Abstract
A simple objective method to quantify embryo dwarfism induced by infectious bronchitis virus in embryonated chicken eggs has been used to determine endpoints in virus titration and neutralization assays. The eggs and the respective embryos were weighed and embryo:egg weight (EE) ratios were calculated. The EE ratios were compared with the uninoculated control eggs and endpoints could be calculated objectively. EE indices were also calculated by dividing the EE ratios of inoculated embryonated chicken eggs by the mean EE ratio of uninoculated controls, or in the case of virus neutralization tests by the mean EE ratio of eggs inoculated with virus alone. Although this mean EE index did not reflect the dwarfing (or lack of it) in individual eggs, it served as a group indicator. This method would be useful to observe embryo lesions especially in field (non-egg adapted) infectious bronchitis virus isolates, which does not cause observable dwarfing until several embryo passages.
Introduction
Infectious bronchitis is an acute, highly contagious viral respiratory disease of chickens characterized by tracheal rales, coughing and sneezing. In laying flocks there is a drop in the quantity and quality of eggs produced. Kidney manifestations are seen with infections of nephropathogenic strains of infectious bronchitis virus (IBV) (Dhinakar Raj & Jones, Citation1997). IBV grows well in the developing chicken embryos as compared with chicken kidney or tracheal organ cultures (Cook et al., Citation1976). Upon initial inoculation of embryos, dwarfing of a few embryos with survival of 90% eggs through to day 19 of incubation is characteristic of IBV growth (De Wit, Citation2000). The embryo is curled into a spherical form, with feet deformed and compressed over the head with a thickened amnion adhered to it. The virus titres are highest in embryos at 24 to 48 h post-inoculation (p.i.), although embryo lesions are seen only after 5 to 6 days p.i. (De Wit, Citation2000).
In infectious bursal disease (IBD) virus-infected chickens, bursal atrophy is usually quantified as a ratio of the bursal weight to body weight in grams (BB ratio) (Lucio & Hitchner, Citation1979; Thangavelu et al., Citation1998). Similarly spleen:body weight ratios are also used to assess spleenic atrophy (Hassan et al., Citation2002). The term bursal index is used to indicate the ratio obtained by dividing the BB ratio of infected/vaccinated chickens by the BB ratio of uninfected/unvaccinated chickens. This study deals with an attempt to quantify embryo dwarfing induced by IBV in embryonated chicken eggs (ECE) on lines similar to quantification of bursal or spleen atrophy induced by IBD virus.
Materials and Methods
Embryonated chicken eggs, viruses and antisera
ECE were obtained from a local source that was shown to be free of IBV antibodies using the commercial enzyme-linked immunosorbent assay kit (Idexx, USA). A commercial H120 vaccine belonging to the Massachusetts (Mass) serotype and a virulent IBV strain 008 also belonging to the Mass genotype, were used. Antisera raised against three other field isolates of IBV (S3, U4 and 177) also belonging to the Mass genotype were used in cross-neutralization tests. Genotyping was done by sequencing a portion of the S1 gene (Keeler et al., Citation1998) (data not shown). Median embryo infectivity titres (EID50) were calculated by inoculating IBV at 10-fold dilutions and calculating 50% endpoint titres by the Reed & Muench (Citation1938) method.
Embryo:egg weight ratio and embryo:egg weight index
The embryo:egg weight (EE) ratios and EE indices were calculated following infection of ECE with vaccine or virulent strains of IBV using the following formulae:
![]()

In the case of virus neutralization (VN) tests, the EE index was calculated using the following formula:
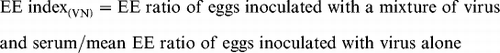
A minimum of at least three eggs was used at each time and the ratios were compared statistically by the Student's t test (P<0.05).
Experimental designs
Experiment 1. Ten-day-old ECE were inoculated via the intra-allantoic route with 103 EID50 of H120 or IBV-008. At 24-h intervals, eggs were chilled overnight and then weighed. After removal of the embryos these were also weighed immediately and EE ratios were calculated for each egg.
Experiment 2. The EE ratio and the EE index were applied to determine the endpoints for the calculation of virus infectivity titres. Diluted H120 vaccine virus and IBV-008 were inoculated intra-allantoically into 10-day-old ECE at 10-fold dilutions and the live eggs were chilled on day 6 p.i. The mean EE ratios of inoculated and control (uninoculated) ECE were calculated on that day.
Experiment 3. The EE ratio and EE index(VN) were applied to determine the endpoints for the calculation of serum neutralization titres. In the cross-neutralization tests 102.0 EID50 H120 IBV was neutralized with two-fold dilutions of sera prepared against the homologous virus and four heterologous viruses (IBV-008, S3, U4 and 177). Controls included inoculation of 102.0 EID50 H120 virus without serum. The mean EE ratio of ECE inoculated with a virus and serum mixture for various antisera, or for virus alone, was calculated on day 6 p.i.
Results
Experiment 1
Until day 3 p.i., no differences in the EE ratios were seen between uninoculated and IBV-inoculated ECE (). However, while the EE ratios started increasing from day 4 p.i. in the mock-inoculated group, they did not increase in the IBV-inoculated groups. The difference was statistically significant (compared with uninoculated controls) in the virulent IBV inoculated eggs from day 4 p.i. onwards and in the vaccine virus inoculated eggs from day 5 p.i. onwards. The EE ratio was lowest on day 5 p.i. in the virulent IBV inoculated group and on day 6 in the vaccine virus inoculated group.
Table 1. Mean embryo egg weight ratios of IBV-inoculated embryonated chicken eggs at different days p.i.
Experiment 2
The mean EE ratios of inoculated and control (uninoculated) ECE are presented in . The EE ratios were similar to those of the control eggs from the 10−4 and 10−5 dilutions for the vaccine and virulent IBV, respectively. The EE index for the H120 vaccine inoculated group was less than 0.4 until the 10−3 dilution and was close to 1.0 for the other dilutions, clearly showing the endpoints. Therefore the titre is determined to be 103.5 EID50. In the IBV-008-inoculated group, the EE index was around 0.4 until the 10−3 dilution and was close to 1 from the 10−5 dilution and thereafter. The individual EE ratios of the three eggs inoculated with the 10−4 dilution of 008 virus were 0.890, 0.423 and 0.387. Although the mean EE index was 0.567, it is clear from the individual values that two of the three eggs inoculated with the10−4 dilution only showed embryo dwarfing. Hence the calculated titres would be 104.24 EID50.
Table 2. Infectious bronchitis virus titration: EE ratios of chicken eggs inoculated with IBV at different dilutionsa
Experiment 3
The mean EE ratio of ECE inoculated with the virus and serum mixture using various antisera is presented in . The mean EE ratio for the ECE inoculated with the virus alone was 0.114. Using this value, the EE index(VN) values were calculated and serum dilution having an EE index(VN) of 1.0 or close to 1.0 can be taken as having no detectable neutralizing antibodies, and hence the previous dilution is taken as the endpoint titre of that serum. In most of the dilutions the EE index(VN) was evenly distributed. However, in the H120 group neutralized with 1:2560 dilution of antisera against 008 virus, the EE index(VN) values of individual eggs were 2.11, 0.877 and 0.947 (mean EE index(VN)=1.31). Again this indicates that this dilution of serum could neutralize the virus in only one of the three eggs.
Table 3. Mean EE ratios of embryonated chicken eggs inoculated with antisera against different isolates of IBV and 100 EID50 H120 vaccine strain
Discussion
From the present study, it appears that the EE ratios are an objective method to quantify embryo dwarfism in IBV-inoculated ECE and can be used to accurately determine the endpoints in virus titration or neutralization assays. Although the EE index is also used to quantify embryo dwarfism, it is especially useful when the EE ratios are evenly distributed. In titration and neutralization tests, it is possible that only a few eggs in each dilution are showing virus growth (or no growth). In those cases, the index calculation may not be a true indicator of lesions in that group although this can be used as a group mean value. It is known that only egg-adapted strains induce clear embryo dwarfism, and field isolates of IBV do not do so until after several embryo passages. In those instances, when serotyping needs to be performed using cross-neutralization tests, quantifying the dwarfism induced by virus alone through the use of EE ratios would be a better measure to compare neutralization by respective antisera.
Acknowledgements
The authors are grateful to the National Agricultural Technology Project, which funded this work.
References
- Cook, JKA , Darbyshire, JH , and Peters, RW , 1976. Growth kinetic studies of avian infectious bronchitis virus in tracheal organ cultures , Research in Veterinary Science 20 (1976), pp. 348–349.
- De Wit, JJ , 2000. Detection of infectious bronchitis virus , Avian Pathology 29 (2000), pp. 71–93.
- Dhinakar Raj, G , and Jones, RC , 1997. Infectious bronchitis virus: immunopathogenesis of infection in the chicken , Avian Pathology 26 (1997), pp. 677–706.
- Hassan, MK , Afify, M , and Aly, MM , 2002. Susceptibility of vaccinated and unvaccinated Egyptian chickens to very virulent infectious bursal disease virus , Avian Pathology 31 (2002), pp. 149–156.
- Keeler, Jr., CL , Reed, KL , Allan Nix, W , and Gelb, Jr., J , 1998. Serotype identification of avian infectious bronchitis virus by RT-PCR of the peplomer (S1) gene , Avian Diseases 42 (1998), pp. 275–284.
- Lucio, B , and Hitchner, SB , 1979. Infectious bursal disease emulsified vaccine: effect upon neutralizing antibody levels in the dam , Avian Diseases 23 (1979), pp. 466–478.
- Reed, LJ , and Muench, H , 1938. A simple method for estimating 50% endpoints , American Journal of Hygiene 27 (1938), pp. 493–497.
- Thangavelu, A , Dhinakar Raj, G , Elankumaran, S , Murali Manohar, B , Koteeswaan, A , and Venugopalan, AT , 1998. Pathogenicity and immuno suppressive properties of infectious bursal disease virus field isolates and commercial vaccines in India , Tropical Animal Health and Production 30 (1998), pp. 167–176.