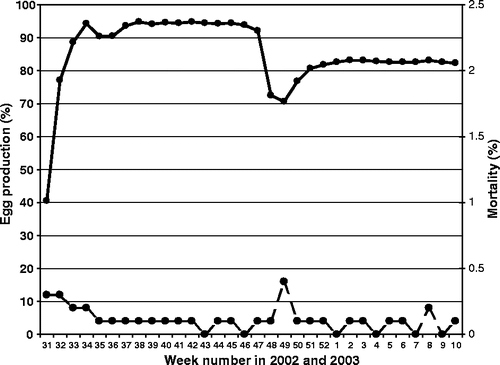
Farm 3 housed a flock of 14,060 free-range layers, 52 weeks old at time of sampling. All 30 sera were positive in the ELISA, of which 29 were also positive in the H7 HI test (). The history of the flock showed a slight, transient drop in egg production, some mortality and peritonitis with Escherichia coli at 25 to 26 weeks of age (weeks 35 to 36 of 2002; see ). At 39 to 41 weeks of age (weeks 49 to 51 of 2002), a drop (20%) in egg production and feed intake was observed. The hens were too quiet and several were reluctant to move. The mortality was slightly increased in week 49. Dead birds submitted for postmortem examination (PME) in this period showed peritonitis, airsacculitis, mild pneumonia, some degenerated ova and an Ascaridia gallinarum infection.
Figure 2. Egg production (solid line) and weekly mortality (dotted line) of the LPAI-infected free-range layer flock of farm 3.
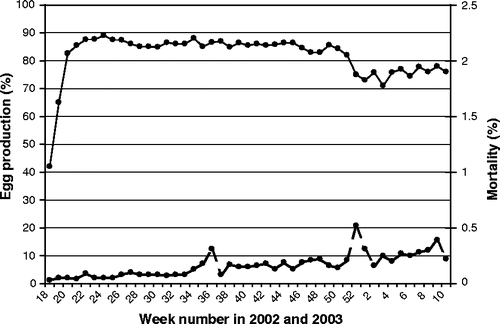
Farm 4 housed a flock of 19,300 free-range layer hens, 67 weeks old at the time of sampling. All 30 sera were positive in the ELISA, of which 28 were also positive in the H7 HI test (). The history of the flock showed an increased mortality in week 36 of 2002 (45 weeks of age) with associated peritonitis and inflammation of the ovary. In weeks 51 and 52 of 2002, the mean feed intake decreased from 123 g/day per bird to 108 g/day per bird and was accompanied by a 10% decrease in egg production and an increase in mortality (). Birds submitted for PME exhibited inflammation of the ovaries. From week 6 in 2003, pale/whitish and soft-shelled eggs were produced.
Figure 3. Egg production (solid line) and weekly mortality (dotted line) of the LPAI-infected free-range layer flock of farm 4.
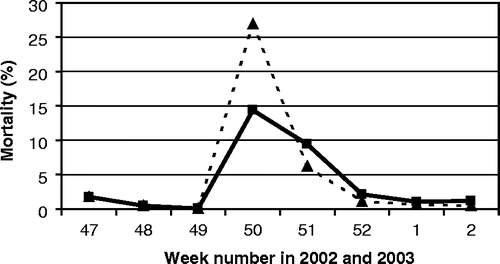
Farm 5 was a turkey farm with two separate houses. In week 48 of 2002, the feed intake of the 19-week-old male turkeys (females were already slaughtered) decreased sharply to 50% within 2 days. Two days later (week 49), the birds showed a sharp respiratory sound. On PME, large quantities of thick mucus without haemorrhages combined with fibrinous airsacculitis were observed. The flock was slaughtered in week 50, still exhibiting respiratory disease. By the end of week 50, an increase in mortality was observed accompanied by digestive problems in 18,000 3-week-old turkeys that were located in the other house. Antibiotic treatment (doxycyclin) was given. In the days after, mortality increased () and the turkeys showed depression and were reluctant to move. In a PME performed by the veterinary practitioner in the beginning of week 51 of 2002, the following abnormalities were observed: trachea with mucus, pericarditis, airsacculitis, and one turkey had a swollen head. The problems were diagnosed to be likely caused by Ornithobacterium rhinotracheale with a secondary bacterial infection of E. coli. A treatment with enrofloxacin (Baytril®) was started the same day. In the next 2 days, enhanced mortality was seen and the birds huddled together under the heaters. With the assistance of a poultry specialist and six students from the Faculty of Veterinary Medicine all severely sick birds (approximately 400 animals) were individually injected with enrofloxacin. Some dead birds were frozen for demonstration purposes for students. By the afternoon, mortality seemed to stabilize, but in the evening mortality increased again. The next day (end of week 51), a total of 12 dead turkeys were submitted for PME to the AHS. In 11 birds, an airsacculitis and in four birds a necrotic pneumonia was noticed. A preliminary diagnosis was an O. rhinotracheale infection. Supplementary investigation revealed the following results: E. coli was isolated, and immunofluorescence tests for O. rhinotracheale and avian pneumovirus were negative. The final diagnosis indicated an airsacculitis caused by E. coli. By the end of week 52 of 2002, mortality returned to normal proportions. When this flock was identified as positive for antibodies against AI subtype H7 in week 11 of 2003 during the cross-sectional serological survey, the frozen turkeys (sampled at week 51 of 2002) were sent to the national reference laboratory at CIDC-Lelystad, where an AI virus of H7N3 subtype was isolated (G. Koch, personal communication).
Discussion
In this study, we have presented the results of a cross-sectional serological survey of commercial poultry farms for the presence of LPAI infections in the Netherlands. The results of this survey indicate that the HPAI H7N7 virus epidemic was not preceded by circulation of an H7N7 LPAI variant.
The serological screening of 1193 at randomly selected poultry farms, which included all turkey, free-range and duck farms outside restriction zones, showed that H7 LPAI virus had circulated in only three farms, all located in the same region of the Netherlands. The ELISA used and the agar gel precipitation test are not suited to test duck sera and therefore these sera were directly tested in the HI test on H5 and H7 HA antigens. None of the duck sera reacted positive in the HI test. However, reportedly, ducks are considered poor antibody responders upon infection with AI viruses (Kida et al., Citation1980) or antibodies are not detected when using the HI test (Lu et al., Citation1982). Therefore, the (zero) prevalence of infected duck flocks may be an underestimation, and also because no virus isolations attempts on cloaca swabs of ducks were made.
From one (farm 5) of the three farms that showed positive for antibodies against H7, a H7N3 virus was isolated (G. Koch, personal communication) from material from diseased turkeys in week 51 of 2002. The other two H7 and N3 antibody-positive but N7 antibody-negative farms had shown a drop in feed intake and egg production at about the same time period as farm 5 (farm 3, weeks 49 to 51; farm 4, weeks 51 to 52). Since there were professional contacts between these three farms, together with the fact that these three farms were situated closely to each other, it seems likely that farm 3 and farm 4 were infected with the same H7N3 virus. Thus, because of the different N subtype of the virus, a direct relation between this epizootic in the South Western part of the Netherlands and the large H7N7 HPAI epidemic in the central part of the Netherlands is unlikely.
The limited spreading of the H7N3 virus compared with the extensive spreading of the HPAI H7N7 virus is caused either by the much lower farm density in the southwest of the Netherlands compared with the high density of poultry farms in the Gelderse Vallei and North Limburg or by different transmission rates of both viruses.
The conclusion that the HPAI H7N7 epidemic was not preceded by a detectable circulation of the LPAI H7N7 variant is further supported by very few positive samples that were detected in flocks that were located within the infected areas (G. Koch, unpublished data). Although the immediate source of H7N7 HPAI virus is not clear, re-assortment of two different wild waterfowl LPAI viruses is a plausible hypothesis for the origin of the H7 and N7 genes in the current H7N7 HPAI virus, because the H7 is phylogenetically closely related to an H7N3 LPAI virus and the N7 to an H10N7 LPAI virus both isolated from mallards in 1999 to 2000 (Fouchier et al., Citation2003, Citation2004). Although, the host species responsible for the final H7 and N7 re-assortment is not known, the free-range chickens of the 2003 H7N7 outbreak were most probably infected with a re-assorted virus since antibodies against H10 and N3 were not detected in serological-positive birds in the surveillance zones of the 2003 outbreak (G. Koch, unpublished data). Therefore, the H7N3 infection and H7N7 epidemic are probably the result of two separate introductions of an AI virus into commercial poultry flocks by contact between wild waterfowl and free chickens.
In a serological survey performed in 2000 using more than 18,000 sera from Dutch poultry farms, no indication for the presence of LPAI strains in the Dutch poultry industry was found at that time (Fabri, Citation2000). During our study in 2003, we also detected antibodies against an Influenza A H1 subtype virus in turkeys of farm 1. The Influenza A H1 subtype virus may have originated from swine raised in a separate house of the same farm. Swine Influenza A H1N1 and H3N2 viruses were isolated from chickens in 1980 in the Netherlands (Siebenga & de Boer, Citation1988). The true status of farm 2 remains uncertain. Because of the high number of sera that were ELISA-positive (mostly strong) (29 out of 30), the ELISA results were considered to be most probably truly positive. However, this could not be confirmed by the HI test using influenza virus of the H1, H3, H4, H5, H6, H7, H8, H9, H10, H11 or H12 subtypes. Possibly another subtype was involved (H2, or H13 to H15). Unfortunately, these tests cannot be performed anymore due to the lack of serum for further testing.
Birds from farms 3, 4 and 5 submitted for PME showed predominantly peritonitis and/or tracheitis, which was equivalent to what was seen during PME in birds submitted during the HPAI H7N7 epidemic in 2003 (Elbers et al., Citation2004b). Observation of peritonitis or tracheitis or oedema of the neck and/or wattles, especially if accompanied by an anamnesis describing increased mortality and/or a decrease in feed and/or water intake and/or a decrease in egg production, should consistently result in a routinely follow-up action to exclude AI in the differential diagnosis as cause of the disease problems by testing tissue samples with an H5 or H7 antigen-specific laboratory test at the national reference laboratory. Preferably, the exclusion of AI is performed with a quick test such as the reverse transcription-polymerase chain reaction to reduce the duration of the stand still at the farm.
Wild waterfowl are considered to be the natural reservoir of LPAI viruses (Fouchier et al., Citation2003; Swayne & Halvorson, Citation2003). AI viruses are spreading with these birds all over the world and are subject to genetic changes (van Tongeren & Voous, Citation1987). The results of the 2003 surveillance and close phylogenetic relationship between the Dutch H7N7 HPAI and recent duck AI strains (Fouchier et al., Citation2004) exemplifies the well-known risks formed by the permanent presence of AI viruses in wild water fowl. The risk is also demonstrated by frequent AI outbreaks in the turkey industry in Minnesota, USA of different LPAI viruses that in time and location correlated with viruses present in migrating water fowl (Alexander, Citation2002). No outbreaks occurred after the contact between turkeys and wild waterfowl were securely excluded (D. Swayne, personal communication.). Since LPAI infections cause no or very mild clinical signs, which can very easily be confused with disease symptoms caused by other (endemic) pathogens, introduction may remain unnoticed for some time. Because the number of poultry farms with free-range housing systems is expected to increase in the Netherlands in the near future due to public demand for more ecological-friendly production systems, and the experiences of the HPAI epidemic in 2003 in the Netherlands, we present the following three recommendations. (1) To develop and execute a continuous monitoring system to detect LPAI virus infections in domestic poultry. (2) To develop a centralized national reporting system where poultry farmers and their veterinarians have the obligation to report any increased mortality and/or a significant decrease in feed and/or water intake or decrease in egg production on a daily basis. Collected data should be analysed daily (e.g. on geographical clustering) by a specialized group of poultry veterinarians. It should be noted that this reporting system is not a replacement of the reporting of a serious suspicion of an AI or Newcastle disease outbreak to the veterinary authorities. (3) To perform routinely a reverse transcriptase-polymerase chain reaction test on AI genome in tissues from postmortem material of chickens or turkeys with a non-specific anamnesis of increased mortality and/or a significant decrease in feed and/or water intake or decrease in egg production to exclude AI as the cause of the clinical problems.
Acknowledgments
The authors would like to thank Dr G. Cattoli for the generous gift of plates coated with N1-expressing and N3-expressing insect cells, and J. J. Voermans for performing the iIFAT.
References
- Alexander , DJ . (2002) . A review of avian influenza in different bird species . Veterinary Microbiology , 74 : 3 – 13 .
- Bean , WJ , Kawaoaka , Y , Wood , JM , Pearson , JE and Webster , RG . (1985) . Characterisation of virulent and avirulent A/Chicken/Pennsylvania/83 influenza A viruses: potential role of defective interfering RNA's in Nature . Journal of Virology , 54 : 151 – 160 .
- Capua , I and Marangon , S . (2000) . The avian influenza epidemic in Italy, 1999–2000 . Avian Pathology , 29 : 289 – 294 .
- Capua , I , Terregino , C , Cattoli , G , Mutinelli , F and Rodriguez , JF . (2002) . Development of a DIVA (Differentiating Infected from Vaccinated Animals) strategy using a vaccine containing a heterologous neuraminidase for the control of avian influenza . Avian Pathology , 32 : 47 – 55 .
- Elbers , ARW , Fabri , THF , De Vries , TS , De Wit , JJ , Pijpers , A and Koch , G . (2004a) . The highly pathogenic avian influenza A (H7N7) virus epidemic in the Netherlands in 2003—lessons learned from the first five outbreaks . Avian Diseases , 48 : 691 – 705 .
- Elbers , ARW , Kamps , B and Koch , G . (2004b) . Performance of gross lesions at post-mortem for the detection of outbreaks during the avian influenza A virus (H7N7) epidemic in the Netherlands in 2003 . Avian Pathology , 33 : 418 – 422 .
- Fabri , THF . (2000) . More than 18,000 blood samples examined for antibodies against Avian Influenza (in Dutch) . GD Pluimvee , 16 : 6
- Fouchier , RAM , Olsen , B , Bestebroer , TM , Herfst , S , van der Kamp , L , Rimmelzwaan , GF and Osterhaus , ADME . (2003) . Influenza A virus surveillance in wild birds in Northern Europe in 1999 and 2000 . Avian Diseases , 47 : 857 – 860 .
- Fouchier , RA , Schneeberger , PM , Rozendaal , FW , Broekman , JM , Kemink , SA , Munster , V , Kuiken , T , Rimmelzwaan , GF , Schutten , M , Van Doornum , GJ , Koch , G , Bosman , A , Koopmans , M and Osterhaus , AD . (2004) . Avian Influenza A virus (H7N7) associated with human conjunctivitis and a fatal case of acute respiratory distress syndrome . Proceedings of National Academy of Science USA , 101 : 1356 – 1361 .
- Garcia , M , Crawford , JM , Latimer , JW , Rivera-Cruz , E and Perdue , ML . (1996) . Heterogenecity in the haemagglutinin gene and emergence of the highly pathogenic phenotype among recent H5N2 avian influenza viruses from Mexico . Journal of General Virology , 77 : 1493 – 1504 .
- Kida , H , Yanagawa , R and Matsuoka , Y . (1980) . Duck influenza lacking evidence of disease signs and immune response . Infection and Immunity , 30 : 547 – 553 .
- Lu , BL , Webster , RG and Hinshaw , VS . (1982) . Failure to detect hemagglutination-inhibiting antibodies with intact avian influenza virions . Infection and Immunity , 38 : 530 – 535 .
- Rojas , H , Moreira , R , Avalos , P , Capua , I and Marangon , S . (2002) . Avian Influenza in poultry in Chile . Veterinary Record , 151 : 188
- Siebinga , JT and De Boer , GF . (1988) . Influenza A viral nucleoprotein detection in isolates from human and various animal species . Archives of Virology , 100 : 75 – 87 .
- Swayne DE Halvorson DA (2003) Influenza In Y.M. Saif, H.J. Barnes, A.M. Fadly, J.R. Glisson, L.R. McDougald & D.E. Swayne (Eds.) Diseases of Poultry 11th edn pp. 135–160 Ames Iowa State University Press
- Swayne DE Suarez DL (2000) Highly pathogenic avian influenza Revue Scientificque et Technique (International Office of Epizootics) 19 463 482
- Van Tongeren , HAE and Voous , KH . (1987) . Epidemiology and ecology of influenza virus with special reference to species of birds associated with water . Tijdschrift voor Diergeneeskunde , 112 : 1337 – 1354 .