Abstract
Immunohistochemical and flow cytometric analyses of the bursa, spleen and thymus following infection with the very virulent infectious bursal disease virus (vvIBDV) strain UK661 revealed discrete differences from classical virulent infectious bursal disease virus strains. Bu-1+, immunoglobulin (Ig)M+ and IgG+ cells were all depleted from the bursa, spleen and thymus, suggesting loss of both immature and mature B lymphocytes. Small numbers of Bu-1+ cells repopulated the bursa 14 days post-infection but few of these expressed IgM or IgG. A transient increase in macrophages at 3 to 5 days post-infection was followed by a later influx of CD4+ and CD8+ T cells into the bursa. Loss of cortical thymocytes during the acute phase of infection suggested disruption of the T-cell system. The results showed that vvIBDV strain UK661 caused earlier and more severe pathology than classical virulent strains of infectious bursal disease virus. The marked influx of T cells into the infected bursa indicates that cell-mediated immunity is likely to be important in the clearance of vvIBDV.
Une immunopathologie augmentée induite par un virus hypervirulent de la bursite infectieuse aviaire (vvIBDV)
Les analyses immunohistochimiques et de cytométrie de flux de la bourse de Fabricius, de la rate et du thymus suivant l'infection par la souche UK 661 de vvIBDV ont révélé des différences originales comparées aux souches virulentes classiques d'IBDV. Les cellules Bu-1+, immunoglobulines (Ig)M+ et IgG+ ont toutes disparu de la bourse, de la rate, et du thymus suggérant la perte des lymphocytes B aussi bien matures qu'immatures. Un petit nombre de cellules Bu-1+ ont repeuplé la bourse 14 jours après infection (dpi) mais peu d'entre elles exprimaient des IgM ou des IgG. Une augmentation transitoire des macrophages 3 à 5 dpi a été suivie par un afflux plus tardif de cellules T CD4+ et CD8+ dans la bourse. La perte de thymocytes au niveau du cortex durant la phase aiguë de l'infection a suggéré une perturbation au niveau du système des cellules T. les résultats ont montré que la souche UK 661 de vvIBDV a entraîné une pathologie plus précoce et plus sévère que les souches virulentes classiques d'IBDV. L'afflux important de cellules T dans la bourse infectée indique que l'immunité à médiation cellulaire est probablement importante dans la clairance du vvIBDV.
Verstärkte immunpathologische Veränderungen durch hochvirulentes Virus der infektiösen Bursitis (vvIBDV)
Immunhistochemische und durchflusszytometrische Untersuchungen von Bursa, Milz und Thymus nach einer Infektion mit dem vvIBDV-Stamm UK661 ließen diskrete Unterschiede zu den klassischen virulenten IBDV-Stämmen erkennen. Alle Bu-1+-, Immunglobulin (Ig)M+- und IgG+-Zellen verschwanden aus Bursa, Milz und Thymus, was den Verlust von sowohl der unreifen als auch der reifen B-Lymphozyten vermuten lässt. 14 Tage nach der Infektion (dpi) besiedelte eine geringe Zahl von Bu-1+-Zellen wieder die Bursa, aber nur wenige von diesen Zellen exprimierten IgM oder IgG. Einer vorübergehenden Zunahme von Makrophagen 3–5 dpi folgte später ein Einstömen von CD4+- und CD8+-T-Zellen in die Bursa. Der Verlust der kortikalen Thymozyten während der akuten Phase der Infektion gab einen Hinweis auf eine Störung des T-Zellsystems. Die Ergebnisse zeigten, dass der vvIBDV-Stamm UK661 frühere und schwerwiegendere pathologisch-anatomische Veränderungen verursacht als klassische virulente Stämme des IBDV. Das deutliche Einströmen von T-Zellen in die infizierte Bursa weist daraufhin, dass die zellvermittelte Immunität wahrscheinlich eine wichtige Rolle bei der Clearance des vvIBDV spielt.
Incremento de la inmunopatología causada por virus de bursitis infecciosa muy virulento (vvIBDV)
Los análisis inmunohistoquímicos y de citometría de flujo de la bolsa, bazo y timo tras la infección con una cepa vvIBDV, la UK661, revelaron discretas diferencias entre ésta y las cepas virulentas clásicas de IBDV. Se observó depleción intensa de las células Bu-1+, inmunoglobulina (Ig)M+ e IgG+ de la bolsa , bazo y timo, lo cual sugiere una pérdida tanto de los linfocitos B maduros como inmaduros. Algunas células Bu-1+ repoblaron la bolsa a los 14 días post infección (dpi), pero pocas de ellas expresaron IgM o IgG. Un incremento transitorio de macrófagos a los días 3–5 dpi fue seguido por una llegada de células T CD4+ y CD8+ en la bolsa. La pérdida de timocitos corticales durante la fase aguda de la infección sugirió una alteración del sistema de células T. Los resultados demostraron que la cepa vvIBDV UK661 causó lesiones más tempranas y graves que las cepas virulentas clásicas de IBDV. La entrada marcada de células T en la bolsa infectada indica que la inmunidad celular es probablemente importante en la eliminación de vvIBDV.
Introduction
Infectious bursal disease virus (IBDV), a member of the Birnaviridae, can cause an acute disease (infectious bursal disease [IBD] or Gumboro disease) in young chickens (Lukert & Saif, Citation2003). IBD is characterized by lymphoid pathology, and in chicks older than 3 weeks is characterized by morbidity and in some cases death (Sharma et al., Citation1989; Vervelde & Davison, Citation1997). Chickens that recover are severely immunosuppressed (Allan et al., Citation1972; Faragher et al., Citation1972, Citation1974; Hirai et al., Citation1974; Butter et al., Citation2003) due to the loss of B cells, especially the developing B-cell population in the bursa of Fabricius. Only serotype 1 viruses cause IBD, and pathotypes have been classified in increasing order of virulence as mild, intermediate, classical virulent, very virulent (vv) or hyper-virulent—and, in North America, variant virulent strains (van den Berg, Citation2000). Over the past two decades Europe and Asia have seen the emergence of strains of vvIBDV (Chettle et al., Citation1989; van den Berg et al., Citation1991; Parede et al., Citation2003) that, unlike the classical virulent strains such as F52/70, can break the protection of high titres of maternally-derived antibody and cause high rates of mortality in young birds (Wyeth & Chettle, Citation1990).
It is clearly important to gain an understanding of pathological changes caused by vvIBDV infection and the immune responses evoked. Van den Berg (Citation2000) suggested that vvIBDV causes similar disease signs to those of classical virulent strains with the same incubation time of 4 days but with an exacerbated acute phase. The mechanisms underlying the pathology caused by classical virulent and variant IBDV and of those relating to the immune clearance of the virus have begun to be elucidated. Tanimura & Sharma (Citation1997) and Kim et al. (Citation2000) have suggested that T cells are induced during an IBDV infection in the bursa, but phenotypic characterization is still required.
Rodenberg et al. (Citation1994) studied changes in the T-cell subsets within the blood, bursa, spleen and thymus after infection with classical virulent IBDV and suggested that, although the number of immunoglobulin (Ig)M+ cells in the bursa and spleen decreased significantly, the relative proportions of CD4+ and CD8+ T cells did not change. Immunohistochemical analysis of chicken lymphoid tissues to determine the location of the virus and various leukocyte subsets (Nieper & Muller, Citation1996; Vervelde & Davison, Citation1997) showed an influx of T cells into the bursa. Bursal CD4+ and CD8+ αβ1-TCR+ cells increased (Vervelde & Davison, Citation1997), and some expressed the activation marker CD25 (the interleukin-2 receptor-α) (Kim et al., Citation2000). It still remains a matter of contention whether these cells are a migratory or resident bursal population.
Most studies have been restricted to vaccine or classical virulent strains of IBDV (Rautenshlein et al., Citation2003); therefore the question remains of which cell types influence the outcome of a vvIBDV infection. This paper provides an immunohistochemical and flow cytometric examination of immunopathological changes in the bursa, spleen and thymus caused by UK661, the benchmark strain for vvIBDV (van den Berg, Citation2000), from the time of infection up to 14 days post-infection (d.p.i.).
Materials and methods
Animals and experimental procedure
Rhode Island Red (RIR) chicks were obtained from an unvaccinated flock maintained in isolation accommodation at the Institute for Animal Health (IAH). Parents were confirmed to be free of antibodies to IBDV, chicken infectious anaemia virus, Marek's disease virus, reovirus and a number of other pathogens. The experiments were carried out using colony cages in isolation rooms with the infected birds kept in a separate room from their uninfected counterparts. The experiments met with ethical guidelines of the UK Home Office. Infection of 2-week-old to 3-week-old RIR chickens was achieved by dropping into each of the nares 50 μl phosphate-buffered saline (PBS) (pH 7.6) containing 20 median embryo infective dose (EID50) vvIBDV isolate UK661 (a gift from Dr M. Skinner, IAH, UK). The EID50 of the virus stock was determined by Dr Adriaan van Loon (Intervet BV, The Netherlands) and the dose of virus used to cause clinical IBD in 2-week-old to 3-week-old RIR chicks determined in a preliminary experiment (data not shown).
In the first experiment, 70 2-week-old chicks were infected with UK661 and 10 chicks were sampled every 12 h, from 24 to 96 h post-infection (h.p.i.). The 10 chickens sampled included those exhibiting clinical signs at the time of sampling. Five uninfected control birds were sampled at 0, 48 and 96 h.p.i. In a second experiment, 45 2-week old RIR chicks were infected with UK661 and five birds from the infected group sampled at 1, 2, 3, 4, 5, 6, 7, 10 and 14 d.p.i. as described earlier. Five uninfected control birds were sampled on days 0, 4, 7 and 14. At each timepoint birds were killed by cervical dislocation and the bursa, spleen and thymus removed. A portion of each tissue was frozen for cryosectioning as described by Vervelde & Davison (Citation1997) or fixed in 4% formalin in PBS (pH 7.6) for histopathology. Portions of the bursae were also snap-frozen in liquid nitrogen for later quantitative reverse transcription-polymerase chain reaction (RT-PCR) analysis. Cell suspensions for flow cytometry were obtained from the remaining portions of the tissues.
Histological analysis and pathological scoring system
Earlier scoring systems developed for the study of IBD (Muskett et al., Citation1979; Henry et al., Citation1980) did not take into account the full range of acute pathology caused by vvIBDV strains so the following modification was used for the bursa (Williams, Citation2002): 0, normal architecture; 1, minor lymphocyte depletion from isolated follicles; 2, lymphocyte depletion from most follicles, heterophilia and hyperaemia; 3, cystic cavity formation in most follicles, necrotic foci, vacuolation and frequent pyknotic nuclei; 4, severe lymphocytic depletion from all follicles, hyperaemia and severe heterophilia, disruption of plical membranes; 5, complete loss of bursal architecture, fibroplasia.
A shorter scoring system that incorporated the severity of disease progression was devised for the spleen: 0, normal structure; 1, hyperaemia with heterophilia; 2, severe hyperaemia and heterophilia, minor lymphocyte depletion and pyknotic nuclei; 3, normal architecture lost with heterophilia, oedema and necrotic foci throughout. For the thymus, scoring was as follows: 0, normal; 1, hyperaemia and heterophilia with few necrotic foci in cortex; 2, severe hyperaemia and heterophilia with necrotic foci and frequent pyknotic nuclei; 3, large necrotic foci, numerous pyknotic nuclei and lymphocyte depletion.
Quantitative RT-PCR assay for IBDV RNA in bursal sample
Total RNA was extracted from bursal samples (20 to 60 mg) under liquid nitrogen using a Qiagen RNeasy extraction kit and according to the manufacturer's guidelines (Qiagen, Crawley, UK). UK661-specific RNA was measured by RT-PCR with the TaqMan™ fluorescent real-time quantitative PCR 7700 sequence detector (Applied Biosystems, Warrington, UK). The amount of IBDV RNA was measured as the (40 – CT) value, the number of cycles of the PCR when the fluorescent signal crossed the mean detectable threshold set by the 7700 detector, and compared with the amount of cellular 28s ribosomal RNA. The UK661-specific primer and probe set contained the following sequences from UK661 (Brown & Skinner, Citation1996): forward primer, 5′-ACTCGAGAGCGCCGTCAG-3′; reverse primer, 5′-CTGAGCGCAGATTGGAACAG-3′; and probe, 5′-AGCAGCAGCCAACGTGGACCC-3′ labelled with FAM (reporter dye) and TAMRA (quenching dye). A single 25 μl RT-PCR reaction composed of the following reagents: 6.25 μl H2O, 5 μl EZ Buffer (Applied Biosystems), 3 μl manganese acetate (25 mM), 0.75 μl dATP, dCTP, dGTP, dUTP each (10 mM), 1 μl rTh polymerase (2.5 U/μl), 0.25 μl AmpErase UNG (1 M/μl), 0.5 μl IBDV forward and reverse primer each (10 μM), 0.5 μl IBDV probe (5 μM), and 5 μl RNA.
The quantity of transcripts for the ribosomal 28s protein was measured using the following primer/probe set: 28s RNA forward, 5′-GGCGAAGCCAGAGGAAACT-3′; 28s RNA reverse, 5′-GACGACCGATTTGCACGTC-3′; and 28s RNA probe, 5′-AGGACCGCTACGGACCTCCAC-3′ labelled with FAM as the reporter dye and TAMRA as the quenching dye. The same EZ RT-PCR reagents were used for 28s RNA with the following differences: 4.25 μl H2O, 1.5 μl 28s RNA forward and reverse primers each (4 μM), and 0.5 μl 28s probe (5 μM). All reactions were performed in triplicate. The thermal cycle consisted of: stage 1 as 1 min at 54°C, 30 min at 60°C, 1 min at 95°C; and stage 2 as 30 sec at 94°C and 20 sec at 52°C with 40 cycles. On each reaction plate a standard curve for both IBDV and 28s RNA was incorporated, as were no-template controls for IBDV and 28s RNA. Results were analysed using ABI sequence analysis software (Applied Biosystems) and CT values set accordingly. CT values for both UK661 and 28s were recorded and the total UK661 RNA levels quantified as the UK661:28s ratio.
Immunohistochemical analysis
Preparation of cryosections and immunostaining has been described by Vervelde & Davison (Citation1997) with endogenous peroxidase activity quenched by incubating with 0.1% H2O2 in PBS for 30 min at ambient temperature. Primary monoclonal antibodies recognizing leukocyte antigens and immunoglobulins were as follows: the B-cell marker Bu-1 (AV20; Rothwell et al., Citation1996); CD4 (AV29; Davison, unpublished); CD8α (AV12; Tregaskes et al., Citation1995); chicken IgM (M1; Mockett, Citation1986), chicken IgG (G1; Mockett, Citation1986) and chicken macrophages (KUL-01; Mast & Goddeeris, Citation1998). The VP2 protein of IBDV was identified with the R63 monoclonal antibody (Snyder et al., Citation1988). Monoclonal antibodies were diluted optimally in PBS containing 2% bovine serum albumin and 0.1% sodium azide (PBA).
Flow cytometric analysis
Whole lymphoid tissues (bursa, spleen and thymus) were removed from a chicken post mortem and were immediately placed in ice-cold PBS, disrupted and passed through a 40 μm nylon filter (Becton-Dickenson, New Jersey, USA). The cell suspension was layered over Ficoll-Paque (Pharmacia, Amersham, UK) and centrifuged at 1000×g for 20 min then re-suspended in PBS at 2×107 cells/ml. One million cells were incubated with specific anti-leukocyte monoclonal antibodies. These antibodies were directly conjugated to one of the following fluorochromes: allophycocyanin (APC), fluorocein-isothiocyanate (FITC), phycoerythrin (PE) or peridinin chlorophyll protein (PerCP). Conjugated monoclonal antibodies were purchased from Southern Biotechnology Associates Inc. (Birmingham, Alabama, USA) as: CD3-FITC (CT3 antibody), Bu-1-FITC (AV20 antibody), CD4-PE (CT4 antibody), γδTCR-PE (TCR1 antibody) or in the case of CD8-PerCP conjugated by Pharmingen (San Diego, California, USA) using the AV14 monoclonal antibody (Tregaskes et al., Citation1996). The anti-IgM-APC was prepared using the M1 antibody and APC-conjugation kit (Europa Bioproducts Ltd, Ely, UK). Conjugated antibodies were optimally diluted in PBA accordingly and incubations were for 10 min at ambient temperature. After washing in PBS the cells were washed three times, re-suspended cells in 300 μl PBS and fluorescence analysed using a FACSCaliber flow cytometer (Becton-Dickenson), collecting at least 30 000 events.
Statistical analysis
Data generated from the flow cytometric analysis were analysed using the FCSExpress software (De Novo Software, Ontario, Canada), were subjected to a two-tailed Student's t test assuming unequal variance and the standard error of the mean calculated for each time-point.
Results
vvIBDV causes rapid onset of pathology
vvIBDV UK661 caused severe disease, and chicks reached clinical endpoints in a relatively short time. Clinical signs (ruffled feathers, hunched posture, lethargy) first became evident at 48 h.p.i. and were most severe between 56 and 72 h.p.i. (data not shown). Based on the lesion score, it was clear that bursal pathology developed rapidly, with a complete loss of tissue architecture by 96 h.p.i. (). In a similar manner, the pathology observed in the spleen and the thymus also progressed rapidly, with considerable damage to both tissues (). The quantity of viral RNA in bursal samples obtained immediately post mortem was compared with the bursal lesion score (). Most viral RNA was detected in bursae with damage corresponding to the median lesion score (+3). This degree of bursal damage usually preceded the onset of the most severe pathology (). The level of viral RNA decreased as the lesion score increased above +3. In the second experiment the levels of the IBDV VP2 antigen in the bursa were investigated using immunohistochemistry over a more extended time. VP2 was first detected in the bursa 2 to 3 d.p.i. (), and was maximal at 5 d.p.i. (). The amount of detectable IBDV decreased thereafter, even though the bursal pathology continued to progress ().
Figure 1. Development of bursal, splenic and thymic pathology caused by IBDV UK661, assessed using the lesion scoring method developed for vvIBDV infection and described in Materials and Methods. Values expressed as the mean±standard error of the mean, n=10.
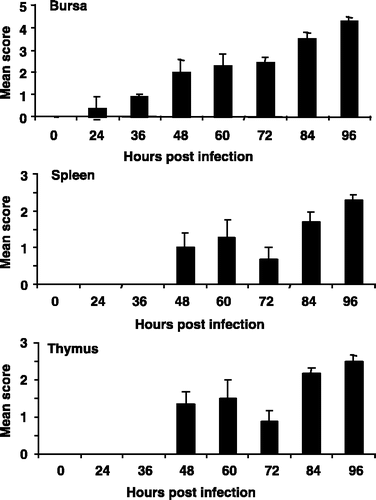
Figure 2. 2A: Relative amount of UK661-specific RNA measured by quantitative RT-PCR (Taqman™) and expressed as the 40–CT value, after adjustment for the 28s ribosomal RNA. The value was related to bursal lesion score. 2B: The VP2 protein of UK661 was first detected at 3 d.p.i. (×100), within the medulla (arrow) of bursal follicles. 2C: The greatest amount of VP2 was detected 5 d.p.i. throughout the entire bursa (×100). 2D: At 14 d.p.i. little VP2 could be detected and productive virus was assumed to be negligible (×400). Values expressed as the mean±standard error of the mean, n=10.
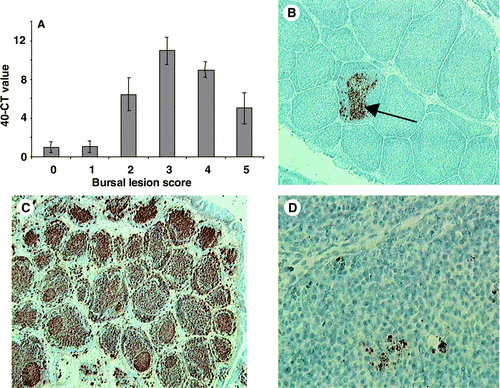
Immunohistochemistry was also employed to detect the VP2 antigen in the spleen and thymus. Although VP2 was detected in these tissues, its presence was more transient than in the bursa and fewer positive cells were detected (data not shown). In the spleen VP2 was first detected 4 d.p.i., was most abundant at 5 d.p.i. but could not be detected by 7 d.p.i.. VP2 became detectable in the thymus at 4 d.p.i., was most frequent at 7 d.p.i. but not at 10 d.p.i. or thereafter.
vvIBDV induces chronic depletion of bursal B cells
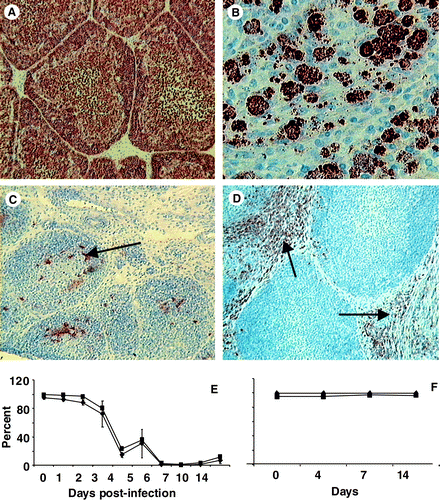
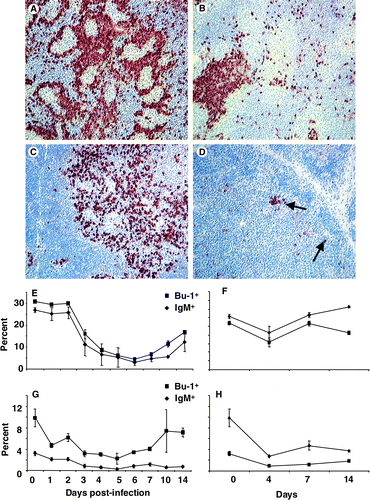
Changes in various lymphoid cells were analysed, both spatially and temporally, over the 2 weeks after infection using both immunohistochemistry and flow cytometry. Bu-1+ cells were rapidly depleted from both the medulla and the cortex of bursal follicles () and had not returned to normal numbers, even by 14 d.p.i. Spherical bodies were often seen in the bursal follicles at 6 to 7 d.p.i. (). Furthermore, bursal populations of both the IgM+ and IgG+ cells were completely depleted and had not recovered after 14 days, suggesting long-term disruption of the B-cell system (data not shown). The macrophage (KUL-01+) population transiently increased between 3 and 5 d.p.i. (). Flow cytometry data confirmed that Bu-1+ and IgM+ populations significantly (P<0.05) decreased between 3 and 4 d.p.i., and remained low in number up to 14 d.p.i. ().
Figure 3. 3A: Uninfected bursa containing Bu-1+ cells densely packed in the cortex and more dispersed in the medulla (×200). 3B: Spherical bodies were often seen in bursal follicles 6 to 7 d.p.i. 3C: Very few Bu-1+ cells (arrow) remained in both the medulla and cortex at 10 d.p.i. (×200). 3D: The KUL0-1+ macrophage (arrow) subset transiently increased, peaking at 4 d.p.i. (×400). 3E: Flow cytometry analysis of bursal lymphocytes from IBDV-infected chicks showing the rapid and significant (P<0.05) decrease in both Bu-1+ (▪) and IgM+ (♦) populations. These cell populations were not recovered by 14 d.p.i. 3F: Bu-1+ (▪) and IgM+ (♦) populations in bursal cells from uninfected bursa. All values expressed as the mean±standard error of the mean, n=5.
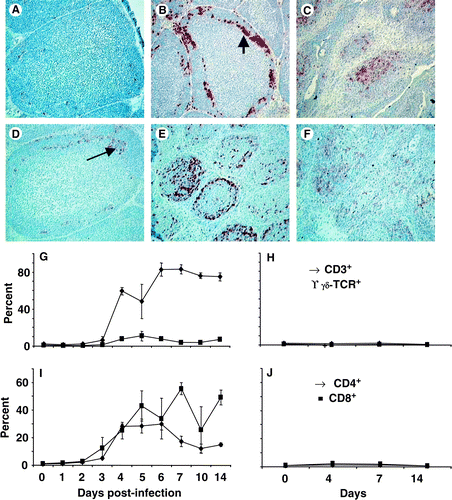
CD4+ and CD8+ T cells increase throughout the bursa
CD4+ and CD8+ T-cell populations were analysed to elucidate the lymphoid cells involved in bursal pathology, viral clearance and/or immunosuppression. Neither CD4+ () nor CD8+ (data not shown) cell populations were detected in significant numbers in uninfected bursae. Neither the proportion nor number of CD4+ and CD8+ cells altered in uninfected control birds (see ). CD4+ cells were obvious at the cortico-medullary boundary by 3 d.p.i. () and were found throughout the bursa until at least 14 days post infection (). CD8+ cells were most obvious at 6 d.p.i. (), throughout entire bursal follicles, and remained within the bursa up to 14 d.p.i. (). Flow cytometry analysis revealed that the bursal CD3+ T-cell population significantly (P<0.05) increased from 3 to 4 d.p.i. () and remained increased until 14 d.p.i.. The γδTCR+ T-cell population showed a slight increase (P<0.05) only at 5 d.p.i. (). Both the proportion of CD4+ and CD8+ cells increased from 3 to 4 d.p.i. (P<0.05) in the bursa (). The proportion of CD4+ cells had decreased by 7 d.p.i. (P<0.05), although it remained higher than in the uninfected bursa (), while the proportion of CD8+ cells remained high ().
Figure 4. 4A: Few CD4+ cells were detected in the bursa at 0 d.p.i. (×200). 4B: The first CD4+ cells were detected 3 d.p.i., mostly at the cortico-medullary boundary (arrow) and in the cortex (×200). 4C: The number of CD4+ cells remained higher than normal until 14 d.p.i. (×100). 4D: Few CD8+ cells (arrow) detected at the cortico-medullary boundary at 3 d.p.i. (×200). 4E: By 6 d.p.i. the number of CD8+ cells increased and could be found throughout the cortex and medulla of follicles (×100). 4F: Many CD8+ cells remained in the bursa at 14 d.p.i. (x100). 4G: Flow cytometry data indicate an increase in the proportions (○) CD3+ T cells (♦) in the infected burs and a transient increase (P<0.05) in γδ TCR+ T cells (▪) at 5 d.p.i. 4H: Proportions of CD3+ (♦) and γδTCR+ T (▪) cells in uninfected. 4I: The proportion of CD4+ (▴) and CD8+ (•) cells in the bursa increased significantly (P<0.05) by 4 d.p.i. 4J: The proportion CD4+ (▴) and CD8+ (•) T cells in uninfected bursae. All values expressed as the mean±standard error of the mean, n=5.
B cells are severely depleted in the spleen and thymus. Although not as severely affected as the bursa, both the spleen () and the thymus () exhibited depletion of Bu-1+ cells. Over the course of the experiment the number and proportion of Bu-1+ cells showed some variations in both the spleen or thymus in uninfected control chicks, with a significant (P<0.05) decrease in the proportion of Bu-1+ and IgM+ cells in the infected spleen 2 to 4 d.p.i. (), which began to recover by 10 d.p.i. although remaining markedly lower than in uninfected controls. The proportion of Bu-1+ cells in the infected thymus decreased between 0 and 3 d.p.i. () but this appeared to be matched by a similar significant (P<0.05) decrease in the uninfected control chicks (), and there was evidence of recovery with a significant (P<0.05) increase in Bu-1+ cells in the recovering chicks at 14 d.p.i. The proportion of IgM+ cells in infected chicks was significantly (P<0.05) lower than in the uninfected thymus (). These data suggest that the B-cell system is detrimentally affected in lymphoid tissues apart from the bursa.
Figure 5. 5A: B cells were frequent around the peri-ellipsoid sheath in an uninfected spleen with few germinal centres (×100). 5B:By 14 d.p.i. few Bu-1+ cells were present in the spleen with small aggregates resembling germinal centres (×200). 5C: In an uninfected thymus, Bu-1+ cells were mainly in the medullary region and infrequent in the cortex (×200). 5D: By 14 d.p.i. few Bu-1+ cells (arrow) were present in the thymus (×200). 5E: Flow cytometric analysis of spleen cells demonstrated the significant (P<0.05) decrease 2 to 4 d.p.i. in both Bu-1+ (▪) and IgM+ (♦) cell populations, which only partially began to increase by 14 d.p.i. 5F: Values for splenocytes from uninfected chicks, Bu-1+ (▪) and IgM+ (♦) cell populations. 5G: Flow cytometric analysis of thymus cells showing a transient decrease (P<0.05) in Bu-1+ (▪) 2 to 7 d.p.i. and a partial recovery to pre-infection levels by 14 d.p.i. The IgM+ (♦) population in the thymus is relatively low in uninfected chicks, and decreased further by 14 d.p.i. 5H: Values from uninfected, Bu-1+ (▪) and IgM+ (♦) cell populations. Values expressed as the mean±standard error of the mean, n=5.
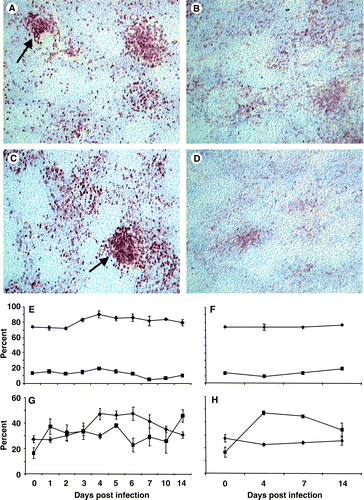
vvIBDV infection alters the spleen and thymus T-cell compartments
The CD4+ cell population appeared to increase in the spleen () while the CD8+ population appeared to decrease after infection (). Flow cytometry suggested that these changes were only transient with both the CD3+ and γδTCR+ T-cell populations showing little change (). The change in the CD4+ population in the infected spleen was transiently increased at 4 and 7 d.p.i. compared with the population in uninfected chicks (). The CD8+ population in the infected spleen was significantly (P<0.05) lower at 4 and 7 d.p.i., but had recovered and then increased above the number in uninfected spleens at 14 d.p.i. ().
Figure 6. 6A: Scattered CD4+ cells throughout the uninfected spleen forming aggregations (arrow) around the vasculature (×100). 6B: After infection with UK661 few spatial changes occurred, although by 14 d.p.i. the CD4+ population was larger than in uninfected spleens (×100). 6C: The CD8+ population in an uninfected spleen was mostly aggregated in the T-cell zones (arrow) around blood vessels (×100). 6D: The number of CD8+ cells decreased slightly after infection, and by 14 d.p.i. less dense aggregations were detected (×100). 6E: Flow cytometry analysis revealed little change in either the CD3+ (♦) or γδ TCR+ T-cell (▪) populations. 6F: Proportions of CD3+ (♦) and γδ TCR+ T cell (▪) cells in the spleens of uninfected chicks. 6G: Proportion of CD4+ (▴) cells transiently increased and was maximal (P<0.05) at 4 to 6 d.p.i., while the proportion of CD8+ (•) cells increased slightly and was greatest at 14 d.p.i. 6H: The proportion of CD4+ (▴) and CD8+ (•) cells in uninfected spleens. Values expressed as the mean±standard error of the mean, n=5.
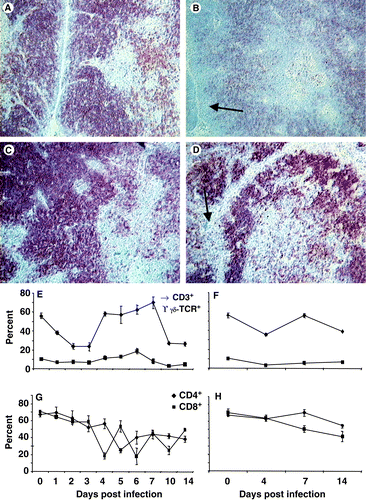
The T-cell population within the thymus was markedly affected as both the CD4+ and CD8+ populations decreased, particularly within the cortex where the more immature T cells reside (). Although the CD4+ population remained spatially abnormal, the CD8+ cell population had returned to normal by 14 d.p.i. (data not shown). Flow cytometry analysis revealed a significant (P<0.05) decrease in the proportion of CD3+ T cells by 4 d.p.i., with a rebound at 5 d.p.i. but a decrease again at 10 and 14 d.p.i. (). The γδTCR+ population was not severely affected (). The proportion of both CD4+ and CD8+ cells in the infected thymus showed marked variations from day to day (). Both populations decreased over time () and following a similar decline in the same populations in the uninfected thymus (). However, at 4, 7 and 10 d.p.i. there were significantly (P<0.05) fewer CD4+ cells in the infected than the uninfected thymus.
Figure 7. 7A: In a normal thymus, CD4+ thymocytes are densely packed in the cortex and dispersed throughout the medulla (×200). 7B: By 14 d.p.i. no CD4+ cells were observed at the outer edge of the cortex (arrow) and fewer were observed in the medulla (×200). 7C: In a normal thymus, CD8+ thymocytes existed as densely packed cells in the cortex and were more dispersed in the medulla (×200). 7D: By 7 d.p.i. CD8+ cells were depleted from the cortex (arrow) (×200). 7E: Flow cytometry data indicated that CD3+ T cells (♦) are affected in a bi-phasic manner, initially by transiently decreasing (P<0.05), recovering to pre-infection levels and then deceasing 10 d.p.i. (P<0.05). The γδTCR+ T cell (▪) population transiently increases (P<0.05) at 6 d.p.i. 7F: CD3+ (♦) and γδTCR+ T-cell (▪) population in uninfected thymus. 7G: Both the CD4+ (▴) and CD8+ (•) populations declined gradually over time so as to be proportionally lower after infection than before (P<0.05). 7H: CD4+ (▴) and CD8+ (•) populations in uninfected thymus. Values expressed as the mean±standard error of the mean, n=5.
Discussion
Van den Berg (Citation2000) suggested that vvIBDV produces similar disease signs to those caused by classical virulent IBDV, with a similar incubation period of 4 days but with an exacerbated acute phase. In the present study, young chicks lacking maternal antibodies, infected with strain UK661, developed clinical signs of IBD within 48 h.p.i. and severe signs of IBD between 56 and 72 h.p.i. The disparity between the two sets of observations could be due to the presence of maternal antibodies in commercial poultry and/or differences in the amount of challenge virus. The rapidity and severity of the pathogenesis caused by UK661, particularly in the bursa, was considerable compared with earlier studies using similar infecting doses of the classical virulent strain F52/70 (Vervelde & Davison, Citation1997; Shaw & Davison, Citation2000). Not only was severe bursal pathology present earlier with UK661, but its severity greatly exceeded that of less virulent strains. Likewise, the spleen and thymus pathology progressed much farther than with classical virulent strains (Tanimura et al., Citation1995).
Analysis of viral RNA, although not a precise measure of infective virus, provided a measure of the amount of bursal virus present. The highest level of UK661 RNA corresponded with the median lesion score and not with the most damaged bursae. This is probably because follicles with moderate damage contain larger numbers of IBDV-infected B cells than those severely depleted of B lymphocytes. The amount of detectable VP2 decreased as the pathology progressed, suggesting that pathological mechanisms independent of direct viral-lysis must contribute to pathogenesis. Similar immunopathologies have been attributed to cell-mediated killing and an increase in type I interferons (Biron, Citation1999; Barry & Bleackley, Citation2002).
UK661 VP2 antigen was most prevalent in the bursa, followed by the spleen and then the thymus, as reported for classical virus strains (Tanimura et al., Citation1995; Vervelde & Davison, Citation1997). However, the distribution of VP2 antigen within the bursa was different from that with less virulent IBDV. VP2 antigen was first detected at the cortico-medullary boundary, which probably corresponds to the site of viral entry. UK661 is probably carried to the bursa by migratory cells (e.g. macrophages) from the site of primary replication (intestine), and once in the medulla is able to colonize individual follicles (Ivanyi & Morris, Citation1976). It is widely accepted that IBDV primarily targets immature B cells located in the bursal cortex. However, UK661 VP2 antigen was mostly present in the medulla, a site where more mature B cells are found (Paramithiotis & Ratcliffe, Citation1996); only later was UK661 disseminated into the cortex.
The rapid depletion of virus from the bursa was associated with distinctive spherical bodies accumulating in the medulla () that resembled highly phagocytosing, activated macrophages (von Bulow et al., Citation1986; Mast et al., Citation1998). Macrophages appeared to play a major role in the clearance of dead (apoptotic and necrotic) cells and cell debris. A large accumulation of cells expressing the macrophage marker KUL-01 () and major histocompatability complex class II invariant chain (data not shown) was present around 3 to 4 d.p.i. This is consistent with the transient elevation of a number of inflammatory cytokines reported to occur during the acute phase of IBDV infection (Kim et al., Citation1998). The contemporaneous decrease in virus could partly be related to the action of phagocytosing macrophages, although also to the exhaustion of target cells.
Depletion of medullary Bu-1+, IgM+ and IgG+ cells, prior to their depletion from the cortex, and depletion of IgM+ cells before IgG+ cells, is consistent with the suggestion that more immature B cells are preferred targets, although it is important to note that both populations became depleted. This will have profound consequences both for the immune responses against UK661 itself and the consequent immunosuppression after viral clearance. The extended range of target lymphocytes could partly explain the increased virulence of vvIBDV isolates.
After destruction of the bursal architecture by UK661, Bu-1+ B cells began to return to the depleted bursal follicles by 14 d.p.i. However, very few cells with surface IgM+ or IgG+ were detected with the monoclonal antibodies M1 and G1 (). Kim et al. (Citation1999) also reported some bursal recovery of the IgM+ cell population at 14 d.p.i. with classical virulent IBDV. In the present study it was clear that the number of IgM+ cells were fewer than the Bu-1+ B cells (). One explanation is that the Bu-1 antigen is being expressed by a subpopulation of macrophages (Houssaint, Citation1987), but we were unable to detect such a population using the KUL-01 antibody. Another explanation is that IBDV-induced conformational changes in the Ig molecules result in alterations in those epitopes recognized by the M1 and G1 monoclonal antibodies. IBDV-induced changes in chicken IgM giving rise to a monomeric form in the circulation of convalescent chickens have been reported Ivanyi & Morris (Citation1976). It was recently reported (Pike et al., Citation2004) that only B cells expressing a surface immunoglobulin are able to colonize bursal follicles, whereas those B cells with a non-productive V-D-J recombination lacking Ig expression are deleted. If this is the case, it would suggest that Bu-1+ cells repopulating the bursa are likely to be non-functional and subsequently deleted. The lack of expression of surface Ig on bursal B cells is consistent with the convalescent chick being severely immunocompromised (Ivanyi & Morris, Citation1976). Lack of Ig-producing B cells would explain the poor antibody response to secondary antigenic challenge in chickens recovering from IBDV infection. The situation after vvIBDV infection seems likely to be far more severe than with less virulent strains (Higashihara et al., Citation1991; Butter et al., Citation2003).
The number of CD4+ and CD8+ T cells increased after infection and were first detected at the cortico-medullary boundary, probably reflecting their route of entry (Vervelde & Davison, Citation1997). The cortico-medullary boundary is rich in arterioles and venules, which allow migratory lymphocytes entry into the follicles in response to an appropriate chemo-attractant(s). Although CD4+ T cells had increased by 7 d.p.i., their location was restricted to the cortico-medullary boundary and the cortex, even though most IBDV+ cells were present in the medulla. Later, CD8+ T cells were detected throughout the follicles. The presence of numerous T cells suggests that cell-mediated immunity has a very important role to play in recovery from an infection with UK661. Poonia & Charan (Citation2001) reported consistently higher titres of IBDV in chickens lacking a fully functional T-cell-mediated immune system. This increase in bursa T cells appears to be a consequence of migration and not an artefact due to the loss of B cells or the result of an expansion of resident T cells (Vervelde & Davison, Citation1997).
An important aspect of the pathogenesis and clinical disease caused by vvIBDV is the involvement of other lymphoid organs (Inoue et al., Citation1994). The extensive damage in the thymus caused by UK661 was far greater than that reported for classical virulent stains (Tanimura et al., Citation1995). At the height of thymic pathogenesis much of the cortex contained pyknotic nuclei within large vacuolated spaces, many of which merged. This suggests either that vvIBDV has a direct apoptotic or necrotic effect on thymocytes (Jungmann et al., Citation2001) or that immunopathology is acting through bystander activity (Welsh et al., Citation2000).
Pathology in the spleen was less evident and the transient nature of detectable virus (data not shown) may be related to lymphocyte and macrophage populations restricting the spread of the IBDV. The spleen is the major secondary lymphoid organ in the chicken where most systemic T-cell and B-cell responses are initiated. The B-lymphocyte population was most severely affected, suggesting that peripheral B cells are highly susceptible to vvIBDV. This further supports the notion that immature B cells are not the only targets for vvIBDV, as both IgM+ and IgG+ B cells decreased.
The IgM+ cell population recovered in the spleen, reaching a higher number than before infection, in association with germinal centres. This is most probably a consequence of expansion of the peripheral B-cell pool and not due to seeding from the bursa. The IgG+ population also recovered and was associated with germinal centres, suggesting isotype switching of the IBDV-reactive IgM+ population. The lack of bursal architecture (and probably function) and the increase in splenic IgM+ and IgG+ cells is consistent with the B-cell compartment being severely suppressed, while at the same time IBDV-reactive B cells are present (Becht & Muller, Citation1991; Ramm et al., Citation1991). The T-cell compartment within the spleen seemed to be unaffected after infection.
In the thymus, IBDV antigen was only transiently detected at 4 to 7 d.p.i. (data not shown). The rapid depletion of Bu-1+ medullary B cells probably follows the loss of viral target cells and restricted viral distribution. Few, if any, Bu-1+, IgM+ or IgG+ cells were detected in the thymic cortex even though VP2 antigen was detected there. The presence of IBDV antigen on the surface of numerous, adjacent cortical thymocytes suggests that IBDV is able to adhere to immature T cells. However, it seems unlikely that IBDV directly infects and replicates within these cells as Hirai & Calnek (Citation1979) were unable to show replication of IBDV in chicken T cells. The proportion of CD4+ and CD8+ T cells in the thymus altered little following infection.
Clear differences between classical virulent IBDV strains (e.g. F52/70) and the vvIBDV strain UK661 are highlighted in this report. Further work is underway to determine activities of T cells by measuring the expression of cytokine transcripts during the course of infection. Such information should provide clues to the immune responses taking place at the sites of infection and viral replication, so improving understanding of protective immunity.
This work was supported by the British Egg Marketing Board Research and Education Trust, who supported A.E.W., and the Biotechnology and Biological Science Research Council. The KUL-01 monoclonal antibody was a gift from Prof. Bruno Goddeeris (University of Leuven, Belgium) and the R63 antibody from Dr D. Lutticken (Intervet BV, The Netherlands). The authors are grateful to Dr Adriaan van Loon (Intervet BV, The Netherlands) for determining the EID50 of the IBDV UK661 stock and to Sue Hacker and Helen Mason (IAH) for their histological expertise.
Additional information
Notes on contributors
A. E. Williams Footnote†
Present address: The Kennedy Institute of Rheumatology division, ARC Building, Charing Cross Campus,Imperial College London, 1 Aspenlea Road, London W6 8LH, UK.Notes
Present address: The Kennedy Institute of Rheumatology division, ARC Building, Charing Cross Campus,Imperial College London, 1 Aspenlea Road, London W6 8LH, UK.
References
- Allan , WH , Faragher , JT and Cullen , GA . (1972) . Immunosuppression by the infectious bursal agent in chickens immunized against Newcastle disease . Veterinary Record , 90 : 511 – 512 .
- Barry , M and Bleackley , RC . (2002) . Cytotoxic T lymphocytes: all roads lead to death . Nature Review in Immunology , 2 : 401 – 409 .
- Becht , H and Muller , H . (1991) . Infectious bursal disease-B cell dependent immunodeficiency syndrome in chickens . Behring Institute Mitteilungen , 89 : 217 – 225 .
- Biron , CA . (1999) . Initial and innate responses to viral infections—pattern setting in immunity or disease . Current Opinions in Microbiology , 2 : 374 – 381 .
- Brown , MD and Skinner , MA . (1996) . Coding sequences of both genome segments of a European 'very virulunt' infectious bursal disease virus . Virus Research , 40 : 1 – 15 .
- Butter , CD , Sturman , TD , Baaten , BJ and Davison , TF . (2003) . Protection from infectious bursal disease virus (IBDV)-induced immunosuppression by immunization with a fowlpox recombinant containing IBDV-VP2 . Avian Pathology , 32 : 597 – 604 .
- Chettle , NJ , Stuart , JC and Wyeth , PJ . (1989) . Outbreaks of virulent infectious bursal disease in East Anglia . Veterinary Record , 125 : 271 – 272 .
- Faragher , JT , Allan , WH and Cullen , GA . (1972) . Immunosuppressive effect of the infectious bursal agent in the chicken . Nature New Biology , 237 : 118 – 119 .
- Faragher , JT , Allan , WH and Wyeth , PJ . (1974) . Immunosuppressive effect of infectious bursal agent on vaccination against Newcastle disease . Veterinary Record , 95 : 385 – 388 .
- Henry , CW , Brewer , RN and Edgar , SA . (1980) . Studies on infectious bursal disease in chickens. 2. Scoring microcopic lesions in the bursa of Fabricius, thymus, spleen, and kidney in gnotobiotic and battery reared white leghorns experimentally infected with infectious bursal disease virus . Poultry Science , 59 : 1006 – 1017 .
- Higashihara , M , Saijo , K , Fujisaki , Y and Matumoto , M . (1991) . Immunosuppressive effect of infectious bursal disease virus strains of variable virulence for chickens . Veterinary Microbiology , 26 : 241 – 248 .
- Hirai , K and Calnek , BW . (1979) . In vitro replication of infectious bursal disease virus in established lymphoid cell lines and chicken B lymphocytes . Infection and Immunity , 25 : 964 – 970 .
- Hirai , K , Shimakura , S , Kawamoto , E , Taguchi , F , Kim , ST , Chang , CM and Iritani , Y . (1974) . The immunodepressive effect of infectious bursal disease virus in chickens . Avian Diseases , 18 : 50 – 57 .
- Houssaint , E . (1987) . Cell lineage segregation during bursa of Fabricius ontogeny . Journal of Immunology , 138 : 3626 – 3634 .
- Inoue , M , Fukuda , M and Miyano , K . (1994) . Thymic lesions in chicken infected with infectious bursal disease virus . Avian Diseases , 38 : 839 – 846 .
- Ivanyi , J and Morris , R . (1976) . Immunodeficiency in the chicken. IV. An immunological study of infectious bursal disease . Clinical and Experimental Immunology , 23 : 154 – 165 .
- Jungmann , A , Nieper , H and Muller , H . (2001) . Apoptosis is induced by infectious bursal disease virus replication in productively infected cells as well as in antigen-negative cells in their vicinity . Journal of General Virology , 82 : 1107 – 1115 .
- Kim , IJ , Karaca , K , Pertile , TL , Erickson , SA and Sharma , JM . (1998) . Enhanced expression of cytokine genes in spleen macrophages during acute infection with infectious bursal disease virus in chickens . Veterinary Immunology and Immunopathology , 61 : 331 – 341 .
- Kim , IJ , Gagic , M and Sharma , JM . (1999) . Recovery of antibody-producing ability and lymphocyte repopulation of bursal follicles in chickens exposed to infectious bursal disease virus . Avian Diseases , 43 : 401 – 413 .
- Kim , IJ , You , SK , Kim , H , Yeh , HY and Sharma , JM . (2000) . Characteristics of bursal T lymphocytes induced by infectious bursal disease virus . Journal of Virology , 74 : 8884 – 8892 .
- Lukert PD Saif YM (2003) Infectious bursal disease In Y.M. Saif (Ed.) Diseases of Poultry 11th edn (pp. 161–179) Ames Iowa State Press
- Mast , J and Goddeeris , BM . (1998) . CD57, a marker for B-cell activation and splenic ellipsoid-associated reticular cells of the chicken . Cell and Tissue Research , 291 : 107 – 115 .
- Mast , J , Goddeeris , BM , Peeters , K , Vandesande , F and Berghman , LR . (1998) . Characterisation of chicken monocytes, macrophages and interdigitating cells by the monoclonal antibody KUL01 . Veterinary Immunology and Immunopathology , 61 : 343 – 357 .
- Mockett , APA . (1986) . Monoclonal antibodies used to isolate IgM from cjicken bile and avian sera and to detect specific IgM in chicken sera . Avian Pathology , 15 : 337 – 348 .
- Muskett , JC , Hopkins , IG , Edwards , KR and Thornton , DH . (1979) . Comparison of two infectious bursal disease vaccine strains: efficacy and potential hazards in susceptible and maternally immune birds . Veterinary Record , 104 : 332 – 334 .
- Nieper , H and Muller , H . (1996) . Susceptibility of chicken lymphoid cells to infectious bursal disease virus does not correlate with the presence of specific binding sites . Journal of General Virology , 77 : 1229 – 1237 .
- Paramithiotis , E and Ratcliffe , MJ . (1996) . Evidence for phenotypic heterogeneity among B cells emigrating from the bursa of Fabricius: a reflection of functional diversity? . Current Topics in Microbiology and Immunology , 212 : 29 – 36 .
- Parede , LH , Sapats , S , Gould , G , Rudd , M , Lowther , S and Ignjatovic , J . (2003) . Characterization of infectious bursal disease virus isolates from Indonesia indicates the existence of very virulent strains with unique genetic changes . Avian Pathology , 32 : 511 – 518 .
- Pike , KA , Baig , E and Ratcliffe , MJ . (2004) . The avian B-cell receptor complex: distinct roles of Ig alpha and Ig beta in B-cell development . Immunological Reviews , 197 : 10 – 25 .
- Poonia , B and Charan , S . (2001) . T cell suppression by cyclosporin-A enhances infectious bursal disease virus infection in experimentally infected chickens . Avian Pathology , 30 : 311 – 319 .
- Ramm , HC , Wilson , TJ , Boyd , RL , Ward , HA , Mitrangas , K and Fahey , KJ . (1991) . The effect of infectious bursal disease virus on B lymphocytes and bursal stromal components in specific pathogen-free (SPF) White Leghorn chickens . Developmental and Comparative Immunology , 15 : 369 – 381 .
- Rautenschlein , S , Yeh , H-Y and Sharma , JM . (2003) . Comparative immunopathogenesis of mild, intermediate and virulent strains of classic infectious bursal disease virus . Avian Diseases , 47 : 66 – 78 .
- Rodenberg , J , Sharma , JM , Belzer , SW , Nordgren , RM and Naqi , S . (1994) . Flow cytometric analysis of B cell and T cell subpopulations in specific-pathogen-free chickens infected with infectious bursal disease virus . Avian Diseases , 38 : 16 – 21 .
- Rothwell , CJ , Vervelde , L and Davison , TF . (1996) . Identification of chicken Bu-1 alloantigens using the monoclonal antibody AV20 . Veterinary Immunology and Immunopathology , 55 : 225 – 234 .
- Sharma , JM , Dohms , JE and Metz , AL . (1989) . Comparative pathogenesis of serotype 1 and variant serotype 1 isolates of infectious bursal disease virus and their effect on humoral and cellular immune competence of specific-pathogen-free chickens . Avian Diseases , 33 : 112 – 124 .
- Shaw , I and Davison , TF . (2000) . Protection from IBDV-induced bursal damage by a recombinant fowlpox vaccine, fpIBD1, is dependent on the titre of challenge virus and chicken genotype . Vaccine , 18 : 3230 – 3241 .
- Snyder , DB , Lana , DP , Cho , BR and Marquardt , WW . (1988) . Group and strain-specific neutralization sites of infectious bursal disease virus defined with monoclonal antibodies . Avian Diseases , 32 : 527 – 534 .
- Tanimura , N and Sharma , JM . (1997) . Appearance of T cells in the bursa of Fabricius and cecal tonsils during the acute phase of infectious bursal disease virus infection in chickens . Avian Diseases , 41 : 638 – 645 .
- Tanimura , N , Tsukamoto , K , Nakamura , K , Narita , M and Maeda , M . (1995) . Association between pathogenicity of infectious bursal disease virus and viral antigen distribution detected by immunohistochemistry . Avian Diseases , 39 : 9 – 20 .
- Tregaskes , CA , Kong , FK , Paramithiotis , E , Chen , CL , Ratcliffe , MJ , Davison , TF and Young , JR . (1995) . Identification and analysis of the expression of CD8 αβ and CD8 αα isoforms in chickens reveals a major TCR- CD8 γδ subset of intestinal intraepithelial lymphocytes . Journal of Immunology , 154 : 4485 – 4494 .
- Tregaskes , CA , Bumstead , N , Davison , TF and Young , JR . (1996) . Chicken B-cell marker chB6 (Bu-1) is a highly glycosylated protein of unique structure . Immunogenetics , 44 : 212 – 217 .
- van den Berg , TP . (2000) . Acute infectious bursal disease in poultry: a review . Avian Pathology , 29 : 175 – 194 .
- van den Berg , TP , Gonze , M and Meulemans , G . (1991) . Acute infectious bursal disease in poultry: isolation and characterization of a highly virulent strain . Avian Pathology , 20 : 409 – 421 .
- Vervelde , L and Davison , TF . (1997) . Comparison of the in situ changes in lymphoid cells during infection with infectious bursal disease virus in chickens of different ages . Avian Pathology , 26 : 803 – 821 .
- von Bulow , V , Rudolph , R and Fuchs , B . (1986) . Enhanced pathenogicity of chicken anemia agent (CAA) in dual infections with Marek's disease virus (MDV), infectious bursal disease virus (IBDV) or reticuloendotheliosis virus (REV) . Zentralblatt für Veterinärmedezin [B] , 33 : 93 – 116 .
- Welsh , RM , McNally , JM , Brehm , MA and Selin , LK . (2000) . Consequences of cross-reactive and bystander CTL responses during viral infections . Virology , 270 : 4 – 8 .
- Williams AE (2002) The pathogenesis of very virulent infectious bursal disease and its modulation by DNA vaccination Ph.D. thesis University of London
- Wyeth , PJ and Chettle , NJ . (1990) . Use of infectious bursal disease vaccines in chicks with maternally derived antibodies . Veterinary Record , 126 : 577 – 578 .