Abstract
The 99323 Egyptian isolate of infectious bursal disease (IBD) virus (IBDV) was identified during an international survey of acute IBD cases. Its unique antigenicity was characterized by a markedly reduced binding of neutralizing monoclonal antibodies 3, 4, 5, 6, 8 and 9 in an antigen-capture enzyme-linked immunosorbent assay. Nucleotide sequencing of the genome region encoding the VP2 major immunogenic domain in 99323 revealed amino acid changes occurring at positions critical for antigenicity, but phylogenetic analysis demonstrated that 99323 was related to typical, very virulent IBDV (e.g. isolate 89163). Protection experimentally afforded by an antigenically classical live IBD vaccine was investigated in specific pathogen free chickens challenged with 99323 or 89163. Both viruses were similarly controlled, as evaluated by clinical signs, growth retardation, bursa-to-body weight ratios and histological lesions of the bursa after challenge. These results document that an active antibody response to a classical live antigen may clinically control infection by an antigenically atypical very virulent IBDV.
1 Introduction
Infectious bursal disease (IBD) virus (IBDV) of chickens belongs to the genus Avibirnavirus in the family Birnaviridae, which groups non-enveloped icosahedral viruses with a bi-segmented double-stranded RNA genome (Pringle, Citation1999). Two IBDV serotypes have been defined on the basis of the lack of cross-neutralization (McFerran et al., Citation1980). Serotype 1 IBDV replicates in and induces lysis of the IgM-bearing B lymphocytes (Hirai et al., Citation1981), mainly in the bursa of Fabricius, thus causing a major fatal or immunosuppressive condition in young chickens. The disease has been recognized worldwide since the mid-1970s (Cosgrove, Citation1962 Citation; for a review, see Lasher & Shane, Citation1994). Although both live and inactivated vaccines have been developed to control IBD in intensively grown poultry, difficulties may occur in implementing these vaccines in combination with efficient sanitary measures under field conditions. Such difficulties may explain why IBD-induced immunosuppression is still frequently encountered and represents a major threat to the control by vaccination of other infectious diseases affecting intensively grown poultry (Lasher & Shane, Citation1994).
Two major events have marked the past two decades of IBD epidemiology. The first was the demonstration of an antigenic drift among serotype 1 viruses. Indeed, since 1984, several generations of IBDV isolates gradually accumulating antigenic changes have been identified in the US (Snyder et al., Citation1988). These viruses induced little if any acute signs of infection, but they had a marked immunosuppressive potential, all the more as they were able to infect chickens with maternally derived antibody levels that would normally protect them against IBDV strains with a classical antigenicity (Müller et al., Citation1992). These viruses have been referred to as “variant” IBDV. It has been proposed that they emerge because escaping the vaccine-induced immunity confers to them a selective advantage, as compared with the “older” classical viruses (Snyder et al., Citation1992). To cope with the emergence of the variant viruses, the poultry industry has tried to adapt the antigenic content of the IBD vaccines in the affected areas (Müller et al., Citation1992).
The second major epidemiological event occurred in Europe in 1987, when serotype 1 IBDV isolates with an increased pathogenicity—now known as the “very virulent” (vv) IBDVs—emerged (Chettle et al., Citation1989; for a review, see van den Berg, Citation2000). These viruses caused mortality at least twice as high as that induced by the classical serotype 1 viruses (van den Berg et al., Citation1991; Eterradossi et al., Citation1992). It has been shown that some Ivorian isolates excepted (Eterradossi et al., Citation1999), the vvIBDV isolates share close genetic relationships in both genome segment A (which encodes IBDV capsid proteins) (Brown et al., Citation1994) and genome segment B (which encodes virus polymerase) (Islam et al., Citation2001). Some nucleotide (nt) and the resulting amino acid (aa) changes occurring in segment A have been proposed as the basis for diagnostic tests for the putative vvIBDV, either on an antigenic basis (Eterradossi et al., Citation1997a,bCitation) or on a molecular basis (Jackwood & Sommer, Citation1999; Zierenberg et al., Citation2001). However, recent progress in the reverse genetics of IBDV (Mundt & Vakharia, Citation1996) have allowed the demonstration that VP2 alone is not responsible for vvIBDV increased pathogenicity (Boot et al., Citation2000). Hence, it is not clear whether the conserved features among vvIBDV should be considered as pathogenicity markers, or merely as an indication that all vvIBDVs, which have now spread almost worldwide, North-America, Australia and New-Zealand excepted (for a review, see van den Berg, Citation2000), may have a clonal origin.
The emergence of variant and vvIBDV has caused considerable concern regarding the vaccine control of IBD. As far as vvIBDVs are concerned, isolates collected in Europe in the late 1980s were shown to be clinically controlled by live vaccines with a classical antigenicity (van den Berg & Meulemans, Citation1991; Eterradossi et al., Citation1992). In order to check whether an antigenic drift could occur among vvIBDVs and lead to the emergence of vvIBDV with an atypical antigenicity, the authors have been engaged since 1999 in a worldwide survey of IBDV isolates collected during acute IBD outbreaks. In this paper, we report on the isolation of vvIBDV isolate 99323, which exhibits extensive antigenic changes, and we describe the experimental evaluation of the clinical protection afforded against this IBDV isolate by a live IBD vaccine with a classical antigenicity. The results suggest that active immunization with a classical live IBD vaccine may clinically control the infection by the antigenically modified vvIBDV.
2 Materials and Methods
2.1 Viruses
The 99323 isolate was kindly provided by Prof. I. Reda, with the authorization of the then OIE delegate for Egypt, Dr A.A.A. Aidaros. It was obtained as a bursal homogenate prepared from samples collected in 1999 in Egypt (Giza province), from a broiler flock that exhibited signs of acute IBD at 23 days of age. The original 99323 homogenate was inoculated into 5-week-old specific pathogen free (SPF) White Leghorn chickens in the containment facilities (negative pressure, filtered air) of AFSSA-Ploufragan. Bursae collected 4 days post-inoculation were used as described previously (Eterradossi et al., Citation1992) to prepare a stock virus that was used in the subsequent steps of the present study.
The 89163 virus was isolated in France in 1989 from broilers with acute IBD. The 89163 virus was initially cloned by limiting dilution in SPF hen eggs and has since been maintained by propagation in SPF chickens. The cloned virus has been identified by its pathogenicity, antigenicity and genome sequence as a typical vvIBDV (Eterradossi et al., Citation1992, 1999Citation).
The CEVAC IBD L vaccine (batch number 3007H1U; CEVA santé animale, Libourne, France) was used in the experimental study. For the antigenic study, a bursal homogenate containing the vaccine virus was prepared with the same protocol as used with isolate 99323.
All viruses were titrated by inoculating serial 10-fold dilutions (0.1 ml per egg, chorioallantoic membrane route, seven eggs per dilution) to 9-day-old to 10 day-old SPF hen eggs (AFSSA-Ploufragan). Virus titres were calculated according to the method of Reed & Muench (Citation1938) and expressed as median embryo infectious doses (EID50).
2.2 Antigen-capture enzyme-linked immunosorbent assay
Bursal homogenates were studied in an antigen-capture enzyme-linked immunosorbent assay (AC-ELISA), as previously described (Eterradossi et al., Citation1997b). Briefly, IBDV antigens contained in a bursal homogenate were captured on polystyrene ELISA microplates coated with an anti-IBDV chicken polyclonal serum. The captured virus particles were then detected with a panel of anti-IBDV neutralizing mouse monoclonal antibodies (mAbs), or with a mouse polyclonal antibody. The bound mouse antibodies were finally detected with a goat anti-mouse IgG-alkaline phosphatase conjugate. The binding of the mAbs to each tested virus was expressed as a percentage of the binding of the polyclonal reference reagent (the higher the percentage, the higher the binding of the mAb and the reactivity of the epitope in the tested virus). As a preliminary step, the captured homogenates were calibrated by serial dilution so that a standardized antigen amount was tested in AC-ELISA. As a second step, the binding of the mAbs to a standardized amount of antigen was studied.
The panel of neutralizing mAbs used in AC-ELISA included mAbs 1, 3, 4, 5, 6, 7, 8 and 9. All bind to the IBDV outer capsid protein VP2. mAb pairs (3 and 4) and (6 and 7) are targeted at two overlapping antigenic sites, with the binding of mAbs 3 and 4 depending on the presence of Proline and Glycine residues at aa positions 222 to 223 and the binding of mAbs 6 and 7 depending on aa changes at positions 318 to 323. VP2 major hydrophilic peak B is recognized by mAb 8, the binding of which depends on a Glutamine residue at aa position 324 (Eterradossi et al., Citation1997a, 1998Citation). mAb 5 probes an epitope involving the first minor hydrophilic peak 1 (249Q) (Eterradossi et al., unpublished results 1998–2000). The binding sites of mAbs 1 and 9 are not known.
2.3 Nucleotide sequencing
The middle part of the VP2 gene, encoding what is known as VP2 variable domain (Bayliss et al., Citation1990), was reverse transcribed and amplified as described previously. Briefly, chimeric oligonucleotide primers, obtained by coupling the sequence of M13 and 21M13 standard primers to the IBDV-specific L2 and U2 sequences, respectively, were used to generate a 604 base pair (bp) reverse transcriptase-polymerase chain reaction (RT-PCR) product, 516 bp of which were IBDV-specific. The RT-PCR products were purified with the Qiaquick Gel purification kits (Qiagen, Chatsworth, CA, USA) and sequenced as described previously (Eterradossi et al., Citation1998, 1999Citation). The resulting nt sequences were submitted to the EMBL database under accession numbers AJ583500 and AJ632141 for the 99323 and CEVAC IBD L viruses, respectively.
2.4 Phylogenetic analysis
The nt sequences (nt positions 746 to 1190, numbering according to the full-length sequence of segment A of serotype I strain P2; Mundt & Müller, Citation1995) were aligned using the Clustal W programme. The phylogenetic analysis were performed as described previously (Eterradossi et al., Citation1999), with a set of 500 bootstrap-generated aligned sequences and using both the neighbour joining and the maximum parsimony methods. In both approaches, the OH strain (serotype 2) was used as an extra group. Consensus trees were calculated and are presented (all programmes from the Phylip 3.52c suite, by J. Felsenstein, Department of Genetics, University of Washington, Seattle, Washington, USA, 1993).
2.5 Experimental study of vaccine protection
Fourteen-day-old SPF White Leghorn chickens (AFSSA-Ploufragan) were weighed and housed in groups of 15 (four groups) or 25 (two groups) birds, in separate filtered-air negative-pressure isolation units. Ten chickens in each group were blood sampled for serological testing on the first day. On the same day, the birds in three of the 15-bird groups were vaccinated by intranasal administration of one dose (0.1 ml=101.6 EID50) of the CEVAC IBD L vaccine. Ten chickens in each group were blood sampled 3 weeks after vaccination, then one 25-bird group (unvaccinated) and one vaccinated 15-bird group were challenged by intranasal administration of the 99323 virus (0.1 ml per bird=104.24 EID50). Two similar groups were challenged similarly with the 89163 virus (0.1 ml per bird=104.58 EID50). One unvaccinated unchallenged 15-bird group was kept as a SPF control. One vaccinated unchallenged 15-bird group was kept as a vaccinated control. The birds were observed daily for clinical signs until 10 days post-inoculation. On the last day, the surviving birds were weighed then humanely killed. Their bursae were weighed and 10 bursae were randomly chosen in each group for histological examination (fixation in 10% formaldehyde).
2.6 Serological testing
Micro virus neutralization (VN) tests were performed as described previously (Eterradossi et al., Citation1992): serial two-fold dilutions of the sera were mixed with 100 median tissue infective doses (TCID50) of the CT IBDV strain (serotype 1 antigen, cell culture adapted; final volume of mix, 50 μl). The classical antigenicity of strain CT and the nt sequence encoding the variable domain of VP2 in this virus strain have been described previously (Eterradossi et al., Citation1997a, 1998Citation). Virus neutralization was allowed for 45 min at room temperature. Neutralization was tested by adding to the mix 250 μl of a suspension of chicken embryo fibroblasts. A cytopathogenic effect typical for cell-culture-adapted IBDV was observed after 5 days of incubation at 37°C with a 2% CO2 atmosphere. The virus neutralizing titre was expressed as log2 of the last dilution resulting in 100% neutralization of the cytopathogenic effect.
2.7 Histological lesions
Histological examinations were performed by Dr M. Lagadic (Maisons-Alfort, France). The severity of the IBD-induced lesions was quantified according to the scale by Skeeles et al. (Citation1979), which is based on five degrees: 0=no lesions; 1=mild scattered cell depletion in a few follicles; 2=moderate, one-third to one-half of the follicles have atrophy or depletion of cells; 3=diffuse, atrophy of all follicles; and 4=acute inflammation and acute necrosis typical of IBD.
3 Results
3.1 Antigenic characterization of the challenge and vaccine viruses
presents the antigenic reactivity of the 89163, 99323 and vaccine viruses, as compared with the Faragher 52/70 strain (Bygrave & Faragher, Citation1970), used as a reference for the “classical” IBDVs, and with the 91168 and 94432 atypical vvIBDV strains (Eterradossi et al., Citation1997b, 1998Citation). The Faragher 52/70 virus reacted strongly with all mAbs. The CEVAC IBD L vaccine also reacted with all mAbs, although it exhibited a weaker binding of mAb5 (26%) than Faragher 52/70 (78%). Neither the 89163 nor the 99323 viruses reacted with mAbs 3 and 4, the lack of reactivity of which has been found in all vvIBDVs that have been studied so far. As compared with all other viruses, including the atypical vvIBDVs, the 99323 virus further exhibited a unique combination of lack of reactivity, or of strongly reduced reactivity (< 20%), with mAbs 3, 4, 5, 6, 8 and 9.
Compared antigenicity of IBDV strains Faragher 52/70 (classical serotype 1), 89163 (typical vvIBDV), 91168 (atypical vvIBDV), 94432 (atypical vvIBDV), 99323 (Egyptian isolate) and CEVAC IBD L (live vaccine)
3.2 Partial nucleotide sequence of CEVAC IBD L and 99323 viruses, and phylogenetic analysis
The nt sequence determined for the CEVAC IBD L vaccine was mostly similar to that of classical virus F52/70 (12 nts differed, 97.3% nt identity). Four of the observed nt changes resulted in aa changes. Only one of these changes occurred in one of VP2 hydrophilic peaks (S217 → L; ) and AC-ELISA results showed that the binding of mAbs 3 and 4 was unaffected. The nt sequence of the 99323 isolate was mostly similar with that of reference vvIBDV strain 89163 (only nine nt positions differed, 98.0% nt identity). Four of these nt changes were silent mutations, hence the deduced aa sequence was also very similar to that of 89163 () and presented the four aa positions 222A, 256I, 284I and 299S, which have been found in every vvIBDV so far characterized, with the two exceptions of an early IBDV strain from Ivory Coast that has not been reisolated since 1988 (Brown et al., Citation1994; Cao et al., Citation1998; Eterradossi et al., Citation1999; To et al., Citation1999; Zierenberg et al., Citation2000) and of Indonesian strain Tasik94, which differs from typical vvIBDVs by lacking residue 222A (Rudd et al., Citation2002). However, the 99323 isolate also exhibited three non-silent nt changes, which encoded three aa changes that have not so far been found in any other vvIBDV-like viruses; namely, Y220 → F, G254 → S and A321 → T. These changes occurred in regions that are known to be important for antigenicity: VP2 major hydrophilic peak A (position 220), VP2 first minor hydrophilic peak (position 254) and VP2 second major hydrophilic peak (position 321) (Schnitzler et al., Citation1993; Vakharia et al., Citation1994; van den Berg et al., Citation1996).
Fig. 1 Amino acid sequence of the VP2 variable domain in IBDV strains F52/70, Cevac IBD L, 89163, 91168, 94432 and isolate 99323, from aa positions 183 to 353 (numbering according to Bayliss et al., Citation1990). Dots indicate positions where the sequence is identical to F52/70. The sequence of the antigenically atypical vvIBDV isolates 91168 and 94432 (Eterradossi et al., Citation1998) has been indicated for comparative purposes. VP2 major and minor hydrophilic peaks are boxed with or without grey shading, respectively.
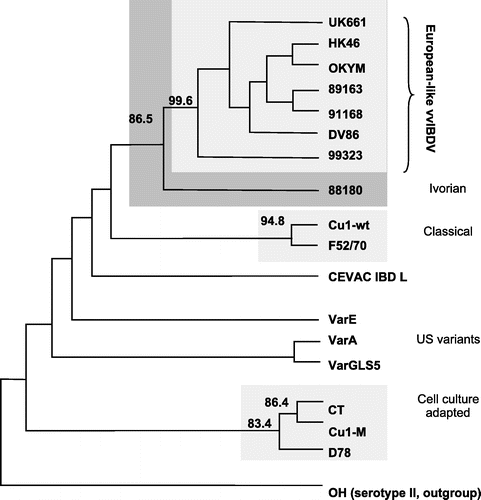
The significant genetic relationships of isolate 99323 with vvIBDVs were confirmed by the phylogenetic analysis (). The neighbour-joining and maximum parsimony approaches produced consensus trees with a similar topology, both showing that the 99323 Egyptian isolate significantly clustered together with the European-like vvIBDV strains (in 99.4% and 99.6% of the bootstrap generated trees, as analysed by neighbour joining and parsimony, respectively).
Fig. 2 Phylogenetic consensus tree of the studied IBDV strains, as obtained with the parsimony method. Phylogenetic analysis was performed as described in Materials and Methods. The nucleotide sequence encoding the VP2 variable domain of isolate 99323 (nts 746 to 1190 according to the full-length sequence of serotype 1 strain P2) was compared with representatives of very virulent, classical, variant, cell culture adapted or serotype 2 IBDVs from various geographical origins. Accession numbers for these sequences: OH (D00867), F52/70 (Y14958), Cu1WT (AF159219), CT (Y14961), Cu-1M (AF362771), D78 (Y14962), Var A (Y14959), Var E (D10065), Var GLS (Q82628), 89163 (Y14956), OKYM (D49706), DV86 (Z25482), UK661(X92760), 88180 (AJ001941), 91168 (Y14957). The OH strain was used as an outgroup. Branch length has no special meaning. The figures at the forks are bootstrap values (i.e. they measure the likelihood that viruses to the right of that fork are indeed genetically related). Only bootstrap values higher than 80% have been indicated, strains in these groups appear with homogeneous shading.
3.3 Experimental assessment of the vaccine-induced protection ()
Results until challenge. On the first day of the experiment, the weights of the chickens in the different groups were not significantly different (according to Kruskal–Wallis’ one-way analysis of variance) and none of the tested chickens had detectable anti-IBDV neutralizing antibodies. Three weeks after vaccination, VN antibodies were found in the vaccinated flocks only. Their titres in the different groups did not differ significantly (geometric mean ranging from 11.5 to 12.4 log2).
Compared effects of the 89163 or 99323 challenges, in 5-week-old SPF chickens receiving or not the CEVAC IBD L live vaccine at 14 days of age (3 weeks prior to challenge)
3.3.1 Results after challenge
Typical IBD signs were observed from 48 to 72 h post challenge (p.c.) onwards in the unvaccinated challenged groups. Morbidity at day 3 was similar for the two challenge viruses (18 and 17 birds out of 25 inoculated with the 89163 or 99323 viruses, respectively). Mortality was observed on days 3 and 4 p.c., but was not significantly different in the two groups receiving the 89163 or 99323 viruses. Five days p.c., four birds still exhibited signs following the 89163 challenge, compared with eight in the 99323-challenged group. Four birds in this group still had not fully recovered 7 days p.c. Neither signs nor mortality were observed in the SPF control group or in the groups that had been vaccinated.
3.3.2 Results at necropsy
The weight gains (expressed as the percentage of the initial weight) were statistically different 10 days p.c., with the unvaccinated challenged groups showing a significantly reduced growth (330% and 344% in the groups challenged with the 99323 and 89163 viruses, respectively, compared with 404 to 420% in all other groups). The SPF controls and the three previously vaccinated groups did not show significantly different growths.
Gross lesions as observed at necropsy are summarized in . Typical chronic IBD lesions (bursal atrophy and mild nephritis) were observed in the two unvaccinated challenged groups. However, quite unexpectedly, eight birds out of 15 challenged with the 99323 virus also exhibited clear thymus atrophy. This was not observed in the 99323-challenged birds that had been previously vaccinated. Pale bone marrow in one to two birds was also observed in the 99323-challenged group and in the previously vaccinated groups.
Bursal atrophy, as measured by the bursa-to-body weight ratio was observed in all groups, as compared with the SPF controls. Statistical analysis showed the birds receiving the vaccine alone or the vaccine followed by the 89163 challenge to have significantly smaller bursae, as compared with the groups receiving the challenge viruses alone, or the vaccine followed by the 99323 virus.
3.3.3 Histological examination
Examination of the bursae revealed no lesions in the SPF controls, moderate to diffuse IBD lesions in the three groups that had received the vaccine in combination or not with a challenge virus (mean lesion scores not significantly different, ranging from 2.0 to 2.4) and extensive lesions in the two challenged groups (mean lesion scores 2.9 and 3.0; significantly higher than in the previous groups).
Histological examinations of the thymus were performed on three birds challenged with the 99323 virus and showing thymus atrophy at necropsy. The cortical area of the thymus lobules was markedly atrophied, thin and depopulated. However, it did not exhibit lymphocyte necrosis. These lesions were said to be “neither typical of nor incompatible with” the hypothesis of an infection by the chicken infectious anaemia virus (CIAV).
4 Discussion
Highly conserved antigenic and genetic features of the vvIBDV isolates have suggested that these viruses might find a clonal origin with the vvIBDV that first emerged in Europe in 1987 (for a review, see van den Berg, Citation2000) and were shown to be experimentally controlled by intermediate live vaccines with a classical antigenicity (van den Berg et al., Citation1991; Eterradossi et al., Citation1992). Hence the lack of control of vvIBDVs in the field was more due to difficulties in inducing an early antibody response with the available live vaccines, rather than to antigenic changes of the field virus. However, the possible emergence of antigenically modified vvIBDVs would further complicate the vaccine control of these viruses. So far, antigenically atypical vvIBDV isolates have only been isolated occasionally (Eterradossi et al., Citation1997b; Domanska et al., Citation2002). There are also some reports of vvIBDV-related genetic sequences, encoding some aa changes located in the regions of VP2 known to be critical for antigenicity (Scherbacova et al., unpublished data 1997, presented in databanks; Cao et al., Citation1998; Rudd et al., Citation2002). The 99323 isolate was identified from Egypt during a worldwide survey of antigenic variation among IBDV strains causing acute IBD outbreaks. Such outbreaks have been reported to be a cause for continuing problems in the field in Egypt since the early 1990s, in spite of extensive and multiple administrations of live vaccines with various degrees of attenuation (Hassan et al., Citation2002).
The 28% mortality induced by isolate 99323 following an experimental 104.24 EID50 challenge in SPF chickens (not significantly different from that induced by strain 89163), the presence in the VP2 protein of 99323 of the four aa found in the typical vvIBDV, and the genetic relatedness between the nt sequences of 99323 and of typical vvIBDVs (as demonstrated by high bootstrap values in the consensus trees) all corroborate the fact that isolate 99323 is indeed a vvIBDV. However in AC-ELISA, the antigenic reactivity of the 99323 isolate was most unusual, as typical vvIBDVs studied until now bound all mAbs but mAbs 3 and 4 (Eterradossi et al., Citation1997b, 1999Citation; Di Fabio et al., Citation1999; Islam et al., Citation2001). Only three other vvIBDV isolates had been found so far with a modified antigenicity. These were French isolates 91168 and 94432 (Eterradossi et al., Citation1997b, 1998Citation) and Polish isolate 93/35, which appears to be nearly identical with 91168 at the genetic level (Domanska et al., Citation2002, 2003Citation). Hence, the 99323 isolate presents the most extensive antigenic variation so far recognized among vvIBDVs. The full length VP2 gene of isolate 99323 was not sequenced, so that the molecular basis for the modified antigenicity of this isolate is not yet known with certainty. However, it is apparent from the partial sequence encoding VP2 variable domain that this isolate exhibits aa changes in three of the hydrophilic regions previously reported to be critical for IBDV antigenicity (Schnitzler et al., Citation1993; Vakharia et al., Citation1994; van den Berg et al., Citation1996; Eterradossi et al., Citation1998).
The experimental challenge study was designed to check whether the antigenic changes found in strain 99323 would allow the virus to escape the immune response induced by a live IBDV vaccine with a classical antigenicity. The study focused on the CEVAC IBD L vaccine, which has been used in the field in Egypt. The vaccine can be described as exhibiting a “classical” antigenicity, as shown by the fact that most mAbs, especially mAbs 3 and 4 that probe VP2 major hydrophilic peak A, bound to the vaccine virus in AC-ELISA. The fact that mAb 5 yielded a low percent reactivity against the vaccine virus is consistent with previous results showing that mAb 5, possibly due to a low affinity (Eterradossi et al., Citation1997a), may present a wide range of reactivity when tested against viruses with a typical minor hydrophilic peak 1 (Eterradossi et al., unpublished observations 1998).
The protection afforded by vaccination was studied in SPF chickens challenged either with the 99323 isolate or with the typical 89163 vvIBDV isolate. The lack of signs and mortality in the challenged groups that had been previously vaccinated and the fact that growth retardation was observed only in the unvaccinated groups are consistent with the vaccine providing a good clinical protection against both challenge viruses. The vaccine alone induced a degree of bursal atrophy quite similar to that seen with the two challenge viruses, a result that is an indication of the “invasive” character of the vaccine. The mean histological lesion score was significantly lower in the group receiving the vaccine alone as compared with those receiving the challenge viruses alone; however, it should be taken into account that the delay between infection and histological examination was different in the two cases, the challenged groups being analysed 10 days p.c., whereas the vaccinated controls were analysed 31 days post-vaccination. Such a difference in delay before histological analysis could have allowed the birds in the vaccine control group to recover from bursal lesions. Nevertheless, as the bursal lesion score in the vaccinated then challenged groups is similar to that found in the birds receiving the vaccine alone, and significantly lower than in the challenged controls, it can be concluded that the previous vaccination protected the birds from further challenge-induced bursal atrophy. A similar study would profitably be performed with a vaccine virus with a classical antigenicity but a more limited “intermediate” invasiveness.
Interestingly, thymus atrophy was apparent in the 99323-challenged group (), but not in the birds that have been previously vaccinated. A PCR for CIAV performed on the 99323 virus stock according to Rodenberg et al. (Citation1994) failed to detect any CIAV (data not shown); however, it should be mentioned that the sensitivity of this method has not been evaluated in the authors’ laboratory. Additionally, two serial passages of the 99323 inoculum on cultures of chicken embryo hepatocytes did not result in the isolation of any contaminating virus, such as adenoviruses (data not shown). Thus, although in the authors’ experience thymus lesions are not frequently seen following challenge with vvIBDVs, the 99323-induced thymus lesions could indeed be due to IBDV alone. This observation is reminiscent of the findings by Tanimura et al. (Citation1995), who demonstrated severe thymic cortical atrophy from day 5 to day 10 in chickens inoculated with the Japanese Ehime/91 or the European DV86 vvIBDV strains.
Overall, our data suggest that under the implemented experimental conditions the 99323 challenge was at least as well controlled by vaccination as the 89163 more typical vvIBDV challenge. This statement seems consistent with previous data from the authors’ laboratory showing that an antiserum produced after inoculation by a natural route with the classical F52/70 IBDV strain still neutralizes to very high titre laboratory-selected escape mutants of IBDV, which exhibit up to six modified neutralizing epitopes (those epitopes probed by mAbs 3, 4, 5, 6, 7 and 8) (Eterradossi et al., unpublished observations 1998). Conversely, it had been reported that immunization with live IBDV escape mutants harbouring three modified neutralizing epitopes still induce a protective immunity against vvIBDV, although the modified epitopes are all present in the studied vvIBDV isolate (van den Berg et al., Citation1994). These observations might suggest that antibodies elicited by an active immune response react against a broad antigenic spectrum, and may neutralize even extensively modified IBDVs. Alternatively, another explanation could be that an additional and major neutralizing epitope is maintained in all these viruses, and represents the molecular basis for an efficient cross-neutralization.
An interesting practical development of the present work would be to test whether the extensive antigenic changes found in 99323 allow this virus to escape the antibodies passively transmitted to the chick by the hens that have been immunized with an inactivated IBD vaccine. Another important question is to investigate whether the 99323-like viruses are still prevalent in the field, and how widespread such viruses may be. Preliminary work in this respect allowed us to identify IBDV isolates with an antigenic profile and/or a genetic sequence similar to the 99323 isolate among a few Egyptian samples submitted in 2002 (Eterradossi et al., unpublished observations 2003). This finding deserves further examination, as the other atypical vvIBDV had been found only occasionally, and had been shown not to replace the more typical vvIBDV isolates (Eterradossi et al., Citation1997b).
Translations of the abstract are available in French, German and Spanish on the Avian Pathology website.
Acknowledgments
The authors would like to thank Dr H.A.A Aidaros, OIE delegate for Egypt, for his kind support to this work.
References
- Bayliss , C.D. , Spies , U. , Shaw , K. , Peters , R.W. , Papageorgiou , A. , Müller , H. and Boursnell , M.E.G. 1990 . A comparison of the sequences of segment A of four infectious bursal disease virus strains and identification of a variable region in VP2 . Journal of General Virology , 71 : 1303 – 1312 .
- Boot , H.J. , Ter Huurne , A.A. , Hoekman , A.J.W. , Peeters , B.P.H. and Gielkens , A.L.J. 2000 . Rescue of very virulent and mosaic infectious bursal disease virus from cloned cDNA: VP2 is not the sole determinant of the very virulent phenotype . Journal of Virology , 74 : 6701 – 6711 .
- Brown , M.D. , Green , P. and Skinner , M.A. 1994 . VP2 sequences of recent European “very virulent” isolates of infectious bursal disease virus are closely related to each other but are distinct from those of classical strains . Journal of General Virology , 75 : 675 – 680 .
- Bygrave , A.C. and Faragher , J.T. 1970 . Mortality associated with Gumboro disease . Veterinary Record , 86 : 758 – 759 .
- Cao , Y.C. , Yeung , W.S. , Law , M. , Bi , Y.Z. , Leung , F.C. and Lim , B.L. 1998 . Molecular characterisation of seven Chinese isolates of infectious bursal disease virus: classical, very virulent, and variant strains . Avian Diseases , 42 : 340 – 351 .
- Chettle , N.J. , Stuart , J.C. and Wyeth , P.J. 1989 . Outbreaks of virulent infectious bursal disease in East Anglia . Veterinary Record , 125 : 271 – 272 .
- Cosgrove , A.S. 1962 . An apparently new disease of chickens—avian nephrosis . Avian Diseases , 6 : 385 – 389 .
- Di Fabio , J. , Rossini , L.I. , Eterradossi , N. , Toquin , D. and Gardin , Y. 1999 . European-like pathogenic infectious bursal disease viruses in Brazil . Veterinary Record , 145 : 203 – 204 .
- Domanska , K. , Rivallan , G. , Smietanka , K. , Toquin , D. , de Boisseson , C. , Minta , Z. and Eterradossi , N. 2002 . Antigenic characterization of Polish infectious bursal disease virus strains . Bulletin of the Veterinary Institute in Pulawy , 46 : 45 – 52 .
- Domanska , K. , Rivallan , G. , Smietanka , K. , Toquin , D. , de Boisseson , C. , Minta , Z. and Eterradossi , N. 2003 . Genetic characterization of Polish infectious bursal disease virus strains . Bulletin of the Veterinary Institute in Pulawy , 47 : 61 – 69 .
- Eterradossi , N. , Picault , J.P. , Drouin , P. , Guittet , M. , L'Hospitalier , R. and Bennejean , G. 1992 . Pathogenicity and preliminary antigenic characterisation of six infectious bursal disease virus strains isolated in France from acute outbreaks . Journal of Veterinary Medicine , B39 : 683 – 691 .
- Eterradossi , N. , Toquin , D. , Rivallan , G. and Guittet , M. 1997a . Modified activity of a VP2-located neutralizing epitope on various vaccine, pathogenic and hypervirulent strains of infectious bursal disease virus . Archives of Virology , 42 : 255 – 270 .
- Eterradossi , N. , Rivallan , G. , Toquin , D. and Guittet , M. 1997b . Limited antigenic variation among recent infectious bursal disease virus isolates from France . Archives of Virology , 142 : 2079 – 2087 .
- Eterradossi , N. , Arnaud , C. , Toquin , D. and Rivallan , G. 1998 . Critical aminoacid changes in VP2 variable domain are associated with typical and atypical antigenicity in very virulent infectious bursal disease viruses . Archives of Virology , 143 : 1627 – 1636 .
- Eterradossi , N. , Arnaud , C. , Tekaia , F. , Toquin , D. , Le Coq , H. , Rivallan , G. , Guittet , M. , Domenech , J. , van den Berg , T.P. and Skinner , M.A. 1999 . Antigenic and genetic relationship between European very virulent infectious bursal disease viruses and an early West-African isolate . Avian Pathology , 28 : 36 – 46 .
- Hassan , M.K. , Afify , M. and Aly , M.M. 2002 . Susceptibility of vaccinated and unvaccinated Egyptian chickens to very virulent infectious bursal disease virus . Avian Pathology , 31 : 149 – 156 .
- Hirai , K. , Funakoshi , T. , Nakai , T. and Shimakura , S. 1981 . Sequential changes in the number of surface immunoglobulin-bearing B lymphocytes in infectious bursal disease virus-infected chickens . Avian Diseases , 25 : 484 – 496 .
- Islam , M.R. , Zierenberg , K. , Eterradossi , N. , Toquin , D. , Rivallan , G. and Müller , H. 2001 . Molecular and antigenic characterization of Bengladeshi isolates of infectious bursal disease virus demonstrate their similarities with recent European Asian and African very virulent strains . Journal of Veterinary Medicine, B , 48 : 211 – 221 .
- Jackwood , D.J. and Sommer , S.E. 1999 . Restriction fragment length polymorphisms in the VP2 gene of infectious bursal disease viruses from outside the United States . Avian Diseases , 43 : 310 – 314 .
- Lasher , H.N. and Shane , S.M. 1994 . Infectious bursal disease . World's Poultry Science Journal , 50 : 134 – 166 .
- McFerran , J.B. , Mc Nulty , M.S. , McKillop , E.R. , Connor , T.J. , McCracken , R.M. , Collins , D.S. and Allan , G.M. 1980 . Isolation and serological studies with infectious bursal disease viruses from fowl, turkeys and ducks: demonstration of a second serotype . Avian Pathology , 9 : 395 – 404 .
- Müller , H. , Schnitzler , D. , Bernstein , F. , Becht , H. , Cornelissen , D. and Lütticken , D.H. 1992 . Infectious bursal disease of poultry: antigenic structure of the virus and control . Veterinary Microbiology , 33 : 175 – 183 .
- Mundt , E. and Müller , H. 1995 . Complete nt sequences of 5′ and 3′ non-coding regions of both genome segments of different strains of infectious bursal disease virus . Virology , 209 : 10 – 18 .
- Mundt , E. and Vakharia , V.N. 1996 . Synthetic transcripts of double-stranded Birnavirus genome are infectious . Proceedings of the National Academy of Sciences of the USA , 93 : 11131 – 11136 .
- Pringle , C.R. 1999 . The universal system of virus taxonomy, updated to include the new proposals ratified by the International Committee on Taxonomy of Viruses during 1998 . Archives of Virology , 144 : 421 – 429 .
- Reed , L.J. and Muench , H. 1938 . A simple method of estimation of fifty per cent end-points . American Journal of Hygiene , 27 : 493 – 497 .
- Rodenberg, J., De Wannemaeker, C., Heeren, J., Colau, D. Thiry, G. & Nordgren, R. (1994). Comparison of MSB1 isolation and polymerase chain reaction to determine the presence of CAV in avian biological products. In Prof E. Kaleta (Ed.), Proceedings of the international symposium on infectious bursal disease and chicken infectious anaemia, 21–24 June (pp. 421–424). Rauischholzhausen, Germany.
- Rudd , M.F. , Heine , H.G. , Sapats , S.I. , Parede , L. and Ignjatovic , J. 2002 . Characterisation of an Indonesian very virulent strain of infectious bursal disease virus . Archives of Virology , 147 : 1303 – 1322 .
- Schnitzler , D. , Bernstein , F. , Müller , H. and Becht , H. 1993 . The genetic basis for the antigenicity of the VP2 protein of the infectious bursal disease virus . Journal of General Virology , 74 : 1563 – 1571 .
- Skeeles , J.K. , Lukert , P.D. , Fletcher , O.J. and Leonard , D.J. 1979 . Immunization studies with a cell culture adapted infectious bursal disease virus . Avian Diseases , 23 : 456 – 465 .
- Snyder , D.B. , Lana , D.P. , Savage , P.K. , Yancey , F.S. , Mengel , S.A. and Marquardt , W.W. 1988 . Differentiation of infectious bursal disease viruses directly from infected tissues with neutralizing monoclonal antibodies: evidence for a major antigenic shift in recent field isolates . Avian Diseases , 32 : 527 – 534 .
- Snyder , D.B. , Vakharia , V.N. and Savage , P.K. 1992 . Naturally occuring-neutralizing monoclonal antibody escape variants define the epidemiology of infectious bursal disease viruses in the United States . Archives of Virology , 127 : 89 – 101 .
- Tanimura , N. , Tsukamoto , K. , Nakamura , K. , Narita , M. and Maeda , M. 1995 . Association between pathogenicity of infectious bursal disease virus and viral antigen distribution detected by immunochemistry . Avian Diseases , 39 : 9 – 20 .
- To , H. , Yamaguchi , T. , Nguyen , N.T.P. , Nguyen , O.T.K. , Nguyen , S.V. , Agus , S. , Kim , H.J. , Fukushi , H. and Hirai , K. 1999 . Sequence comparison of the VP2 variable region of infectious bursal disease virus isolates from Vietnam . Journal of Veterinary Medical Sciences , 61 : 429 – 432 .
- Vakharia , V.N. , He , J. , Ahamed , B. and Snyder , D.B. 1994 . Molecular basis of antigenic variation in IBDV . Virus Research , 31 : 265 – 273 .
- van den Berg , T.P. 2000 . Acute infectious bursal disease in poultry: a review . Avian Pathology , 29 : 175 – 194 .
- van den Berg , T.P. and Meulemans , G. 1991 . Acute infectious bursal disease in poultry: protection afforded by maternally derived antibodies and interference with live vaccination . Avian Pathology , 20 : 409 – 421 .
- van den Berg , T.P. , Gonze , M. and Meulemans , G. 1991 . Acute infectious bursal disease in poultry: isolation and characterisation of a highly virulent strain . Avian Pathology , 20 : 133 – 143 .
- van den Berg, T.P., Gonze, M., Morales, D. Meulemans, G. (1994). Relevance of antigenic variation for protection in infectious bursal disease In Prof E. Kaleta (ED)., Proceedings of the international symposium on infectious bursal disease and chicken infectious anaemia, 21–24 June (pp. 22–36). Rauischholzhausen, Germany.
- van den Berg , T.P. , Gonze , M. , Morales , D. and Meulemans , G. 1996 . Acute infectious bursal disease in poultry: Immunological and molecular basis of antigenicity of a highly virulent strain . Avian Pathology , 25 : 751 – 768 .
- Zierenberg , K. , Nieper , H. , van den Berg , T.P. , Ezeokoli , C.D. , Voss , M. and Müller , H. 2000 . The VP2 variable region of two German and 11 African isolates of infectious bursal disease viruses (IBDV): comparison of the amino acid sequences with very virulent, “classical” virulent, and attenuated tissue culture-adapted strains . Archives of Virology , 145 : 113 – 125 .
- Zierenberg , K. , Raue , R. and Müller , H. 2001 . Rapid identification of “very virulent” strains of infectious bursal disease virus by reverse transcription-polymerase chain reaction combined with restriction enzyme analysis . Avian Pathology , 30 : 55 – 62 .