Abstract
Data on the effects of Plasmodium gallinaceum on domesticated fowl are sparse, justifying a full investigation of its pathology. Clinical signs following blood-induced infections with the Wellcome line of strain 8A included depression, fever, anorexia, reduced weight gain, poor feed conversion, anaemia, green faeces and often death. After administration of 106 erythrocytic parasites, mortality 5 to 10 days after infection was 10% to 93% in chickens 7 to 84 days old. The older the birds, the lower the mortality and the longer the time to death. Onset of detectable parasitaemia occurred mostly during the second day after infection (59% of birds). Peak parasitaemia (∼70%) occurred on the sixth day in 85% of surviving birds. The patent period was usually 7 to 19 days. Abnormally low haematocrit values of ≤24% and high colonic temperatures of ≥42°C were recorded. A febrile response is demonstrated conclusively here in P. gallinaceum malaria for the first time. Weight gain of malarious birds was reduced by ∼18% to 51%, and feed conversion efficiency was often reduced by ∼12% to 41%. Growth reduction was due entirely to anorexia. Liver weight relative to body weight (normally ∼2% to 3%) increased to ∼4.5% by 8 days, and relative spleen weight (normally ∼0.2%) increased to 1.6% by 12 days. Specific gravities of livers and spleens in healthy and infected birds were ∼1.09. Gall bladder volume in malarious birds 8 days after infection was approximately four times that of normal birds. Statistically significant changes occurred in the proportions of plasma proteins in malarious birds 8 days after infection; albumin and α2-globulin were reduced, while γ1-globulin and γ2-globulin were increased. Those changes coincided with significant increases in concentrations of plasma total protein and the enzymes aspartate aminotransferase, glutamate dehydrogenase and γ-glutamyltransferase, and a decrease in creatinine. Green (biliverdin) colouration of the faeces was a consistent sign of malaria. Birds acquired non-sterile immunity after a single primary infection. The quantitative data presented facilitate selection of the most useful criteria for field diagnosis, estimation of potential economic losses, and assessment of potential avian antimalarial drugs.
Malaria aviaire : pathologie clinique et chimique de Plasmodium gallinaceum chez des poules domestiques Gallus gallus
Les données sur les effets de Plasmodium gallinaceum chez les poules domestiques sont clairsemées, justifiant une investigation complète de sa pathologie. Les symptômes consécutifs à des infections induites par le sang contenant la lignée Wellcome de la souche 8A comprenaient de la dépression, de la fièvre, de l'anorexie, une diminution du gain de poids, un mauvais indice de conversion, de l'anémie, des fèces verdâtres et souvent de la mortalité. Après administration de 106 parasites érytrocytiques la mortalité 5–10 jours après infection a été de 10–93% chez les poulets âgés de 7–84 jours. Plus les oiseaux étaient âgés, plus la mortalité était faible et plus le temps pour mourir était long. Le début de la parasitémie détectable est apparu, le plus souvent, au cours du 2è jour après l'infection (59% des animaux). Le pic de parasitémie (∼70%) est apparu le 6è jour chez 85% des animaux survivants. La période patente a habituellement été de 7–19 jours. Des valeurs d'hématocrite anormalement faibles ≤24% et des températures élevées ≥42°C ont été enregistrées. De façon concluante il a été mis en évidence pour la première fois, une réponse fébrile dans la malaria à P. gallinaceum . Le gain de poids des animaux atteints a été réduit de ∼18−51%, et l'indice de conversion a souvent été réduit de ∼12−41%. La diminution de croissance a été due entièrement à l'anorexie. Le poids du foie relatif au poids du corps (normalement 2−3% environ) a augmenté de ∼4,5% en 8 jours, et le poids relatif de la rate (normalement ∼0.2%) a augmenté de 1,6% en 12 jours. Les poids relatifs des foies et des rates des animaux infectés et non infectés étaient de ∼1,1. Le volume de la vésicule biliaire chez les animaux atteints de malaria 8 jours après infection était environ 4 fois celui des animaux normaux. Des changements significatifs sont apparus dans les proportions des protéines du plasma chez les animaux atteints de malaria 8 jours après l'infection ; l'albumine et l’α2-globuline ont diminué, alors que la γ1- globuline et la γ2-globuline ont augmenté. Ces changements coïncident avec des augmentations significatives des concentrations en protéines totales du plasma et en enzymes ASAT, GDH et GGT, ainsi qu'avec une diminution en créatine. La coloration verdâtre des fèces a été un symptôme compatible avec la malaria. Les animaux ont acquis une immunité non stérile après une seule infection primaire. Les données quantitatives présentées facilitent la sélection des critères les plus utiles pour un diagnostic terrain, une estimation des pertes économiques potentielles, et l’évaluation de médicaments potentiels contre la malaria aviaire.
Hühnermalaria: Klinische und chemische Veränderungen durch Plasmodium gallinaceum im domestizierten Huhn Gallus gallus
Berichte über die Auswirkung einer Plasmodium gallinaceum-Infektion beim domestizierten Huhn sind nur spärlich vorhanden, was eine vollständige Untersuchung seiner Pathologie rechtfertigt. Die klinischen Symptome nach Infektionen mit Blut, das die Wellcome-Linie des 8A-Stammes enthielt, umfassten Störung des Allgemeinbefindens, Fieber, Anorexie, reduzierte Gewichtszunahme, schlechte Futterverwertung, Anämie sowie grüner Fäzes und führte oft zum Tod. Nach Verabreichung von 106 erythrozytären Parasiten betrug die Mortalität 5–10 Tage nach der Infektion 10–93% in 7–84 Tage alten Hühnern. Je älter die Tiere waren desto geringer war die Mortalität und desto länger war die Zeit bis zum Tod. Der Beginn der nachweisbaren Parasitämie lag meistens auf dem zweiten Tag nach der Infektion (59% der Tiere). Der Höhepunkt der Parasitämie (∼70%) trat am 6. Tag nach der Infektion bei 85% der überlebenden Tiere auf. Die Patenzphase war gewöhnlich 7–19 Tage lang. Abnorm niedrige Hämatokritwerte von ≤24% und hohe Temperaturen im Kolon von ≥42°C wurden ermittelt. Zum erstenmal wird hiermit eine fieberhafte Reaktion bei der P. gallinaceum-Malaria schlüssig nachgewiesen. Die Gewichtszunahmen bei den Malaria-kranken Tieren waren um ∼18–51% geringer und die Futterverwertung war oftmals um ∼12–41% reduziert. Die Wachstumsreduktion war hauptsächlich durch Anorexie bedingt. Das Verhältnis von Lebergewicht zu Körpergewicht (normal 2–3%) stieg am 8. Tag auf ∼4,5% an und das relative Milzgewicht (normal ∼0,2%) erhöhte sich auf 1,6% am 12. Tag. Die spezifischen Gewichte von Leber und Milz betrugen in gesunden und infizierten Tieren ∼1,1. Das Volumen der Gallenblase war bei den Malaria infizierten Tieren am 8.Tag nach der Infektion etwa viermal so groß wie bei den gesunden Tieren. Statistisch signifikante Unterschiede traten 8 Tage nach der Infektion bei den infizierten Tieren in den Plasmaproteinanteilen auf; Albumin und a2-Globulin waren reduziert, während ?1-Globulin und ?2-Globulin anstiegen. Diese Veränderungen gingen mit signifikanten Konzentrationserhöhungen des Gesamtplasmaproteins und der Enzyme ASAT, GDH und GGT sowie einer Abnahme des Kreatinins einher. Eine Grünverfärbung des Fäzes durch Bilirubin war ein einheitliches Anzeichen für die Malaria. Die Tiere entwickelten nach einer einzelnen Primärinfektion eine nicht sterile Immunität. Die vorgestellten quantitativen Daten erleichtern die Auswahl der sinnvollsten Kriterien für die Diagnose im Feld, die Abschätzung der potentiellen wirtschaftlichen Verluste und die Beurteilung von Medikamenten für ihre Anwendung gegen die Hühnermalaria.
Malaria aviar: patología clínica y química de Plasmodium gallinaceum en aves domésticas Gallus gallus
Hay muy pocos doatos sobre los efectos de Plasmodium gallinaceum en aves domésticas, lo cual justifica una investigación extensa sobre su patología. Los signos clínicos tras la infección inducida vía sanguínea de la línea Wellcome de la cepa 8A incluyeron depresión, fiebre, anorexia, reducción de la ganancia de peso diaria, bajo índice de conversión, anemia, heces verdosas y, con frecuencia, muerte. Tras la administración de 106 parásitos eritrocíticos, la mortalidad a los 5−10 días tras la infección fue del 10−93% en pollos de 7−84 días de edad. Cuanto más avanzada la edad de las aves, menor era la mortalidad y mayor el tiempo que tardaban en morir. La parasitemia detectable ocurría mayoritariamente hacia el segundo día tras la infección (59% de las aves). El pico de parasitemia (∼70%) ocurrió en el sexto día en un 85% de las aves supervivientes. El periodo de prepatencia fue de 7−19 días. Se detectaron valores de hematocrito de ≤24% y altas temperatures colónicas de ≥42°C. Se demostró pues, por primera vez, que la infección con P. gallinaceum producía un estado febril. La ganancia de peso en aves con malaria se redujo entre ∼18−51%, y la eficiencia de conversión se redujo frecuentemente entre ∼12−41%. La reducción en el crecimiento fue debido a la anorexia. El peso relativo del hígado respecto al peso corporal (normalmente c. 2−3%) se incrementó hasta el ∼4.5% en 8 días, y el peso relativo del bazo (normalmente ∼0.2%) incrementó al 1.6% en 12 días. La gravedad específica de los hígados y bazos de las aves sanas e infectadas fue del ∼1.1. El volumen de la vesícula biliar de las aves con malaria a los 8 días tras la infección fue 4 veces mayor que el de las aves normales. Cambios estadísticamente significativos ocurrieron en las proporciones de las proteínas plasmáticas en aves con malaria a los 8 días tras la infección; la albúmina y la α2-globulina se redujeron, mientras que la γ1-globulina y γ2-globulina se incrementaron. Estos cambios coincidieron con un incremento significativo en las concentraciones de las proteínas plasmáticas totales y en los enzimas ASAT, GDH y GGT, y en una disminución de la creatinina. La coloración verde (biliverdina) de las heces fue un signo consistente de malaria. Las aves adquirieron inmunidad no estéril tras una sola infección primaria. Los datos cuantitativos presentados pueden facilitar la selección de los criterios más útiles para el diagnóstico de campo, la estimación de las pérdidas económicas potenciales y la evaluación de posibles fármacos para tratar la malaria en aves.
Introduction
Plasmodium gallinaceum, a malarial parasite (Apicomplexa: Haemospororida) of birds of the genus Gallus, was first described by Brumpt (Citation1935), and during the ensuing 30 years or so more than 1000 papers were published on the species (Garnham, Citation1966). Many of those publications dealt with P. gallinaceum as a tool for screening potential human antimalarial agents (see Richards, Citation1984), and with its development in tissue culture and its life cycle (see van Riper et al., Citation1994). By the 1970s it was observable that “The amount of recent research in avian malaria has declined somewhat, largely because the discovery of species of Plasmodium which will infect rats and mice has made mammalian hosts inexpensive and easily obtainable” (Seed & Manwell, Citation1977, p.313). That trend has continued apparently unabated. Statements such as “None of these [malarial] parasites are important causes of spontaneous disease in domestic fowl” (Shadduck & Pakes, Citation1978, p. 1612) and “The disease [malaria in birds] has only slight veterinary interest” (Raether, Citation1988, p. 755) have done little to encourage further research by veterinarians into malaria of the domesticated fowl.
Clearly then, the initial interest in P. gallinaceum was largely medically orientated, and work on this parasite from the veterinarian's standpoint was somewhat neglected, and remains so. Recent publications still concentrate on the use of P. gallinaceum for in vitro and in vivo models for solving problems of biochemistry, drug resistance, epidemiology, life cycles and genomics in human malaria. However, no comprehensive study appears to have been made on the potential range of pathological effects in commercial chickens, although it is known that P. gallinaceum can be the cause of serious disease with high mortality in domesticated fowl in Asia (Crawford, Citation1945; Omar, Citation1968). The major objectives of the present study were to establish and quantify the pathogenic effects of P. gallinaceum as a basis for the field diagnosis of avian malaria, for estimating potential economic losses due to the disease, and for the selection of suitable criteria for veterinary drug screening and efficacy trials. It should be noted that there is apparently still no commercially available medication for the control of avian malaria.
The results comprise a new contribution to knowledge of the clinical and chemical pathology of P. gallinaceum in the domesticated fowl (Gallus gallus), and characterize a subline of the parasite for further use in veterinary chemotherapeutic studies. The provenance of the parent strain (8A) has been traced, and criteria for designation and characterization of the strain and line as recommended by Joyner et al. (Citation1978) for other apicomplexans (Eimeriorina) have been parallelled.
Materials and Methods
Designation and provenance of the parasite strain
The parasite used in this study was Plasmodium gallinaceum Brumpt, Citation1935 (strain 8A; Wellcome line; Berkhamsted subline). Brumpt (Citation1935) originally described the species from some blood smears given to him many years before by his pupil, Broussais. In 1936, Brumpt isolated from chickens in Ceylon (now Sri Lanka), by subinoculation of blood, the first viable material (Brumpt, Citation1949, vol. I, p. 520, footnote) that he distributed to numerous colleagues all over the world.
A line was maintained “for a great number of years in the Wellcome Laboratories of Tropical Medicine, both by syringe passage and cyclically through Aedes aegypti” (Richards, Citation1969, p. 8). This is presumably the line that Al-Dabagh (Citation1966) stated had been maintained at the Wellcome Laboratories (UK) since 1936. However, Dr L.G. Goodwin informed me that it was received there, in fact, soon after the Second World War, but the source is uncertain. Dr W.H.G. Richards preserved some blood stabilates in liquid nitrogen in 1965, and these were kept at the Wellcome Research Laboratories at Beckenham, Kent, UK. They were labelled “Normal Ceylon”, and with Dr Richards's help I was able to establish that they are of a strain that was originally designated 8A. According to the late Prof. P.C.C. Garnham, this strain may have been derived from material supplied to Col. S.P. James in 1936 and maintained at the Molteno Institute at Cambridge, UK. However, it seems more likely, because of its original designation (Normal Ceylon) at the Wellcome Laboratories, that it is a sample of strain 8A defined by the Committee on Terminology of Avian Malaria of the American Society of Parasitologists (see McGhee, Citation1949). I have therefore here designated Dr Richards's stabilate as the Wellcome line of strain 8A, to distinguish it from other lines of the same strain that might have been maintained elsewhere in the UK (for example, Ryley & Peters, Citation1970) or in the USA (for example, McGhee et al., Citation1977).
In 1983, Mr B. Maples kindly sent one of Dr Richards's stabilates to the Wellcome Research Laboratories at Berkhamsted, Hertfordshire, UK. The blood was immediately thawed and injected intravenously into three chickens, which developed parasitaemias. Blood samples from these birds were pooled and heparinized, glycerol was added to 10% volume, and stabilates were immediately frozen at −70°C, thus establishing the Berkhamsted subline on 8 February 1983. This was revivified by injection into chickens on 26 August 1983 and was maintained by repeated blood passage until 8 December 1983, when it was frozen with 10% v/v glycerol in liquid nitrogen. It was revivified again on 8 March 1984 and repeated blood passages were again initiated. All experiments described herein involved Berkhamsted subline infections maintained by weekly blood passage in 7-day-old chicks. On completion of this work in November 1985, the remaining material, frozen in liquid nitrogen, was deposited in the collection of Winches Farm Field Station (Department of Medical Protozoology), London School of Hygiene and Tropical Medicine, UK.
Experimental animals and husbandry
The chickens (G. gallus) used in all experiments were males of egg-laying hybrids, 7 to 84 days of age, housed in wire-floored cages, initially at 24°C and 37% relative humidity with 23 h lighting. Various groups of birds in all experiments were either infected or maintained as healthy controls, and temperature and lighting periods were reduced for their comfort as they grew older. Ross Brown chickens were used for maintenance of malarial infections and in experiments 413, 416, 418, 426, 428, 431, 437, 439, 440, 445, 446, 447, 448, 449, 451 and 452. Ross Ranger birds were used in experiments 419 and 423. No differences in their physiology or susceptibility to malaria were observed between the different breeds. Chickens were caged in groups of up to five and had free access to unmedicated drinking water and the unmedicated experimental chick diet (LD5) described by Williams (Citation1996). Bird welfare was monitored at least four times daily. All experiments were carried out in compliance with the contemporary UK legislation.
Procedures for studies of clinical pathology
Malarial infections were produced by injection into a jugular vein of each experimental bird of one million parasitized erythrocytes (taken directly from donor birds) suspended in 0.1 ml physiological saline. A standardized procedure described by Williams (Citation1986a) was used for obtaining erythrocyte haematocrit values. Parasitaemias were assessed by the examination of Giemsa-stained blood smears; the results are shown as percentages of erythrocytes infected, rounded off to the nearest integer. Sometimes there may have been more than one parasite in a cell; no distinction was drawn between the various parasitic stages seen in erythrocytes. Exoerythrocytic stages were examined in Giemsa-stained impression smears from freshly cut surfaces of the liver, spleen, lung, kidney, heart and brain, immediately after examination of the condition of the organ in situ. Whenever appropriate, the timing, relative to other clinical signs, of the production of green faeces was recorded and the condition of the droppings or diarrhoea was described.
During the course of infections in some experiments, the individual chickens were weighed daily or weekly and the food consumption of groups of five was measured daily or weekly for the calculation of feed conversion ratios (FCRs): FCR=weight of feed consumed/body weight gain. If any birds died, due allowance was made in the FCR calculations for relevant groups. Colonic temperatures were taken daily between 08:00 h and 09:00 h, using a standardized method described by Williams (Citation1986b). All birds that died were dissected and examined as soon as possible post mortem; if found dead at 08:00 h (the first welfare check of the day) they were deemed to have died the previous day. Birds found to be in extremis at any time were immediately killed humanely. The mean time to death was calculated for each group of birds. In all experiments, daily observations were made in an attempt to detect eyelid lesions characteristic of pantothenic acid deficiency (Al-Dabagh, Citation1961).
In a pair-feeding experiment (number 449), birds all of the same age were individually weighed every day for 2 weeks after infection. The three treatments comprised five uninfected healthy control birds given feed ad libitum (UUC-ad lib), 15 birds infected when 25 days old and fed ad libitum (IUC), and 15 uninfected healthy birds on restricted feed (UUC-restr). The amount of feed consumed by each group of birds during each 24 h period was weighed and the mean amount eaten on each day by the IUC birds was offered the following day to the UUC-restr birds. All birds were allowed to drink ad libitum.
In experiment 428, infected birds and uninfected controls of the same age were killed at predetermined intervals and the following data were obtained immediately before or after death as appropriate: body weight, haematocrit value, percentage parasitaemia, liver weight and volume, spleen weight and volume, gall bladder volume, and condition of visceral organs. If any bird died before it was due to be killed, all these data (except haematocrit and parasitaemia values) were collected within 1 hour of death if possible; otherwise, the bird was discounted from the experiment. A blood sample was taken from a jugular vein of each bird immediately before it was killed, and plasma was collected from each blood sample for chemical pathology studies.
Volumes of spleens and livers were obtained by a water displacement method. A graduated cylinder of a diameter as close as possible to the maximum dimension of the organ was filled with water to a convenient level, which was recorded after adding a few drops of liquid detergent to flatten the meniscus. An organ was removed from the bird, immediately blotted to remove any surface moisture, then weighed and placed in the cylinder. The displaced volume of water was then removed, down to the original recorded level, using a hypodermic syringe with a fine needle. The smallest possible syringe was used, and the volume of water removed was read against a supplementary intermediate scale with which the syringe barrel was aligned.
Because of the difficulty of removing gall bladders from birds without bursting them, their volumes were estimated from their dimensions in situ. The maximum diameter (d) and length (l) of each were measured to the nearest 0.5 mm. The shape of a fowl's gall bladder is approximately pyriform, but for the purpose of estimating its volume it may be regarded as an ellipsoid. Therefore, the volume of each gall bladder (mm3) was estimated using the formula: (4/3)×π×(l/2)×(d/2)2. Dividing by 1000 converted the volume to millilitres.
Procedures for studies of chemical pathology
The plasma from blood samples taken in experiment 428 was subjected to cellulose acetate strip electrophoresis, using a Gelman Sepratek system at 220 V and staining with Ponceau S to reveal plasma proteins. The slides were then scanned with a DCD16 digital computing densitometer to calculate the area under each peak, which was recorded as a percentage of the total area under the whole curve. The distance migrated by each peak was evaluated relative to the albumin front and expressed as the relative mobility index (RMI) (Sherman & Hull, Citation1960). Because during the present work some pre-albumin peaks were observed, RMIs greater than unity were sometimes obtained.
Other chemical assays were carried out on the same plasma samples using a Hitachi 705E Chemistry Analyzer. The following reagent kits were used: total protein (g/l), Boehringer reagents 147672 and 47699; creatinine (μmol/l), Monitor International product 22.1111; uric acid (mg/dl), Boehringer reagent 620416; urea (mmol/l), S.K. Beckman product 86245; glutamate dehydrogenase (GDH) (IU/l at 25°C), Boehringer reagent 124320; γ-glutamyltransferase (γ-glutamyl transpeptidase) (GGT) (IU/l at 25°C), Boehringer reagents 543098 and 543101; alanine aminotransferase (glutamate pyruvate transaminase) (ALAT) (IU/l at 25°C), S.K. Beckman product 86230; aspartate aminotransferase (aspartate transaminase or glutamate oxaloacetate transaminase) (ASAT) (IU/l at 25°C), S.K. Beckman product 86227; and glucose (mmol/l), Boehringer reagent 245178.
Statistical analyses
Methods and results are indicated in the appropriate places in the text or the tables.
Results
Clinical observations
For the first 4 days after administration of a blood-induced infection of P. gallinaceum, chicks appeared to be healthy. On the fifth to seventh days after infection, they were anorexic and hunched or sitting on their hocks with ruffled, fluffed-out feathers and closed eyes. Their combs and legs were generally pale; the more lively birds had redder combs. Most birds that died did so during this period or, if in extremis, were humanely killed; rather fewer died during days 8 to 10 (). Birds that survived began to recover their normal appearance on the eighth day, but the comb and legs remained very pale. During the ninth to 11th days after infection, surviving birds regained their lively behaviour and from the 12th day onwards, the normal colour of the comb and legs was mostly reattained. No eyelid lesions like those described by Al-Dabagh (Citation1961) were ever observed.
Table 1. Mortalities of chickens after infection, when 15 to 25 days old, with P. gallinaceum (blood-induced)
Mortalities
presents the mortalities that occurred when a total of 223 chickens, 15 to 25 days old, were infected in lots ranging from seven to 32 birds in 13 experiments. The mortality during each experiment resulting from the intravenous injection of one million parasitized erythrocytes varied from 26.7% to 93.3%, with an overall mean of 60.5%. The Pearson product-moment correlation coefficient (r) was −0.6475 (11 degrees of freedom [d.f.], P<0.02) for the association between age of bird lot when infected and percentage mortality; the older the birds, the lower the mortality. Deaths occurred from 5 to 10 days after infection, mostly during days 5 to 7. The overall mean time to death was 6.3 days, with a range of 5.1 to 7.5 days. The value of r was 0.6998 (11 d.f., P<0.01) for the association between age of bird lot when infected and mean time to death; the older the birds, the greater the mean time to death.
Age resistance of birds to malaria
Because of the statistically significant associations between age of birds when infected and mortality or time to death (), experiment 451 was carried out to investigate age resistance over a greater age range of birds. summarizes the mortalities of 119 chickens infected when they were between 7 and 84 days old. Analyses by a log-rank procedure confirmed the negative correlation between bird age when infected and percentage mortality (P<0.001) and the positive correlation between bird age when infected and mean time to death (P=0.008), although there was some departure from linearity in both cases.
Table 2. Mortalities of chickens after infection, when 7 to 84 days old, with P. gallinaceum (blood-induced) (experiment 451)
By combining results from and , the following data may be derived. When 20 birds were infected at 7 days old, their mortality was 80% and their mean time to death was 6.3 days. Similarly, data for 99 birds infected at 15 days old gave a mortality of 72.7% and a time to death of 5.8 days; 72 birds at 20 days old gave 63.9% mortality and 7.0 days to death; 42 birds at 21 days old gave 38.1% mortality and 7.1 days to death; 30 birds at 25 days old gave 36.7% mortality and 7.4 days to death; 20 birds at 35 days old gave 30.0% mortality and 7.5 days to death; 20 birds at 49 days old gave 10.0% mortality and 7.5 days to death; 20 birds at 63 days old gave 15.0% mortality and 9.0 days to death; and 19 birds at 84 days old gave 31.6% mortality and 7.8 days to death. When subjected to Spearman's rank correlation test, statistically significant associations (P<0.005, single-tailed tests) were revealed between bird age and the percentage mortality (negative correlation), between bird age and the mean time to death (positive correlation), and between the percentage mortality and the mean time to death (negative correlation).
Parasitaemia
presents the percentages of parasitaemia followed during development of disease in 35 chickens, 15 or 21 days old when infected. The patent period had generally begun by the third day after infection. In the 13 birds that survived, the peak of parasitaemia occurred 6 days after infection in 11 (85%) of them; on the seventh day in one (8%) bird and on the ninth day in another (8%). The mean peak of parasitaemia in survivors was 70.1%, ranging from 28% to 89%; or if the apparently atypical bird number 7 was excluded, the mean was 73.6%, ranging from 54% to 89%. Parasitaemia was reduced to<0.5% or zero by the ninth to 13th day after infection, except in the case of bird number 34 (17th day). The patent period was usually from 7 to 12 days long, but was 19 days in the case of bird number 30, and at least 25 days in the case of bird number 34.
Table 3. Parasitaemias (%) of chickens after infection, when 15 or 21 days old, with P. gallinaceum (blood-induced)
In the 22 birds that died, the highest level of parasitaemia occurred on the day of death in 20 (91%) of them, and on the day before death in two birds. In birds that died, the mean highest parasitaemia was 64.6%, ranging from 25% to 90%. If bird number 26, which died with an exceptionally low parasitaemia, was excluded, the mean was 66.4%, ranging from 47% to 90%. Hence, parasitaemias in birds that died were not significantly different from those in birds that did not die (t test corrected for unequal variances, P=0.34).
In experiment 439 (results not shown), designed to investigate the time of onset of parasitaemia and patent periods in 14-day-old chicks, patency began 1 day after infection in 10 out of 58 chicks (17%), on the second day in 34 chicks (59%), on the third day in eight chicks (14%), and after the third day in six chicks (10%). Mortality was 89.7%. The patent periods in the six surviving chicks ranged from 11 to 19 days, somewhat longer than previously observed (). There was no association between the day of onset of parasitaemia and the length of the patent period in the surviving chicks (Pearson product-moment correlation coefficient, r=−0.6606, 4 d.f., P>0.1).
Haematocrit values
presents the haematocrit values of the same 35 infected chickens as those presented in . For comparative purposes, a further 34 healthy control chickens of the same breeds and ages were kept under the same conditions, but they were not infected. Based on the results obtained from the uninfected controls, the normal haematocrit range was judged to be from 25% to 32% (Williams, Citation1986a). Nevertheless, 5% of single haematocrit readings from healthy chickens might be expected to fall outside that range. In the present experiments a value ≤24% was taken to be an indication of a severe malarial infection.
Table 4. Erythrocyte haematocrit values (%) of chickens after infection, when 15 or 21 days old, with P. gallinaceum (blood-induced)
Hence, from , in infected birds that survived, significant falls in haematocrit values occurred as early as 3 days after infection on one out of 13 occasions (8%); 4 days after infection, three times (23%); 5 days after infection, four times (31%); 6 days after infection, four times (31%); and 8 days after infection, once (8%). Values returned to normal (i.e. ≥25%) from 9 to 17 days after infection, but the day of return to normal was not correlated with the first day that a subnormal value was obtained; the total numbers of days on which a bird had subnormal values ranged from 4 to 17. The lowest haematocrit value occurred on the sixth day after infection in six out of 13 cases (46%); on the seventh day in four cases (31%); on the eighth day in one case (8%); and on the ninth day in two cases (15%). The mean lowest haematocrit value in survivors was 16.1%, ranging from 12% to 19%.
In birds that died, the lowest value occurred on the day of death in 18 out of 22 cases (82%), and on the day before death or earlier in four cases (18%). In three of those cases (bird numbers 9, 16 and 20), although the haematocrit value fell slightly, it never fell below normal; and in bird number 35 it did not decrease at all. The mean of the lowest values in fatal cases was 22.0%, ranging from 18% to 27%. Hence, haematocrit values of birds that died were significantly higher than those of birds that survived (t test corrected for unequal variances, P<0.0001).
Relating parasitaemias () to haematocrit values () in the 13 birds that survived the infection, the peak level of parasitaemia occurred on the same day as the lowest haematocrit value in seven cases (54%); on the day before in four cases (31%); and 2 days before in two cases (15%). In the birds that died (excluding numbers 16 and 35) the highest level of parasitaemia occurred on the same day as the lowest haematocrit value in 14 out of 20 cases (70%); on the day after in four cases (20%); and on the day before in two cases (10%).
Colonic temperatures
presents the colonic temperatures of the same 35 infected chickens as those presented in and . For comparison, 30 uninfected healthy birds were kept to establish the normal temperature under the same conditions and 510 readings were taken. Based on those data, abnormal temperatures were defined as those ≤41.1°C or ≥42.0°C (Williams, Citation1986b).
Table 5. Colonic temperatures (°C) of chickens after infection, when 15 or 21 days old, with P. gallinaceum (blood-induced)
In the 35 malarious birds () a transient, although definite, febrile response (i.e. ≥42.0°C) occurred in 23 cases (66%). In those 23 birds, fever began 3 days after infection in seven birds (30%); 5 days after in seven birds (30%); 6 days after in three birds (13%); 7 days after in three birds (13%); and 8 or more days after infection in three birds (13%). Of those 23 birds, 11 (48%) died. A further 11 birds died in the 12 cases in which fever did not occur. When fever occurred, it lasted 1 or 2 days only and was sometimes followed, often immediately, by an abnormally low temperature (i.e. ≤41.1°C) for 1 or 2 days if the bird survived long enough. Occasionally (3/22 birds, 14%), only the lower abnormal temperature, with no previous fever, was recorded immediately before a bird died. Of the 22 fatalities, 11 birds (50%) died on the day when their highest temperature was recorded, although only seven (32%) of them were febrile. Another seven (32%) died on the day when their lowest temperature was recorded, and four (18%) died on a day when neither their highest nor lowest temperature occurred.
Relating temperatures () and parasitaemias () in birds that died and in survivors, several associations may be considered. In 11 birds that had a fever and died, the highest temperature occurred on the same day (±1) as the highest parasitaemia in nine (82%) of them. In nine surviving birds that had abnormally high temperatures, the highest temperature occurred on the same day (±1) as the highest parasitaemia in all of them. Even in the 13 birds that did not develop a fever, the highest temperature occurred on the same day (±1) as the highest parasitaemia in 10 (77%) of them.
Production of green faeces
Chickens infected with P. gallinaceum produced green faeces in all experiments. Three phases were specifically identified in experiment 426. During phase I, which lasted only a few hours, the droppings were normally formed with a uric acid portion, and green pigment was confined to the faecal part. In droppings passed early in phase I, the colour was not very marked, but in those passed a little later its intensity was greater, and it tended to be absorbed by the uric acid portion. Phase II was characterized by a thin, mucoid, brilliant green diarrhoea, which lasted about 2 days if a bird survived the acute disease. Birds that died usually did so during phase I or phase II, but occasionally death occurred 1 or 2 days after phase II or, very rarely, just before phase I could commence. Phase III was observable only in birds that did not die; the droppings were produced normally formed, but with a green colour intermediate in intensity between that seen in phases I and II. Droppings began to be produced without green pigment when the parasitaemia became undetectable and the haematocrit value simultaneously returned to normal, or within a day or so either way.
The timing of the phases in seven other experiments involving 122 infected chickens between 14 and 27 days old was as follows. Phase I began almost invariably on the fifth or sixth day after infection. If deaths occurred during an experiment, the first birds succumbed on the day before green droppings were first observed in the survivors in two out of 25 cages (8%), and on the same day that green droppings were observed in 10 cages (40%). Other birds succumbed 1 day after green droppings were observed in 12 cages (48%), and 2 days after in just one cage (4%). In all cases, the percentage parasitaemia had been rising and the haematocrit value had been falling for between 1 and 4 days before any green droppings were produced.
Weight gains
In seven experiments, each lasting 2 weeks from the day of infection, the mean weight gain of infected chickens that survived was 66.2% (ranging from 48.9% to 82.4%) of that of the uninfected controls (). In six of these experiments, the differences between surviving infected and uninfected birds were statistically significant by analysis of variance. In the seventh experiment (number 423), because only one infected bird survived and the difference between the weight gains of infected and uninfected birds was only ∼18%, significance was not demonstrable.
Table 6. Weight gains during 14 days of chickens infected with P. gallinaceum (blood-induced) when 14 or 21 days of age
Feed conversion ratios
FCRs were calculated for cages of chickens (). If any birds died in a group during the 2 weeks over which the FCR was calculated, allowance was made for the food consumed prior to their deaths. Results were submitted to analysis of variance. Surviving infected birds usually had poorer (i.e. numerically higher) FCRs than the respective uninfected controls, and when the differences exceeded +20% they were statistically significant (). However, in three experiments, the difference between the FCRs was not significant. In one (experiment 418) the infected birds had a poorer FCR by only 12%, and in the others (experiments 419 and 423) the infected birds had unexpectedly better FCRs than the uninfected controls (), despite suffering high mortalities.
Table 7. FCRs during 14 days of chickens infected with P. gallinaceum (blood-induced) when 14 or 21 days of age
Cause of weight gain reduction (pair-feeding experiment)
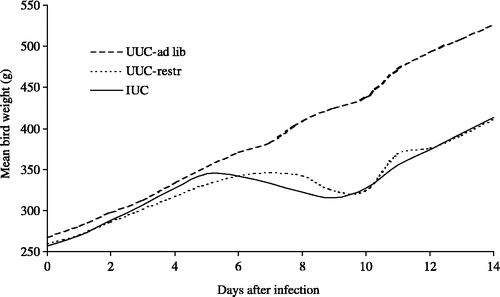
shows the daily weights for 14 days of healthy control birds (UUC-ad lib), infected birds (IUC) and pair-fed healthy birds (UUC-restr) in experiment 449. The treatments were compared by repeated measures analysis of variance. There was no statistically significant difference between the shape of the curves for the IUC and UUC-restr birds (P=0.195), leading to the conclusion that the weight gain reduction of infected birds was due solely to their reduced feed intake.
Figure 1. Growth curves, from 25 days of age, of healthy birds (UUC-ad lib), birds infected on day 0 with blood-induced P. gallinaceum (IUC), and pair-fed healthy birds (UUC-restr) (experiment 449).
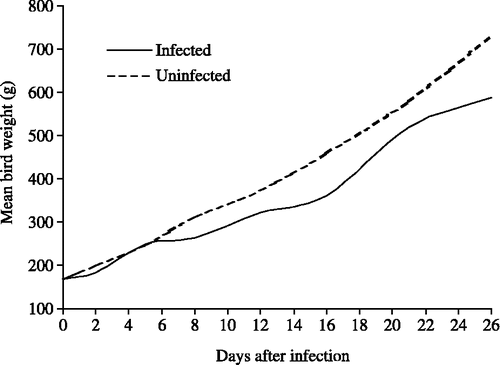
In another experiment (428), the growth rates of uninfected and infected birds were compared during 26 days (). In experiments 428 and 449, the growth rate of infected birds was similar to that of uninfected birds until 5 days after infection, when it slowed down. It reattained the same rate as the uninfected controls between 9 and 16 days after infection, but did not change subsequently, at least up to 26 days ().
Liver size
In experiment 428, the liver weights of surviving chickens, relative to bird body weight and relative to liver volume, were recorded at intervals following infection and were compared with uninfected birds (). Associated clinical data were also obtained (). The liver weight as a percentage of body weight in healthy uninfected birds steadily decreased from ∼3% to 2.29% during 26 days (Pearson product-moment correlation coefficient, r=−0.9115, 6 d.f., P<0.01), whereas in malarious birds it rose to a peak of 4.52% approximately 8 days after infection, decreasing thereafter to 2.92% by 26 days after infection. The peak coincided with the highest level of parasitaemia recorded and a correspondingly low haematocrit value (), and occurred as the weight gain of the infected birds was diverging from that of the healthy controls (). The mean specific gravities of the livers from infected chicks (1.086 overall) and uninfected chicks (1.094 overall) were closely similar throughout the 26 days after infection ().
Table 8. Liver parameters in uninfected or P. gallinaceum-infected chickens (experiment 428)
Table 9. Clinical data related to body weights for uninfected or P. gallinaceum-infected chickens (experiment 428)
Spleen size
Information on spleen sizes was also collected in experiment 428 (). The spleen weight as a percentage of body weight in uninfected birds changed little during 26 days, being around 0.17% overall. In infected birds it increased at first, reaching 1.60% after 12 days, then falling back to 0.84% after 26 days. The greatest percentage weight occurred 4 days after the peak parasitaemia and the corresponding beginning of the haematocrit trough (), and at the time when the growth rate of infected birds was just beginning to recover (). The mean specific gravities of the spleens from infected chicks (1.088 overall) and uninfected chicks (1.108 overall) were closely similar ().
Table 10. Spleen parameters in uninfected or P. gallinaceum-infected chickens (experiment 428)
Gall bladder size
presents the volumes of gall bladders of infected and uninfected chickens of various ages (experiment 428). There is a trend of increasing volume associated with the age of healthy uninfected birds (Pearson product-moment correlation coefficient, r=0.9468, 6 d.f., P<0.01). However, in birds of the same age that had been infected there was no such association (r=0.4972, 6 d.f., P>0.1). Comparing gall bladder volumes on each sampling day, those of infected birds rose to 208% of the size of those of healthy birds by 5 days after infection, peaking at 442% after 8 days, as the weight gain of the infected birds was diverging from that of the healthy controls (), and subsequently falling to 48% after 26 days. By 26 days after infection, the gall bladders of malarious birds had increased in volume by approximately 2.3 times, compared to an increase of approximately 4.8 times in uninfected birds. However, the maximum increase in volume for malarious birds was approximately 3.7 times, although this was not maintained.
Table 11. Gall bladder volumes (ml±standard error) of uninfected or P. gallinaceum-infected chickens (experiment 428)
Condition of visceral organs
presents the condition of the visceral organs of uninfected and infected birds in experiment 428. The subjective assessments of relative sizes of liver, spleen and gall bladder were in accord with the actual measurements presented in and and , respectively. The kidneys became slightly swollen by 5 days after infection, very swollen by day 12, and returned to slightly swollen by day 26. As for the liver, spleen and gall bladder, pathological changes in the kidneys occurred more or less concomitantly with the reduction in body weight gain (). There was a general tendency for the colour of organs to become darker as the disease developed. The colours of the liver and spleen were characteristic, the former becoming a bronzed chocolate-brown and the latter becoming a very dark slate-grey. Serous exudate in the pericardial sac was consistently observed.
Table 12. Condition of visceral organs a of uninfected or P. gallinaceum-infected chickens (experiment 428)
Chemical pathology
In experiment 428, the plasma proteins of uninfected and malarious chickens, sampled during 26 days after infection, were electrophoresed, and the densitometric patterns were compared and analysed ( and ). Usually, the following peaks occurred: pre-albumin, albumin, α2-globulin, β1-globulin, γ1-globulin and γ2-globulin. Occasionally, in some uninfected and some infected birds, a pre-albumin peak did not occur, or extra peaks designated α1-globulin, β2-globulin or γ3-globulin were seen ( and ). There was no apparent consistency in the occurrence of these additional peaks and so they were not included in the analyses of variance carried out for time and treatment effects on each protein. However, when they did occur, their mean RMIs were subjectively similar in uninfected and infected birds.
Table 13. Protein electrophoresis (mean RMIs of albumin and globulins) of plasma samples taken at intervals from uninfected chickens aged between 20 and 46 days (experiment 428)
Table 14. Protein electrophoresis (mean RMIs of albumin and globulins) of plasma samples taken at intervals from chickens aged between 22 and 46 days, infected with P. gallinaceum (blood-induced) when 20 days old (experiment 428)
There was no overall statistically significant difference between the mean RMIs of plasma proteins of the uninfected () and infected birds (), so the same proteins were apparently present in the plasma of birds in both groups. There was, however, a time effect (P<0.001) in the case of albumin only, but since the greatest deviation from the overall mean RMI occurred in the uninfected birds 5 days after the experiment was begun, when the deviation observed in the infected birds was rather less, it seems unlikely that this transient change was related to infection.
With regard to areas under peaks of plasma proteins, there were statistically significant differences between uninfected () and infected () birds in the cases of albumin (lower in infected birds, P<0.001), α2-globulin (lower in infected birds, P<0.001), γ1-globulin (higher in infected birds, P<0.001), and γ2-globulin (higher in infected birds, P<0.001). The time effect was significant at various levels for all of the proteins that were statistically analysed. Particularly important were the sharp fall in albumin in the infected birds 8 days after infection (P<0.001) and the subsequent linear increase up to day 26 (P<0.001). There was a simultaneous dramatic increase (day 8) in γ1-globulin in the infected birds (P<0.001), followed by a linear fall up to day 26 (P<0.001). There was also a slow rise in γ2-globulin, beginning on day 8 and sustained until day 26 in the infected birds (P<0.001). These changes in proportions of proteins were initiated as the growth rate of the infected birds was slowing (), but similar patterns were not apparent in the uninfected birds.
Table 15. Protein electrophoresis (mean % area under peaks of albumin and globulins) of plasma samples taken at intervals from uninfected chickens aged between 20 and 46 days (experiment 428)
Table 16. Protein electrophoresis (mean % area under peaks of albumin and globulins) of plasma samples taken at intervals from chickens aged between 22 and 46 days, infected with P. gallinaceum (blood-induced) when 20 days old (experiment 428)
Results of chemical analyses of the plasma samples from experiment 428 were subjected to analyses of variance carried out for time and treatment effects on each constituent. There were highly significant differences (P<0.001 to P=0.007) between concentrations of all constituents tested for in uninfected () and infected () birds, except creatinine and uric acid. The time effect was significant for all constituents (P<0.001), as were the treatment–time interactions for all constituents (P<0.001 to P=0.048) except glucose. The dramatic increases in concentrations of total protein, ASAT, GDH and GGT in the infected birds 8 days after infection were highly significant (P<0.001), as was the simultaneous reduction in creatinine (P<0.001). The changes in blood chemistry were associated with the slowing of growth rate in infected birds ().
Table 17. Chemical analyses (means±standard deviation) of plasma samples taken at intervals from uninfected chickens between 20 and 46 days old (experiment 428)
Table 18. Chemical analyses (means±standard deviation) of plasma samples taken at intervals from chickens aged between 22 and 46 days, infected with P. gallinaceum when 20 days old (experiment 428)
Long-term effects of blood-induced infections
In experiment 446, six birds that had been used for routine blood passage of infections were maintained for 149 to 184 days after infection. Two weeks after the acute disease that followed a blood-induced infection, all six birds had recovered and appeared healthy until the experiment was terminated. By that time all the birds had had undetectable parasitaemias for at least 93 to 132 days and their haematocrit values were between 35% and 50% (). Furthermore, contact smears taken from the brain, liver, lungs, heart, spleen and kidneys of each bird 149 to 184 days after infection contained no plasmodial parasites. Despite that, when a blood sample was taken from each bird 75 to 110 days after infection, and again 149 to 184 days after infection, and each sample was injected into a 12-day-old naïve bird, a mild parasitaemia developed in each recipient, except for the chick that received blood taken from bird number 2, 110 days after infection. However, blood from bird number 2 was infective 184 days after infection (). This showed that all six of the donor birds were still carrying viable infections for up to 184 days, although no clinical signs were apparent in any of them.
Table 19. Long-term effects, monitored for 132 days, on six 7-day-old chickens that survived infection with P. gallinaceum (blood-induced) (experiment 446)
presents the occurrence of parasites in visceral organs of 66 chickens that had survived a primary blood-induced infection, sampled from 1 to 41 days after infection (experiments 437 and 439). No parasites were detected up to 3 days after infection. From 4 to 11 days after infection, contact smears showed that parasites occurred only in the erythrocytes of blood from the organs. Exoerythrocytic schizonts became evident occasionally in all of the organs from day 13 onwards, being most frequently seen in the brain and to a lesser extent in the lungs. The brain was the most consistently and heavily infected, exhibiting large, irregularly elongated schizonts that had often released merozoites. The kidneys and spleen contained parasites the least frequently. From 28 to 41 days after infection, no parasites were found in any organ. None of these birds, which had survived the acute phase of disease during the first 9 days after infection in this experiment, died or appeared to be ill during the remaining period of observation.
Table 20. Parasites found in contact smears of organs of chickens after infection, when 14 or 20 days old, with P. gallinaceum (blood-induced) (experiments 437 and 439)
Acquired immunity
In experiment 448, acquisition of immunity was assessed by challenging 18 birds that had survived a primary blood-induced infection, together with 10 of the contemporaneous uninfected controls. All 28 birds were challenged with 106 parasitized erythrocytes 15 days after the primary immunizing infection was administered. Of the 18 survivors of the original infection, 10 had negative parasitaemias and eight birds had registered only a<0.5% parasitaemia the day before being challenged. The 10 original naïve controls all had negative parasitaemias. The results of the challenge are presented in , demonstrating that birds had developed a strong immunity after a single primary infection of 106 parasitized erythrocytes. None of the challenged immunized birds died, while 61% of them developed a parasitaemia of ≤0.5%, the remainder exhibiting no erythrocytic parasites. However, of the challenged naïve controls, all of them developed a parasitaemia of between 21% and 74%, and 30% of the birds died. All differences in numbers of birds dying or exhibiting parasitaemias between immunized and non-immunized birds following challenges were statistically significant (χ2 with Yates's correction, one-tailed test, P<0.05).
Table 21. Demonstration of acquired immunity by challenge of chickens 15 days after immunization with a single primary blood-induced infection of P. gallinaceum when 14 days old (experiment 448)
Discussion
This widely ranging study has established the provenance of the Wellcome line of strain 8A of P. gallinaceum, and has fully characterized its biological properties. Furthermore, it has provided a range of criteria that may be used to come to an accurate diagnosis of avian malaria, and on which experimental designs may be based for the assessment of potential avian antimalarial drugs. Quantification of the pathogenic effects of P. gallinaceum has also provided data that may be used in models (for example, Williams, Citation1999) to estimate potential economic losses due to avian malaria. Some critical issues and new findings are now discussed.
Standardization of the method of experimental infection with P. gallinaceum is crucial if results of different studies are to be compared. In the present experiments, in order to obtain consistent results, infections were standardized at one million parasitized erythrocytes per bird injected into a jugular vein. In many previous studies of blood-induced malaria, infections consisted of variable volumes of blood taken haphazardly, so that the actual numbers of infected erythrocytes administered varied considerably (for example, Das et al., Citation1952; Omar & Lim, Citation1962; Al-Dabagh, Citation1966). The effects of different sizes of inoculum of parasitized erythrocytes on various clinical signs of P. gallinaceum malaria have been examined by Permin & Juhl (Citation2002). They found that heavier infections led to increased severity of disease judging by clinical signs, mortality and pathological changes in organs, but not in terms of body weight gain.
The routes of infection used in previous studies have been various. For instance, Das et al. (Citation1952) used the intramuscular or subcutaneous route, Omar & Lim (Citation1962) used the intramuscular or intravenous route, and Al-Dabagh (1966) used the intraperitoneal, intramuscular, intravenous or subcutaneous route. Therefore, the numbers of parasites surviving injection into the host and the development of parasitaemias were probably extremely variable both within and between those experiments.
Other probable sources of variation among experimental studies are: the sex, age and breed of chicken; its diet; and its husbandry. Male chickens, as used in all the present experiments, are rather more resistant to P. gallinaceum than are females (Bennison & Coatney, Citation1948). Results herein ( and ) have shown that younger chickens are more susceptible than older ones, and that this is not related to prior exposure to infection. An age resistance has previously been recorded in chickens infected with P. gallinaceum (see Garnham, Citation1966), and has also been observed with malarias of other birds (van Riper et al., Citation1994). During the present studies, all birds were held at the same temperature during the acute phase of infection, because the severity of disease is known to vary with changes in ambient temperature (Mandarini-Galli et al., Citation1974).
Since the work described here was carried out in England, and furthermore no insect colonies were maintained in this laboratory, there was no risk of extraneous infections being accidentally introduced into the experimental chickens by indigenous or introduced vectors.
The present holistic approach to investigating the pathology of P. gallinaceum has facilitated a complete description of the course of the disease due to blood-induced infections. The general appearance of infected birds was in broad agreement with the observations of Crawford (Citation1945), Das et al. (Citation1952), Omar & Lim (Citation1962) and Omar (Citation1968), although the timing of the course of infection was rather variable among those studies. Based on new results herein, the usual course of blood-induced infections () and the quantitative effects may be summarized as follows. In birds approximately 2–3 weeks old, mortality of ∼60% (range 27% to 93%) occurred, mainly between 5 and 7 days after infection (). The older the birds, the lower the mortality and the longer the time to death. Death frequently occurred on the day of peak parasitaemia. Peak parasitaemias averaged ∼65% in birds that died, ∼70% in birds that recovered, and ∼66% overall (). The onset of parasitaemia occurred usually by the third day after infection, confirming the observation of Lumsden (Citation1989) that the prepatent period after a blood-induced infection is 2.75 days, although Crawford (Citation1945) found it to be 6 to 9 days. In surviving birds, parasitaemia was usually patent for approximately 7 to 19 days ( and experiment 439). Both in birds that survived or succumbed, the lowest haematocrit values and the highest colonic temperatures frequently coincided with the highest parasitaemias ( –).
Figure 3. Diagrammatic representation of the relative timing of clinical signs of P. gallinaceum malaria in chickens following a blood-induced infection, presented as positive and negative deviations from the baseline conditions in healthy birds.
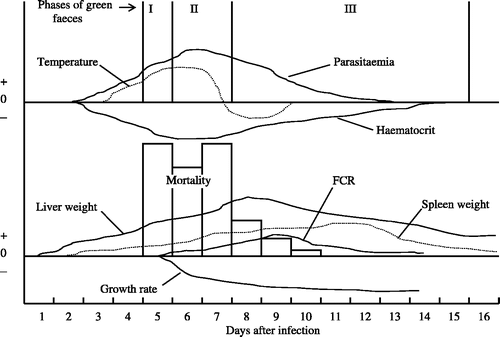
A febrile response (from 42.0°C to 43.3°C), which was weak and transient, lasting only 1 or 2 days, occurred in 66% of birds, and was sometimes followed by an abnormally low temperature (between 39.8°C and 41.1°C) for another 1 or 2 days. Birds were slightly more likely to die on the day of their highest temperature than on the day of their lowest (). A thin, mucoid, brilliant green diarrhoea commenced 2 or 3 days after parasitaemia first became detectable. In birds that did not succumb, this colour disappeared from the droppings when the parasitaemia became negative and the haematocrit value simultaneously returned to normal. The normal haematocrit values in healthy young male birds of those breeds used here ranged from 25% to 32% (Williams, Citation1986a), rather more limited than that recorded for adult fowl by Freeman (Citation1971a) of 20% to 40%, and unexpectedly falling within the range of 20% to 30% given for female chickens (Freeman, Citation1984)—while being quite distinct from the range of 40% to 45% of males (Freeman, Citation1984). The difference may be due to an age factor. This hypothesis is supported by the fact that the six recovered cockerels used in experiment 446 had a mean haematocrit of 42% (ranging from 35% to 50%) when aged from 156 to 191 days ().
The disease had a marked effect on bird weights, the mean weight gains of survivors being ∼66% of uninfected birds 14 days after infection (). Such a weight gain reduction has previously been remarked upon only by Das et al. (Citation1952) and Permin & Juhl (Citation2002), but no statistical significances were demonstrated in their studies. In the present study, malarious birds had not attained the same weights as uninfected controls between 14 and 26 days after infection ( and ), which is in accord with the results of Permin & Juhl (Citation2002), although they found that by 40 days after infection malarious birds were of similar weights to uninfected controls. FCRs have not previously been reported for malarious birds; here, the mean FCR of infected birds was 2.514, compared with 2.216 for uninfected birds during 14 days ().
During the course of infection, liver weight as a percentage of body weight increased to ∼4.5% 8 days after infection, while in uninfected birds it remained at ≤3% up to 26 days after infection (). Spleen weight rose to ∼1.6% of body weight by 12 days, remaining at ∼0.17% in uninfected birds (). The densities of livers and spleens in infected and uninfected chicks were ∼1.09 throughout the experiments ( and ). The failure to observe the eyelid lesions described by Al-Dabagh (Citation1961) may have been due to the fact that the diet (Williams, Citation1996) fed to these birds was more than adequate in pantothenic acid content (National Research Council, US, Citation1984).
Birds that survived the first 2 weeks after infection regained their health, and became immune to clinical malaria (see ). However, as long as 184 days after a primary infection, birds carried parasites capable of infecting a naïve bird injected with a sample of their blood (). No parasites were detected in smears from major visceral organs sampled between 28 and 184 days after infection ( and ).
Effects of various avian malarias on host body temperatures have long been controversial. Seed & Manwell (Citation1977) stated categorically that, except for pigeons with Plasmodium pinottii malaria, no avian species suffers a rise in body temperature resulting from a malarial infection. Crawford (Citation1945) and Neveu-Lemaire (Citation1952) denied that temperature elevation occurs in chicks infected with P. gallinaceum. Omar & Lim (Citation1962) also observed no febrile responses in their experiments with P. gallinaceum. Rao et al. (Citation1951) were rather unclear about whether body temperature was elevated with this disease, but stated that a fall of temperature (sometimes to subnormal) occurred before death, and Levine (Citation1985) simply stated, with no supporting data, that body temperature fluctuates. Hayworth et al. (Citation1987), with regard to Plasmodium relictum infections in canaries, found that a febrile response definitely did not occur, but that birds that died had subnormal temperatures. Das et al. (Citation1952) recorded slightly elevated temperatures for 9 days in response to P. gallinaceum infection, after which recovery was usual unless the temperature then fell dramatically, which often portended death. However, no control data for normal temperatures were presented, and the authors stated that generally P. gallinaceum causes an afebrile disease.
During the present study, controls were included and it was established that the normal temperature range for the male birds used is from 41.2°C to 41.9°C (Williams, Citation1986b). This agrees closely with the range of 41.0°C to 42.0°C for adult birds given by Freeman (Citation1983). In chickens suffering from malaria during the present studies, colonic temperatures of up to 43.3°C were recorded (). Supernormal temperatures were not invariably associated with mortality, however. It is unlikely that high body temperature was the direct cause of death, since the upper lethal temperature of the fowl is>45.0°C (Freeman, Citation1971b). Likewise, the subnormal body temperatures recorded, as low as 39.8°C, were not always associated with mortality. Such temperatures are well above the lower lethal body temperature of approximately 20°C (Freeman, Citation1971b). Adequate control data (Williams, Citation1986b) have now facilitated the first reliable report of a febrile response to P. gallinaceum.
Little attention has previously been paid to the significance of the green faeces produced by chickens infected with P. gallinaceum. The green diarrhoea consistently shown here to be characteristic of malarious birds was also observed by Crawford (Citation1945), Omar & Ismail (Citation1962), Omar & Lim (Citation1962) and Omar (Citation1968), but its cause was not considered. Williams (Citation1985) identified the predominant pigment as biliverdin; in the published coloured plate, the printer has rendered the green pigment much paler than it really is. The green colouration of faeces is not pathognomonic for avian malaria. A similar effect is caused by fowl typhoid (Buxton & Fraser, Citation1977), Doyle's form of Newcastle disease (Albiston & Gorrie, Citation1942), spirochaetosis (Gross, Citation1978), fowl cholera (Heddleston & Rhoades, Citation1978) and leucocytozoonosis (Supekar, Citation1986). Williams (Citation1985) concluded that green droppings produced by chickens are a sign of any disease that causes secondary anaemia. However, their occurrence, together with the characteristic parasitaemia, provides a clear and rapid diagnosis of field outbreaks of malaria in chickens, although the disease would be well advanced in some individuals. The timing of the appearance of green droppings in relation to the course of disease is shown in .
Fallon et al. (Citation2003), using a polymerase chain reaction method of detecting malarial infections of ∼105 parasites in wild birds, concluded that prevalences are higher than previously documented and that blood smears tend to underestimate the extent of parasitism. Nevertheless, the primers used did not reveal all infections detected by other polymerase chain reaction methods and by blood smears, so no assay for avian blood parasites is universally reliable. Although individual malarious birds already producing green droppings may be past saving, initiation of appropriate chemotherapy immediately that green diarrhoea is observed may prevent other birds in earlier stages of malaria from dying.
The significant adverse effects of P. gallinaceum infection on the weight gain and FCR of chickens are demonstrated in and . Clearly, in commercially produced chickens these would be economically important effects in the surviving birds (Williams, Citation1999). A pair-feeding experiment indicated that the reduction of weight gain in malarious birds was due solely to anorexia (). This is to be expected in a parasitic infection that does not involve the intestine (Symons, Citation1985). Although water consumption was not measured, it is most probable that, as with other chicken diseases, it was reduced proportionally with feed intake (Williams, Citation1996), thus contributing to the reduction in weight gain, and adversely affecting digestion, hence the poor FCR.
The range in liver weight as a percentage of body weight of uninfected birds between 20 and 46 days of age was 2.29% to 3.07%, with that of malarious birds attaining a 1.7-fold increase over the controls 8 days after infection (). The results from the healthy controls were similar to those obtained by Al-Dabagh & Abdulla (Citation1963), who recorded a range of 2% to 5% in birds aged 1 to 60 days. In malarious birds aged 22 to 46 days, the range observed here was 2.92% to 4.52% (), compared with 2.8% to 7.6% in the birds aged 6 to 34 days studied by Al-Dabagh (Citation1966). However, the specific gravities of livers in the present study, both in healthy and malarious birds, ranged from 1.060 to 1.168, whereas calculations based on the results of Al-Dabagh & Abdulla (Citation1963) give a range from 0.846 to 1.055 (mean, 0.9705) in uninfected birds, and the results of Al-Dabagh (Citation1966) reveal a range of 0.897 to 1.111 (mean, 1.002) for infected birds. Since livers do not float in water, it is probable that the results of Al-Dabagh & Abdulla (Citation1963) and Al-Dabagh (Citation1966) were inaccurate, most probably with regard to the liver volumes. The method used in the present study is more accurate than reading volumes directly from a graduated cylinder, particularly for smaller volumes.
In uninfected birds, the mean spleen weight as a percentage of body weight ranged from 0.12% to 0.21%, which is in close agreement with the results of Wolfe et al. (Citation1962) who observed a value of 0.2% in 10-week-old birds, and of Al-Dabagh & Abdulla (Citation1963) who recorded a range of 0.09% to 0.21%. The mean spleen weight as a percentage of body weight of malarious birds infected at 20 days of age attained a 9.4-fold increase compared with the uninfected controls 12 days after infection (). In malarious birds aged 22 to 46 days, the range observed here was 0.24% to 1.6% (), compared with 0.23% to 1.4% in the birds aged 6 to 34 days studied by Al-Dabagh (Citation1966). The specific gravities of spleens in uninfected and infected chicks in the present study ranged from 1.046 to 1.292, whereas calculations based on the results of Al-Dabagh & Abdulla (Citation1963) for uninfected chicks give a range from 0.857 to 1.500 (mean, 1.0769), and previous results for infected birds (Al-Dabagh, Citation1966) reveal a range of 0.75 to 1.201 (mean, 0.922). As for the data on specific gravities of livers, Al-Dabagh & Abdulla (Citation1963) and Al-Dabagh (Citation1966) apparently presented some inaccurate results, probably again due to methodological errors in organ volume measurement.
As pointed out by Seed & Manwell (Citation1977) and van Riper et al. (Citation1994), the hepatosplenomegaly in malaria is due to hypercellularity rather than oedema. The similar specific gravities of livers and of spleens (∼1.09), both of infected and uninfected birds, in the present study are consistent with this fact.
The pathological changes in visceral organs () observed here are in general accord with those documented by Permin & Juhl (Citation2002), who used the same route of infection and the same infective dose for their group A as in the present experiments. The general darkening of organ colours was a constant observation, although the specific colours recorded herein are slightly different from those described by Permin & Juhl (Citation2002). However, it is rather surprising that the maximum increases in size of the spleen and gall bladder occurred very much later after infection (28 and 14 to 28 days, respectively) in the experiments of Permin & Juhl (Citation2002) than in the present study (12 and 8 days, respectively). The timing of the swelling of kidneys, however, was similar in both cases.
Little has previously been published on the chemical pathology of P. gallinaceum. Examination of plasma proteins suggested that there were no major changes in the presence of particular types of component during 26 days after infecting birds, and the RMIs of those components were closely similar in healthy and malarious birds ( and ). However, infection appeared to cause various changes in the proportions of albumin and globulin fractions ( and ). The coincidence in the infected chicks of the sharp fall in the proportion of albumin and the sudden increase in γ1-globulin, together with the initiation of the slow rise in γ2-globulin, all occurring on day 8 after infection, and statistically significant, was particularly notable (). There was no difference between the contributions of β-globulins to the total protein in healthy and malarious birds (cf. and ). These results confirm those of Rao & Cohly (Citation1953) on P. gallinaceum.
The occurrence of α1-globulin peaks was less frequent in infected birds (cf. and ), and also α2-globulin contributed statistically significantly less to the plasma proteins than in uninfected birds (cf. and ). However, Rao & Cohly (Citation1953) observed no marked changes in α1-globulin and α2-globulin. Sherman & Hull (Citation1960) recorded significant depression of albumin and rises in β-globulins and γ-globulins, but no change in α-globulin during the crisis period of Plasmodium lophurae infections in chickens. Neither Rao & Cohly (Citation1953) nor Sherman & Hull (Citation1960) observed any pre-albumin peak in uninfected or malarious chickens, but a small pre-albumin peak was seen in uninfected and malarious birds in the present study. Schinazi (Citation1957) recorded a pre-albumin peak in pigeons infected with P. relictum.
Chemical analyses of the plasma revealed marked differences between concentrations of all constituents tested for in uninfected () and infected () chicks, except in the cases of creatinine and uric acid. The dramatic and statistically significant increases in total protein, ASAT, GDH and GGT in birds 8 days after infection () probably reflected the interactions between pathology during the acute phase of disease and the immune responses to it. It appears that the increase in total protein () may have been largely due to the rises in γ-globulins (), which were probably antibody responses to the tissue damage indicated by the plasma enzyme increases (). It is significant that the levels of γ-globulins remained elevated long after the crisis period was passed (cf. and ). Although Rao & Cohly (Citation1953) also observed rises in γ-globulins, they recorded a 34% decrease in total protein. Seed & Kreier (Citation1972) found, in common with the present results, that total protein and urea increased, and glucose decreased, in birds infected with P. gallinaceum.
Acquired immunity to P. gallinaceum is of great interest with regard to field outbreaks in commercial birds. While a marked protection against challenge resulted from a single infection, it was not a sterile immunity (). Birds that survived an acute blood-induced infection and went on to develop exoerythrocytic parasites in the visceral organs survived for at least 41 days (), and even as long as 184 days (). Permin & Juhl (Citation2002) similarly observed exoerythrocytic stages in all organs examined from 14 to 28 days after infection, and only in the kidneys up to 35 days; but from then until 49 days they saw no more parasites. Those authors considered that this was due to a possible cell-mediated immunity against parasite invasion of the visceral organs, and that the infection remained confined to the erythrocytes thereafter (cf. Garnham, Citation1966). The present results support that hypothesis. Naïve recipients of blood taken from birds infected between 75 and 184 days previously developed parasitaemias (), despite the donor birds having no detectable parasitaemias when their blood was sampled. No parasites were found in their visceral organs sampled between 149 and 184 days after infection (). Furthermore, in birds sampled daily, detectable parasitaemias were rarely observed much beyond 14 days after infection ( and ), nor were exoerythrocytic parasites found in any organs later than 27 days after infection ( and ).
Such observations confirm the conclusions of Garnham (Citation1966) and Permin & Juhl (Citation2002), but they may not necessarily be relevant to natural conditions in the field. For instance, Al-Dabagh (Citation1966) has pointed out that exoerythrocytic schizonts occur relatively rarely following blood-induced infections, while after sporozoite-induced infections they may be seen in massive numbers. It is a moot point, therefore, whether the long-term survival of chickens observed in the present study is due solely to the chickens’ immune responses, or to the low numbers of exoerythrocytic stages accruing after the blood-induced infections. Fowl infected by mosquitoes in the field, if they survive the acute anaemia of the initial infection, may succumb eventually to the blockage of capillaries in the brain by exoerythrocytic schizonts (Garnham, Citation1966).
The findings of the present study should be valuable for the selection of criteria for drug efficacy testing, bearing in mind that male chickens, as used in all these experiments, are more resistant to P. gallinaceum than are females (Bennison & Coatney, Citation1948). Although P. gallinaceum has been widely used as a tool for screening potential human antimalarial agents (Peters, Citation1974; Richards, Citation1984), it has not necessarily given results that are in accord with those obtained by using mammalian plasmodia such as Plasmodium berghei in mice. For instance, the second most potent blood schizonticide of 10 quinolones tested against P. berghei exhibited no activity at all against P. gallinaceum using the same sole criterion (Ryley & Peters, Citation1970). Clearly then, P. gallinaceum used in the chicken host is the ideal model for identifying potential antimalarial compounds for veterinary use in commercial fowl. The present study has provided materials from which criteria of potency may be selected for screening or secondary testing of drugs, following intravenous infection with 106 parasitized erythrocytes. The use of the present model for veterinary chemotherapeutic studies will be addressed in a subsequent paper.
The author is grateful to Mrs J. Brackpool, Mr D.S. Brackpool and Mrs R.G. Redding for skilled technical assistance, and to Miss K. Legge and Miss C. Daly for some of the statistical analyses. Dr W.H.G. Richards, Dr L.G. Goodwin, the late Prof. P.C.C. Garnham and the late Dr Ann Bishop all gave valuable help and advice in establishing the provenance of the parasite strain used in this study. The Wellcome Research Laboratories have been subsumed by Schering-Plough Animal Health, whom the author thanks for permission to publish this work.
References
- Albiston , HE and Gorrie , CJR . (1942) . Newcastle disease in Victoria . Australian Veterinary Journal , 18 : 75 – 79 .
- Al-Dabagh , MA . (1961) . Eyelid lesions in chicks infected with Plasmodium gallinaceum . Transactions of the Royal Society for Tropical Medicine and Hygiene , 55 : 351 – 354 .
- Al-Dabagh MA (1966) Mechanisms of Death and Tissue Injury in Malaria with Special Reference to Five Species of Avian Malaria Baghdad Shafik Press
- Al-Dabagh , MA and Abdulla , M . (1963) . Correlation of sizes and weights of livers and spleens to the ages and bodyweights of normal chicks with a note on the histology of these organs in chicks . Veterinary Record , 75 : 397 – 400 .
- Bennison , BE and Coatney , GR . (1948) . The sex of the host as a factor in Plasmodium gallinaceum infections in young chicks . Science, New York , 107 : 147 – 148 .
- Brumpt , E . (1935) . Paludisme aviaire: Plasmodium gallinaceum n. sp. de la poule domestique . Compte Rendu Hebdomadaire des Séances de l'Académie des Sciences, Paris , 200 : 783 – 786 .
- Brumpt E (1949) Précis de Parasitologie I 6th edn Paris Masson
- Buxton A Fraser G (1977) Animal Microbiology 1 (pp. 111–113) Oxford Blackwell
- Crawford , M . (1945) . Plasmodium gallinaceum, a malarial parasite of the domestic fowl . Veterinary Record , 57 : 395 – 396 .
- Das , J , Rao , SBV and Ramnani , DR . (1952) . Studies on Plasmodium gallinaceum . Indian Veterinary Journal , 29 : 14 – 26 .
- Fallon , SM , Ricklefs , RE , Swanson , BL and Bermingham , E . (2003) . Detecting avian malaria: an improved polymerase chain reaction diagnostic . Journal of Parasitology , 89 : 1044 – 1047 .
- Freeman BM (1971a) The corpuscles and physical characteristics of blood In D.J. Bell & B.M. Freeman (Eds.) Physiology and Biochemistry of the Domestic Fowl 2 (pp. 841–852) London Academic Press
- Freeman BM (1971b) Body temperature and thermoregulation In D.J. Bell & B.M. Freeman (Eds.) Physiology and Biochemistry of the Domestic Fowl 2 (pp. 1115–1151) London Academic Press
- Freeman BM (1983) Body temperature and thermoregulation In B.M. Freeman (Ed.) Physiology and Biochemistry of the Domestic Fowl 4 (pp. 365–377) London Academic Press
- Freeman BM (1984) Appendix: biochemical and physiological data In B.M. Freeman (Ed.) Physiology and Biochemistry of the Domestic Fowl 5 (pp. 407–424) London Academic Press
- Garnham PCC (1966) Malaria Parasites and Other Haemosporidia Oxford Blackwell
- Gross WB (1978) Spirochaetosis In M.S. Hofstad, B.W. Calnek, C.F. Helmboldt, W.M. Reid & H.W. Yoder (Eds.) Diseases of Poultry 7th edn (pp. 330–334) Ames Iowa State University Press
- Hayworth , AM , van Riper , C and Weathers , WW . (1987) . Effects of Plasmodium relictum on the metabolic rate and body temperature in canaries (Serinus canarius) . Journal of Parasitology , 73 : 850 – 853 .
- Heddleston KL Rhoades KR (1978) Avian pasteurellosis In M.S. Hofstad, B.W. Calnek, C.F. Helmboldt, W.M. Reid & H.W. Yoder (Eds.) Diseases of Poultry 7th edn (pp. 181–207) Ames Iowa State University Press
- Joyner , LP , Canning , EU , Long , PL , Rollinson , D and Williams , RB . (1978) . A suggested terminology for populations of coccidia (Eimeriorina), particularly of the genus Eimeria (Protozoa: Apicomplexa) . Parasitology , 77 : 27 – 31 .
- Levine ND (1985) Veterinary Protozoology Ames Iowa State University Press
- Lumsden , WHR . (1989) . Sweating it out . British Medical Journal , 299 : 1341
- Mandarini-Galli , L , Brambilla , E and Sacchi , L . (1974) . Influenza della temperatura ambientale sul decorso della infezione da Plasmodium gallinaceum nel pulcino di pollo domestico . Rivista di Parassitologia , 35 : 253 – 260 .
- McGhee , RB . (1949) . Pre-erythrocytic development of Plasmodium gallinaceum in avian embryos . Journal of Infectious Diseases , 84 : 105 – 110 .
- McGhee , RB , Singh , SD and Lushbaugh , WB . (1977) . Plasmodium gallinaceum: changing virulence patterns of malaria parasites during adaptation from neonate chick to chicken embryos . Experimental Parasitology , 43 : 220 – 230 .
- National Research Council US (1984) Nutrient Requirements of Poultry 8th edn Washington DC National Academy Press
- Neveu-Lemaire M (1952) Précis de Parasitologie Vétérinaire 3rd edn Paris Vigot Frères
- Omar , AR . (1968) . Haemoprotozoan infections of poultry in Malaysia . Kajian Veterinar , 1 : 109 – 124 .
- Omar , AR and Ismail , Y . (1962) . Plasmodium gallinaceum infection among chickens in Malaya . Journal of the Malayan Veterinary Medical Association , 3 : 75 – 80 .
- Omar , AR and Lim , SY . (1962) . Experimental Plasmodium gallinaceum infection in chickens of indigenous Malayan breed . Journal of the Malayan Veterinary Medical Association , 3 : 81 – 104 .
- Permin , A and Juhl , J . (2002) . The development of Plasmodium gallinaceum infections in chickens following single infections with three different dose levels . Veterinary Parasitology , 105 : 1 – 10 .
- Peters , W . (1974) . Recent advances in antimalarial chemotherapy and drug resistance . Advances in Parasitology , 12 : 69 – 114 .
- Raether W (1988) Chemotherapy and other control measures of parasitic diseases in domestic animals and man In H. Mehlhorn (Ed.) Parasitology in Focus (pp. 739–866) Berlin Springer-Verlag
- Rao , RR and Cohly , MA . (1953) . Micro-electrophoretic study of serum proteins from normal and malarial chicken (infected with Plasmodium gallinaceum) . Current Science, Bangalore , 22 : 204 – 205 .
- Rao , SBV , Das , J and Ramnani , DR . (1951) . Fowl malaria . Indian Veterinary Journal , 28 : 99 – 101 .
- Richards WHG (1969) Antigenic studies of the class Sporozoa with particular reference to species of Plasmodium Ph.D. thesis University of London
- Richards WHG (1984) Use of avian malarias (in vivo) In W. Peters & W.H.G. Richards (Eds.) Antimalarial Drugs I (pp. 207–224) Berlin Springer-Verlag
- Ryley , JF and Peters , W . (1970) . The antimalarial activity of some quinolone esters . Annals of Tropical Medicine and Parasitology , 64 : 209 – 222 .
- Schinazi , LA . (1957) . Observations on a fast-moving protein in avian malarial serum . Science, New York , 125 : 695 – 696 .
- Seed TM Kreier JP (1972) Plasmodium gallinaceum: erythrocyte membrane alterations and associated plasma changes induced by experimental infections Proceedings of the Helminthological Society of Washington 39 (special issue) 387 411
- Seed TM Manwell RD (1977) Plasmodia of birds In J.P. Kreier (Ed.) Parasitic Protozoa III 1st edn (pp. 311–357) London Academic Press
- Shadduck JA Pakes SP (1978) Protozoal and metazoal diseases In K. Benirschke, F.M. Garner & T.C. Jones (Eds.) Pathology of Laboratory Animals II (pp. 1587–1696) New York Springer-Verlag
- Sherman , IW and Hull , RW . (1960) . Serum alterations in avian malaria . Journal of Protozoology , 7 : 171 – 176 .
- Supekar , PG . (1986) . Leucocytozoonosis . Poultry International , 25 ( 13 ) : 48 – 50 .
- Symons , LEA . (1985) . Anorexia: occurrence, pathophysiology, and possible causes in parasitic infections . Advances in Parasitology , 24 : 103 – 133 .
- van Riper C Atkinson CT Seed TM (1994) Plasmodia of birds In J.P. Kreier (Ed.) Parasitic Protozoa 7 2nd edn (pp. 73–140) San Diego Academic Press
- Williams , RB . (1985) . Biliverdin production in chickens infected with the malarial parasite Plasmodium gallinaceum . Avian Pathology , 14 : 409 – 419 .
- Williams , RB . (1986a) . Packed cell volume of blood from male domestic chicks . British Poultry Science , 27 : 483 – 485 .
- Williams , RB . (1986b) . Body temperatures of chicks of the domesticated fowl (Gallus gallus) . Zootecnica International , 1986 ( 10 ) : 61
- Williams RB (1996) The ratio of the water and food consumption of chickens and its significance in the chemotherapy of coccidiosis Veterinary Research Communications 20 437−447 [see also publisher's erratum: Veterinary Research Communications, 21, 69]
- Williams , RB . (1999) . A compartmentalised model for the estimation of the cost of coccidiosis to the world's chicken production industry . International Journal for Parasitology , 29 : 1209 – 1229 .
- Wolfe , HR , Sheridan , SA , Bilstad , NM and Johnson , MA . (1962) . The growth of lymphoidal organs and the testes of chickens . Anatomical Record , 142 : 485 – 493 .