Abstract
A method for detecting antibody against leucocytozoonosis in chickens by latex agglutination (LA) was developed using latex beads coated with recombinant R7 (rR7), an outer membrane antigen of second-generation schizonts of Leucocytozoon caulleryi. Compared with the agar gel precipitation test, which is widely used in Japan, LA could detect antibody induced by L. caulleryi infection with greater sensitivity. No agglutination was detected with sera from specific pathogen free or layer chickens that had not been vaccinated with oil-adjuvanted rR7 antigen, or infected with L. caulleryi, nor with sera from chickens inoculated with other pathogens, establishing the specificity of the assay. The LA test was also able to be used to quantify serum antibody induced by vaccination, which is not possible with the agar gel precipitation test. This study has shown that LA using the rR7 antigen is a simple, quick, and useful method for detecting antibody against L. caulleryi.
Méthode rapide de détection des anticorps de la leucocytozoonose chez le poulet à l'aide d'un test d'agglutination au latex utilisant l'antigène recombinant R7
Une méthode rapide de détection des anticorps de la leucocytozoonose chez le poulet, à l'aide d'un test d'agglutination au latex (LA), a été développée en utilisant des billes de latex tapissées avec le recombinant R7 (rR7), un antigène de membrane externe de schizontes de seconde génération de Leucocytozoon caulleryi. Par comparaison au test de précipitation en milieu gélosé (AGP) qui est largement utilisé au Japon, le LA peut détecter les anticorps induits par une infection à L. caulleryi avec une sensibilité supérieure. Aucune agglutination n'a été détectée avec des sérums de poulets exempts d'organismes pathogènes spécifiés ou de poulettes qui n'ont pas été vaccinées avec l'antigène rR7 adjuvé huileux, ou infectées par L. caulleryi, ni avec des sérums de poulets inoculés avec d'autres agents pathogènes, mettant en évidence la spécificité de la méthode. Le test LA peut également être utilisé pour quantifier les anticorps sériques induits par la vaccination, ce qui n'est pas possible avec le test AGP. L’étude a montré que le test LA utilisant l'antigène rR7 est une méthode simple, rapide et utile pour la détection des anticorps contre L. caulleryi.
Ein Schnelltest für den Nachweis von Antikörpern gegen Leukozytozoonose in Hühnern mittels eines Latexagglutinationstests unter Verwendung des rekombinanten R7-Antigens
Es wurde eine Methode zum Nachweis von Antikörpern gegen Leukozytozoonose in Hühnern mitttels Latexagglutination (LA) entwickelt, wobei Latexkügelchen mit rekombinantem R7 (rR7), einem Antigen auf der äußeren Membran der Schizonten der zweiten Generation von Leucocytozoon caulleryi , beschichtet wurden. Verglichen mit dem in Japan häufig benutzten Agargelpräzipitationstest (AGP-Test) wies die LA eine größere Sensitivität zum Nachweis von durch L. caulleryi-induzierten Antikörpern auf. Weder mit Seren von spezifisch-pathogenfreien Hühnern und von Legehennen, die nicht mit der rR7-Antigen-Öl-Adjuvansvakzine geimpft oder mit L. caulleryi infiziert worden waren, noch mit Seren von Hühnern, die mit anderen Erregern inokuliert worden waren, wurde eine Agglutination gefunden, was die Spezifität dieses Tests nachweist. Der LA-Test kann außerdem zur Quantifizierung von durch Vakzination induzierten Serumantikörpern verwendet werden, was mit dem AGP-Test nicht möglich ist. Diese Studie hat gezeigt, dass die LA mit rR7-Antigen eine einfache, schnelle und nützliche Methode zum Nachweis von Antikörpern gegen L. caulleryi ist.
Descripción de un ensayo rápido para detectar anticuerpos frente a Leucocytozoon en pollos mediante una prueba de aglutinación en látex utilizando un antígeno recombinante R7
Se desarrolló un método para detectar anticuerpos frente a Leucocytozoon en pollos, basado en la aglutinación en látex (LA), con gotas de látex tapizadas con recombinante R7 (rR7), una antígeno de la membrana externa de la segunda generación de esquizontes de Leucocytozoon caulleryi. En comparación con la técnica de ágar gel precipitación (AGP test), que se usa ampliamente en Japón, la LA pudo detectar anticuerpos inducidos por la infección con L. caulleryi con una mayor sensibilidad. No se detectó aglutinación con el suero proveniente de pollos libres de patógenos específicos o ponedoras que no habían sido vacunados con antígeno rR7 adyuvantado en aceite o infectados con L. caulleryi, ni con los sueros de pollos inoculados con otros patógenos, con lo cual se estableció la especificidad del ensayo. La técnica de LA también pudo ser utilizada para cuantificar los anticuerpos producidos tras la vacunación, lo cual no es posible con el método de AGP. Este estudio ha mostrado que la LA utilizando un antígeno rR7 es un método simple, rápido y útil para detectar anticuerpos frente a L.caulleryi.
Introduction
Leucocytozoon caulleryi, a pathogenic protozoan parasite of chickens, is common in many Asian countries, including Japan (Akiba, Citation1960, Citation1970; Mathis & Leger, Citation1909). Leucocytozoonosis is an economically important disease, causing reduced egg production, weight loss, and sometimes death.
Serological diagnosis of leucocytozoonosis was first reported by Morii (Citation1972), who used agar gel precipitation (AGP) to detect soluble antigen and antibody in sera from infected chickens. Later, counter-immunoelectrophoresis, which can detect antibody more quickly (Fujisaki et al., Citation1980), immunofluorescence using parasites at each stage of the developmental cycle (Fujisaki et al., Citation1981; Isobe & Akiba, Citation1982) and an enzyme-linked immunosorbent assay (ELISA) using sonicated second-generation schizont (2GS) antigen (Isobe & Suzuki, Citation1986, Citation1987a) were also assessed. The ELISA using the 2GS antigen is the most sensitive and specific assay for antibody induced by infection with L. caulleryi. However, this assay has not been used for diagnosis in the field because it is extremely difficult to prepare the 2GS antigen in large quantities. The AGP test is the only assay available commercially and it is widely used in Japan, but has a low sensitivity and is relatively slow. In addition, antibody induced by the vaccine based on oil-adjuvanted recombinant R7 (rR7) protein, which is used to control leucocytozoonosis in Japan, cannot be detected using the AGP test (Itoh & Gotanda, Citation2002, Citation2004).
In this study our aim was to develop a latex agglutination (LA) test using rR7 protein, which could be performed simply and quickly in the field and could also be used to detect antibody induced by both infection and vaccination.
Material and Methods
Purification of rR7 antigen
The rR7 antigen expressed in Escherichia coli using the pMAL system (New England BioLabs, Massachusetts, USA) was purified by ion-exchange chromatography with DEAE-Sephacel (Pharmacia, Uppsala, Sweden) and gel chromatography with Superdex 200 (Pharmacia) as described previously (Itoh & Gotanda, Citation2002). The purified rR7 antigen was dialysed against phosphate-buffered saline (PBS), the amount of protein determined using the BCA Protein Assay Kit (Pierce Chemical, Illinois, USA), and the antigen stored at −20°C until use.
Preparation of rR7 latex suspension
Latex beads (Polysciences Inc., Pennsylvania, USA) with a diameter of 3.219 μm and possessing primary amino surface functional groups (Rajashekara et al., Citation1998) were activated with PBS containing 8% glutaraldehyde (Wako Pure Chemical Industries Ltd, Osaka, Japan), and were resuspended in PBS at a concentration of 2.6% w/v. Forty micrograms of purified rR7 antigen was added to 1.0 ml of the 2.6% latex suspension to coat the beads and the active group was then inactivated using PBS containing 0.20 M monoethanolamine. The latex beads were then blocked with PBS containing 5.0% normal bovine serum (Sigma-Aldrich, Missouri, USA) and then diluted in PBS containing 1.0% normal equine serum (Sigma-Aldrich) and 0.10% sodium azide (Wako Pure Chemical Industries Ltd) to yield a final concentration of latex beads of 0.065%.
Detection of antibody using the LA test
Forty microlitres of latex antigen suspension was mixed with 10.0 μl chicken serum in a circular area approximately 14.0 mm in diameter on a glass slide. The glass slide was incubated at room temperature with shaking at 60 rotations per minute. The assay was considered positive if agglutination occurred within 5 min. To determine LA antibody titres, serial twofold dilutions of serum samples were prepared in PBS and the reciprocal of the highest dilution that still agglutinated the beads was regarded as the LA antibody titre of the serum sample.
Detection of antibody using an ELISA based on 2GS antigen
An ELISA using 2GS antigen as the coating antigen (2GS ELISA), as described by Isobe & Suzuki (Citation1986, Citation1987a), was performed to detect antibody. The 2GS ELISA antibody titre was the reciprocal of the highest dilution of the serum sample that yielded an optical density greater than the threshold value established in our previous study (Itoh & Gotanda, Citation2002).
Detection of antibody using an AGP test
The AGP test using soluble serum antigen (SSA), as described by Morii (Citation1972), was performed. Serial twofold dilutions of serum samples were used and the AGP antibody titre was the reciprocal of the highest dilution at which precipitation lines were still seen.
Verification of the specificity of the LA test
The specificity of the LA test was examined using serum samples from 100 35-day-old to 84-day-old specific pathogen free (SPF) chickens (Nippon Institute for Biological Science, Tokyo, Japan) that had not been vaccinated with the rR7 vaccine nor infected with L. caulleryi, and serum samples from 98 116-day-old layer chickens raised on a commercial farm. The serum samples were also assessed using the 2GS ELISA and the AGP test. The LA test was also performed on serum samples from 85 chickens that had antibodies against other pathogens of chickens induced by either infection or vaccination (Yamamoto & Somersett, Citation1964; Lukert & Davis, Citation1971; Borzemska, Citation1972; Yamaguchi et al., Citation1981a; Nandapalan et al., Citation1982; United States Department of Agriculture, Citation1985; Onaga et al., Citation1986). Some of these serum samples were prepared in our laboratory, and others were obtained from the Saitama Prefecture Agriculture and Forestry Research Centre, from the Nippon Institute for Biological Science and from Shokukanken Co. Ltd.
Detection of antibody induced by infection with L. caulleryi
Seventy-nine serum samples were collected from 251-day-old to 524-day-old commercial layers that had not been vaccinated with the rR7 vaccine and that were showing clinical signs, such as green faeces, anaemia and reduced egg production, typical of disease due to infection with L. caulleryi. The samples were tested by LA, Giemsa-stained blood smears were examined for L. caulleryi (Akiba, Citation1960), and the samples were also tested using the 2GS ELISA and the AGP test, and the results were compared with those obtained with the LA test.
Confirmation of the sensitivity of the LA test in chickens experimentally infected with L. caulleryi
Four 170-day-old SPF chickens were each experimentally infected by intravenous inoculation of 104 sporozoites of the Shizuoka strain of L. caulleryi, which had been passaged in the laboratory (Morii & Kitaoka, Citation1968). Blood was collected at 7, 13, 20, 26, 33, 40, 47 and 68 days after inoculation of the sporozoites and the LA test was performed to detect antibody. The sera were also examined using the 2GS ELISA and the AGP test.
Detection of antibody induced by rR7 vaccine
Serum samples from 100 35-day-old to 147-day-old SPF chickens and 110 134-day-old to 160-day-old commercial layers were tested for antibody induced by vaccination using the LA test. Titres of antibody as assessed by the LA test and the 2GS ELISA was also determined. Serum samples that were used had been collected 14 to 112 days after vaccination. The AGP test was also performed on all samples.
Statistical analysis
The proportions of samples in which antibody was detected in each group of chickens with each test were compared using the chi-squared test. Mean antibody titres in the LA test and the AGP test were compared using Student's t test. Pearson's product moment correlation coefficient was used to analyse the correlation between the LA titres and the 2GS ELISA titres.
Results
Specificity of the LA test
As shown in , none of the sera from SPF or commercial layer chickens agglutinated the rR7 latex. None of the sera yielded positive results in either the 2GS ELISA or the AGP test. None of the sera from chickens infected with, or vaccinated against, other pathogens agglutinated the latex beads ().
Table 1. Specificity of the LA test using sera from SPF and layer chickens
Table 2. Reactivity of sera from chickens with antibody against different pathogens in the LA test with various antisera
Detection of antibody in chickens from a flock naturally infected with L. caulleryi
Clinical signs of leucocytozoonosis were observed in 60 of 79 (75.9%) chickens from the field, and L. caulleryi second-generation merozoites and gametocytes were seen in Giemsa-stained blood smears from 32 birds. In the LA test, 54 samples were positive (68.4%). In the 2GS ELISA, 66 samples were positive (84.8%). In the AGP test, 41 samples were positive (51.9%). All samples that were positive in the LA test or the AGP test were also positive in the 2GS ELISA test. Fourteen samples that were positive in the LA test were negative in the AGP test, and only one sample that was positive in the AGP test was negative in the LA test. The LA test detected a significantly greater proportion of antibody positive birds than the AGP test (P=0.035).
Sensitivity of detection of antibody with the LA test in chickens experimentally infected with L. caulleryi
The four chickens inoculated with L. caulleryi had clinical signs typical of leucocytozoonosis—green faeces, anaemia and reduced egg production. presents the changes in antibody titre induced by L. caulleryi infection. In three chickens the LA test could detect antibody from the seventh day after inoculation with L. caulleryi sporozoites. In the fourth chicken, antibody could be detected from the 13th day. The 2GS ELISA could detect antibody in all four infected chickens from the seventh day after inoculation. In contrast, the AGP test could not detect antibody in any of the infected chickens until the 20th day after inoculation. The highest antibody titres in all these three tests were detected on the 20th day after inoculation, which corresponded with the appearance of gametocytes in the chickens. On the 26th day after inoculation L. caulleryi could no longer be detected in Giemsa-stained blood smears from infected chickens, and the antibody titres as measured by the LA test were much lower; and after that day they continued to gradually decrease. On the 68th day after inoculation, one chicken was negative in the LA test. The antibody titres as measured by the 2GS ELISA and the AGP test also decreased sharply on the 26th day after inoculation and the titres continued to decrease gradually, but all birds were still positive on 68th day after inoculation. The LA antibody titres were significantly higher than the AGP antibody titres until day 13 post-infection (). However, there were no significant differences between the LA antibody titres and the AGP antibody titres after day 20 post-infection.
Table 3. Comparison of sensitivity of different methods for detecting antibodies against L. caulleryi in experimentally infected chickens
Detection of antibody using the LA test in chickens vaccinated with rR7
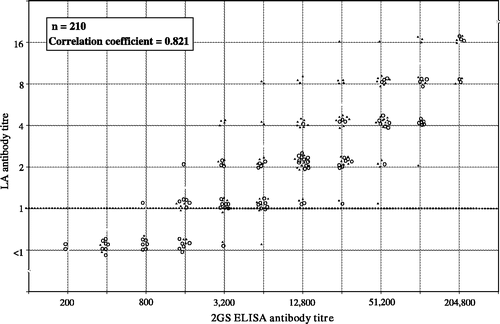
shows a comparison of LA and 2GS ELISA antibody titres in vaccinated chickens. The 2GS ELISA detected antibody in all birds, but antibody was not detected in any birds by the AGP test. One hundred and eighty out of 220 samples (85.7%) were positive in the LA test and 93.7% of samples that had a 2GS ELISA antibody titre of 1600 or greater were positive in the LA test. There was a strong correlation between the titres determined by each test (R 2=0.821, P=0.117 ×10–4) for all samples. Of the samples that were negative in the LA test, 18 had a 2GS ELISA antibody titre under 800, 11 had a 2GS ELISA antibody titre of 1600 to 3200, and one sample had a 2GS ELISA antibody titre of 6400.
Discussion
SSA derived from the 2GS is usually used as an antigen in the AGP test to detect antibody induced by L. caulleryi (Morii, Citation1972) and it is known that antibody against SSA can be found in serum 17 days after infection with L. caulleryi. It has been reported that the AGP test can be used as diagnostic method for detection of SSA from 10 to 13 days after infection (Morii, Citation1972, Citation1974, Citation1978). However, as we have shown previously, it is very difficult to detect SSA in the serum of infected chickens in the field (Ito & Gotanda, Citation2004). The rR7 antigen used in this study is derived from a gene for an outer membrane protein of 2GS. The LA test using rR7-coated latex microspheres could detect antibody induced by L. caulleryi infection from 7 to 13 days after infection. We did not study the type of immunogloblin detected by LA test at these early stages of infection, but it is likely to be IgM, as suggested by the study of Isobe & Suzuki (Citation1987b). When applied to serum samples collected from chickens thought to have been infected with L. caulleryi, the LA test could detect a higher proportion of infected chickens than the AGP test. Thus the LA test is likely to be a useful serological method for diagnosis of leucocytozoonosis. However, the AGP test tended to detect antibody for a longer period than the LA test.
There have been few reports of the development of LA assays for serological diagnosis in chickens, possibly because chicken serum can non-specifically agglutinate latex microspheres (Franklin, Citation1962). The only LA test available in the field is for antibody against infectious bursal disease virus (Yamaguchi et al. Citation1981b; Nakamura et al., Citation1993a,Citationb, Citation1994) and this is a latex agglutination-inhibition test, in which the serum sample is sensitized with a virus antigen suspension and then mixed with latex microspheres coated with a monoclonal antibody against the viral antigen. In our study, we verified the specificity of the LA test using sera from SPF and commercial chickens and sera from birds with antibody against a variety of pathogens, and no non-specific agglutination was observed. This may be because the latex beads used in this study were functionalized, and did not attach to proteins passively, as conventional latex beads do. Additionally, the components of the buffer solution for the LA suspension may have prevented non-specific agglutination.
The LA test could be used to detect and quantify antibody induced by rR7 vaccination, something that was not possible using the AGP test. The LA test was able to detect antibody in most vaccinated chickens with a 2GS ELISA antibody titre over 1600, and did not detect antibody in most birds with a serum 2GS ELISA antibody titre under 1600. Since a 2GS ELISA serum antibody titre of 1600 to 3200 is correlated with protection after vaccination in the field (Itoh & Gotanda, Citation2002), a positive result in the LA test using undiluted serum may be a useful indicator of protective immunity in birds. Further studies are needed to develop assays that can distinguish vaccinated and infected chickens.
The Japanese Ministry of Agriculture, Forestry and Fisheries supported this study. The authors are grateful to Katsumi Kume for many suggestions during this study and to Naomi Himeno for critical review of the manuscript.
References
- Akiba , K . (1960) . Studies on the leucocytozoon found in the chicken in Japan. II. On the transmission of L. caulleryi by Culicoides arakawae . The Japanese Journal of Veterinary Science , 22 : 309 – 317 .
- Akiba , K . (1970) . Leucocytozoonosis of chicken . National Institute of Animal Health Quarterly Supplement (Japan) , 10 : 131 – 147 .
- Borzemska , WB . (1972) . Effect of neutralizing (SN) and hemagglutination inhibiting (HI) antibodies on the course of Newcastle disease infection in chickens . Polskie Archiwum Weterynaryjne , 15 : 495 – 512 .
- Fujisaki , K , Takamatsu , H , Kitaoka , S , Suzuki , K and Kuniyasu , C . (1980) . Rapid detection by counterimmunoelectrophoresis of antigens and antibodies in the sera of chickens infected with Leucocytozoon caulleryi . National Institute of Animal Health Quarterly (Japan) , 20 : 96 – 100 .
- Fujisaki , K , Takamatsu , H , Kitaoka , S , Suzuki , K and Kamio , T . (1981) . Direct immunofluorescent staining of Leucocytozoon caulleryi of different developmental stages . National Institute of Animal Health Quarterly (Japan) , 21 : 73 – 79 .
- Franklin , EC . (1962) . Some physicochemical and biological properties of high molecular weight gamma and beta globulins in chicken sera . Proceedings of the Society for Experimental Biology and Medicine , 109 : 338 – 342 .
- Isobe , T and Akiba , K . (1982) . Indirect immunofluorescent antibody test in chicken leucocytozoonosis . National Institute of Animal Health Quarterly (Japan) , 22 : 163 – 169 .
- Isobe , T and Suzuki , K . (1986) . Enzyme-linked immunosorbent assay for detection of antibody to Leucocytozoon caulleryi . Avian Pathology , 15 : 199 – 211 .
- Isobe , T and Suzuki , K . (1987a) . Detection of serum antibody to Leucocytozoon caulleryi in naturally infected chicken by enzyme-linked immunosorbent assay . The Journal of Veterinary Medical Science , 49 : 165 – 167 .
- Isobe , T and Suzuki , K . (1987b) . Immunoglobulin M and G immune response to Leucocytozoon caulleryi in chickens . The Journal of Veterinary Medical Science , 49 : 333 – 339 .
- Itoh , A and Gotanda , T . (2002) . The correlation of protective effects and antibody production in immunized chickens with recombinant R7 vaccine against Leucocytozoon caulleryi . The Journal of Veterinary Medical Science , 64 : 405 – 411 .
- Ito , A and Gotanda , T . (2004) . Field efficacy of recombinant R7 vaccine against chicken leucocytozoonosis . The Journal of Veterinary Medical Science , 66 : 483 – 487 .
- Lukert , PD and Davis , RB . (1971) . An antigen used in the agar-gel precipitin reaction to detect avian encephalomyelitis virus antibodies . Avian Diseases , 15 : 935 – 938 .
- Mathis , C and Leger , M . (1909) . Leucocytozoon De la poule . Comptes Rendus des Seances de la Societe de Biolgie et de ses Filiales et Associees Paris , 67 : 470 – 472 .
- Morii , T . (1972) . Presence of antigens and antibodies in the sera of chickens infected with Akiba caulleryi . National Institute of Animal Health Quarterly (Japan) , 12 : 161 – 167 .
- Morii , T . (1974) . Characterization of antigens and specific antibodies in the sera of chickens infected with Akiba caulleryi . National Institute of Animal Health Quarterly (Japan) , 14 : 174 – 181 .
- Morii T (1978) Soluble antigens in the sera of chickens infected with Leucocytozoon caulleryi Proceedings of the 1st Japanese–German Cooperative Symposium on Protozoan Diseases Tokyo Japan (pp. 211–217)
- Morii , T and Kitaoka , S . (1968) . The laboratory colonization of Culicoides arakawae (Diptera:Ceratopogonidae) . National Institute of Animal Health Quarterly (Japan) , 8 : 26 – 30 .
- Nakamura , T , Kato , A , Lin , Z , Hiraga , M , Nunoya , T , Otaki , Y and Ueda , S . (1993a) . A rapid quantitative method for detecting infectious bursal disease virus using polystyrene latex microspheres . Journal of Virological Methods , 43 : 123 – 129 .
- Nakamura , T , Otaki , Y , Lin , Z and Kato , A . (1993b) . Rapid and quantitative assay system for measuring anti-infectious bursal disease virus antibody using monoclonal antibody bound to polystyrene latex microspheres . Avian Diseases , 37 : 786 – 792 .
- Nakamura , T , Otaki , Y , Lin , Z , Nunoya , T , Hoshi , S and Kato , A . (1994) . Direct correlation between the titer of infectious bursal disease virus VP2-specific antibody and protection . Avian Diseases , 38 : 251 – 255 .
- Nandapalan , N , Wilcox , GE and Penhale , WJ . (1982) . Enzyme-linked immunosorbent assay for the detection of antibodies to infectious bronchitis virus . Avian Diseases , 26 : 171 – 176 .
- Onaga , H , Saeki , H , Hoshi , S and Ueda , S . (1986) . An enzymed-linked immunosorbent assay for serodiagnosis of coccidiosis in chickens: use of a single serum dilution . Avian Diseases , 30 : 658 – 661 .
- Rajashekara , G , Munir , S , Lamichhane , CM , Back , A , Kapur , V , Halvorson , DA and Nagaraja , KV . (1998) . Application of recombinant fimbrial protein for the specific detection of Salmonella enteritidis infection in poultry . Diagnostic Microbiology and Infectious Disease , 32 : 147 – 157 .
- United States Department of Agriculture (1985) National Poultry Improvement Plan and Auxiliary Provisions Publication APHIS 91-40 (pp. 52–60) Animal and Plant Health Inspection Service, US Department of Agriculture Washington DC
- Yamaguchi , S , Imada , T , Kawamura , H , Taniguchi , S , Saio , H and Shimamatsu , K . (1981a) . Outbreaks of egg-drop syndrome-1976 in Japan and its etiological agent . Avian Diseases , 25 : 628 – 641 .
- Yamaguchi , T , Iwaki , S and Iritani , Y . (1981b) . Latex agglutination test for measurement of type-specific antibody to Haemophilus paragallinarum in chickens . Avian Diseases , 25 : 988 – 995 .
- Yamamoto , R and Somersett , DT . (1964) . Antibody response in chickens to infection with Haemophilus gallinarum . Avian Diseases , 8 : 441 – 453 .