Abstract
Systemic Mycoplasma synoviae infection in 47-day-old broiler chickens with septicaemic lesions and increased carcass condemnation rate is reported. The clinical history included respiratory signs and an enlarged keel bursa. Condemnations at the processing plant were due to airsacculitis and keel bursitis. Involvement of several organs, including the keel bursa, liver, spleen, brain, choroid of the eye, nerves and skeletal muscle associated with vasculitis, and the isolation of M. synoviae from the liver and keel bursa are only occasionally seen in field cases. Random amplified polymorphic DNA analysis of the M. synoviae isolated from the broiler chickens in this study had a different pattern when compared with the reference M. synoviae strains, WVU-1853, MS-H and F10-2AS, and another M. synoviae isolated from broiler breeders from the same company, but had a similar DNA pattern to an M. synoviae isolated from broiler chickens and turkeys owned by the same company. This finding suggests a horizontally acquired infection rather than vertical transmission.
Il est décrit une infection systémique à Mycoplasma synoviae (MS) chez des poulets de chair âgés de 47 jours présentant des lésions de septicémie et une augmentation du taux de saisie des carcasses. Les commémoratifs incluaient des symptômes respiratoires et des ampoules de bréchet hypertrophiées. Les saisies à l'abattoir ont été dues à l'aérosacculite et aux ampoules de bréchet. L'implication de plusieurs organes, incluant les ampoules de bréchet le foie, la rate, le cerveau, la choroïde des yeux, les nerfs et les muscles du squelette associés à une vascularite, et l'isolement de Ms à partir du foie et des ampoules de bréchet ont été observés occasionnellement dans les cas terrain. L'analyse des réactions de RAPD des Ms isolés des poulets de chair dans cette étude montrent qu'ils ont un profil différent de celui des souches MS de référence, WVU-1853, MS-H et F10-2AS, et d'autres souches de MS isolées de reproducteurs de type chair de la même compagnie, mais ont un profil d'ADN similaire à celui des souches de MS isolées à partir de poulets de chair et de dindes appartenant à la même compagnie. Ce résultat suggère une infection acquise horizontalement plutôt qu'une transmission verticale.
Es wird über einen systemische Mycoplasma synoviae (MS)-Infektion mit septikämischen Veränderungen und erhöhter Schlachtkörperverwerfungsrate bei 47 Tage alten Broilern berichtet. Im klinischen Vorbericht wurden respiratorische Symptome und Vergrößerung der Bursa sternalis genannt. Aufgrund von Aerosacculitis und Entzündung der Bursa sternalis kam es im Weiterverarbeitungsbetrieb zu Verwerfungen. Nur selten wurde wie in diesen Feldfällen die Beteiligung verschiedener Organe wie Bursa sternalis, Leber, Milz, Gehirn, Augenchoroid, Nerven und Skelettmuskeln verbunden mit Vasculitis und die Isolierung von MS aus Leber und Bursa sternalis beobachtet. Die im Rahmen dieser Studie aus den Broilern isolierten MS-Stämme zeigten in der zufalls-amplifizierten polymorphen DNS-Analyse beim Vergleich mit den MS-Referenzstämmen WVU-1853, MS-H und F10-2AS sowie mit einem anderen Isolat aus Broilereltern derselben Firma ein unterschiedliches Muster, wiesen aber ein ähnliches DNS-Muster auf wie ein MS-Stamm aus Broilern und Puten derselben Firma. Diese Befunde lassen eher auf eine horizontal als auf eine vertikal übertragene Infektion schließen.
Se describe un caso de infección sistémica con Mycoplasma synoviae (MS) en pollos de 47 días de edad con lesiones septicémicas e incremento en la proporción de canales decomisadas. La historia clínica incluía signos respiratorios e incremento de tamaño de la bolsa pectoral. Los decomisos en la planta de procesado se debieron a la aerosaculitis y a la bursitis pectoral. En los casos de campo, la afectación de diferentes órganos como la bolsa pectoral, hígado, bazo, cerebro, coroides ocular, nervios y músculo esquelético asociado a vasculitis y el aislamiento de MS de hígado y bolsa pectoral sólo se observó ocasionalmente. El análisis de amplificación polimórfica al azar de ADN de MS aislados de pollos de engorde en estos estudios presentó un perfil diferente respecto a las cepas de MS de referencia, WVU-1853, MS-H y F10-2AS, y otro MS aislado de reproductoras de la misma compañía, pero que presentó un patrón de ADN similar a un MS aislado de pollos de engorde y pavos de la misma compañía. Estos hallazgos sugieren una infección horizontal adquirida más que una transmisión vertical.
Introduction
Mycoplasma synoviae infection is an upper respiratory disease of chickens and turkeys. Most of the time, M. synoviae infection occurs subclinically; it can cause airsacculitis when combined with viral infections, such as avian paramyxovirus 1 (APMV-1) infection and infectious bronchitis (Olson, Citation1984; Kleven, Citation2003). Less frequently, M. synoviae infection can become systemic, resulting in exudative synovitis in joints and tendon sheaths, and bursitis (King et al., Citation1973). In such cases, M. synoviae infection can cause significant economic loss as a result of reduced production efficiency and condemnations due to airsacculitis, synovitis, bursitis and septicaemia (Kerr & Olson, Citation1967; Olson & Kerr, Citation1967; King et al., Citation1973; Timms, Citation1978; Kleven, Citation2003; Lockaby & Hoerr, Citation1999).
This report describes M. synoviae infection in broiler chickens that had systemic lesions involving several organs and a high rate of condemnation at processing.
Materials and Methods
Case history
Fifty-nine live birds, two dead and 16 partially processed carcasses with intact viscera of 47-day-old broiler chickens were submitted for laboratory evaluation and testing to the California Animal Health & Food Safety Laboratory System, Fresno Branch, between 20 and 27 December 2000. The clinical history included respiratory signs, keel bursitis, leg problems, increased mortality, and uneven size of birds. The carcass condemnation rate was 15% at processing, mainly due to airsacculitis and keel bursitis (the normal expected condemnation rate at processing is 2% to 3%). The birds had been vaccinated against Marek's disease at 1 day of age, APMV-1 and infectious bronchitis virus at 18 days of age, and infectious bursal disease virus at 12 days of age. The total mortality in birds for the entire growing period until slaughter ranged from 7.54% to 8.33% in affected flocks. The parent flock of the broiler chickens was also infected with M. synoviae as evidenced by positive serology and isolation of M. synoviae.
Pathology
The live birds were observed for clinical signs and humanely euthanized. Blood was collected from the femoral vein for serology. Necropsies were performed on all submitted chickens and carcasses. Gross lesions were recorded on 54 birds, including eight partially processed carcasses. Except for lack of feathers and the head, which had been removed during processing, the processed carcasses were intact. Sections of the trachea, lung, air sac, heart, liver, kidney, spleen, bursa of Fabricius, thymus, crop, proventriculus, gizzard, intestines, pancreas, skeletal muscle, bone, peripheral nerve, brain, ear and eye were fixed in 10% neutral buffered formalin, processed and embedded in paraffin. Sections were cut at 4 to 7 μm, mounted on glass slides, and stained with haematoxylin and eosin. The microscopic lesions in various organs were scored as mild (+) if there was infiltration of a few inflammatory cells with or with out small amounts of fibrin, moderate (++) if 10 to 20 inflammatory cells were present with or with out fibrin, and severe (+++) if more than 20 inflammatory cells were present with or with out fibrin in the entire specimen in each high-power field (40 x).
Bacteriology
Twenty-one keel bursas, 14 tracheas, 14 livers, eight air sacs, four spleens, three joints and one pericardium were cultured on 3% sheep blood agar and MacConkey agar plates (Remel, Lenexa, Kansas, USA), and incubated at 37°C with 8% CO2 for 24 to 48 h. For mycoplasma isolation, 21 keel bursas, 11 air sacs, 10 tracheas, six brains, four livers and three joints were cultured using modified Frey's broth and agar medium supplemented with 12% of equal volumes of horse and swine sera. Thallium acetate (1:4000) and penicillin (1000 IU/ml) were added as bacterial inhibitors. The plates were incubated at 37°C with 8% CO2, 98% relative humidity and checked for 2 weeks for typical mycoplasma colonies.
Random amplified polymorphic DNA analysis for M. synoviae
Six field isolates of M. synoviae were compared by random amplified polymorphic DNA (RAPD) analysis following the procedure described by Fan et al. (Citation1995). Two isolates were from the keel bursa of broilers in this study with keel bursitis. Two isolates were from the trachea of 33-day-old to 39-day-old broiler chickens diagnosed with severe tracheitis and airsacculitis. One isolate was from the trachea of a clinically normal 51-week-old broiler-breeder of the same company as the broilers in this study with no clinical disease. Another isolate was from the trachea of a 15-week-old commercial meat turkey from a flock of turkeys diagnosed with synovitis and tracheitis. The turkey was from the same company as the broilers and broiler-breeder, and the turkey flock was raised in the same general area where the chickens were raised. In addition, three reference strains, MS-H, WVU-1853 and F10-2AS, were analysed by RAPD (Yogev et al., Citation1988).
Serology
Serum from 45 birds was tested for antibodies to Mycoplasma gallisepticum and M. synoviae by the serum plate agglutination test according to the manufacturer's instructions (Intervet Inc., Millsboro, Delaware, USA); serum samples showing agglutination at a dilution of 1:10 or higher were considered positive; serum samples showing agglutination at dilution 1:5 were considered suspect. Sera were tested for antibodies to infectious bronchitis (serotypes Arkansas 99, Connecticut, and Massachusetts 41), M. gallisepticum and M. synoviae using standard procedures for haemagglutination-inhibition (HI) tests (Thayer & Beard, Citation1998). For M. synoviae, serum samples with a HI titre ≥1:80 were considered positive, serum samples with a HI titre of 1:20 or 1:40 were considered suspect. Serology for M. synoviae, APMV-1, infectious bronchitis virus, infectious bursal disease virus and reovirus was performed by enzyme-linked immunosorbent assays (ELISA) (IDEXX Laboratories, Inc., Westbrook, Maine, USA); for M. synoviae, sera with titres in titre group 1 and above were considered positive according to the manufacturer's instructions.
Virology
Samples of trachea and lung were pooled together, and individual samples of caecal tonsil, brain, and proventriculus tissues were collected separately in virus transport medium. Each organ was pooled together and tissues were triturated and the suspensions were clarified by centrifugation at 1780 g for 10 min at 4°C, and then filtered through a 0.2 μm membrane filter. The supernatant was inoculated into the allantoic cavity and onto the chorioallantoic membrane of 10-day-old specific pathogen free embryonating chicken eggs (SPAFAS; Storrs, Connecticut, USA). The eggs were incubated at 37°C and candled daily for up to 7 days. Two serial passages of chorioallantoic fluid in embryonating eggs were performed using standard methods (Senne, Citation1998).
Results
Gross pathology
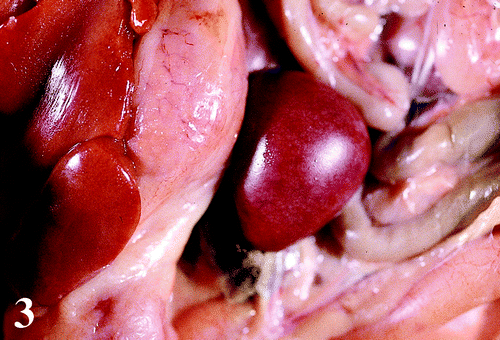
The frequency and severity of the macroscopic lesions in selected organs are presented in . Lesions observed at necropsy included moderate to severe enlargement of the keel bursa with an accumulation of fibrinous and/or caseous exudate and occasional petecchiae (). The livers were mildly to severely enlarged with a prominent reticular pattern, and occasionally had a greenish tinge (). The spleens were moderately to severely enlarged with white mottling (). There were mildly to moderately enlarged hocks with an accumulation of fibrinous and/or caseous exudate in five birds. Other lesions included a mild to severe accumulation of fibrinous to caseous exudate in the air sacs, pericardium and capsule of the liver; congestion of the lungs; moderate to severe atrophy of the bursas of Fabricius and thymus; moderately enlarged and pale kidneys; moderately thickened proventricular wall and a moderate increase in mucus in the trachea.
Figure 1. 1a: Subcutaneous, diffuse accumulation of fibrinous exudate in and around the keel bursa of a broiler chicken carcass infected with M. synoviae. 1b: Extensive localized accumulation of caseous exudate in the keel bursa of a 47-day-old broiler chicken infected with M. synoviae.
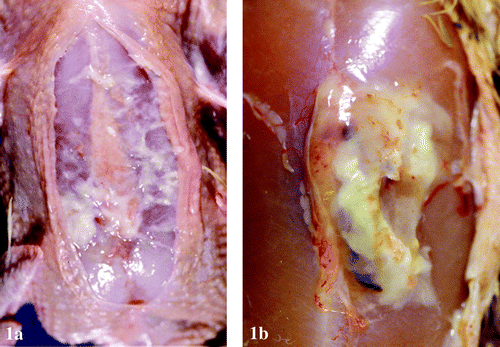
Figure 2. Enlarged liver with greenish tinge and scattered pale reticular pattern from a 47-day-old broiler chicken infected with M. synoviae.
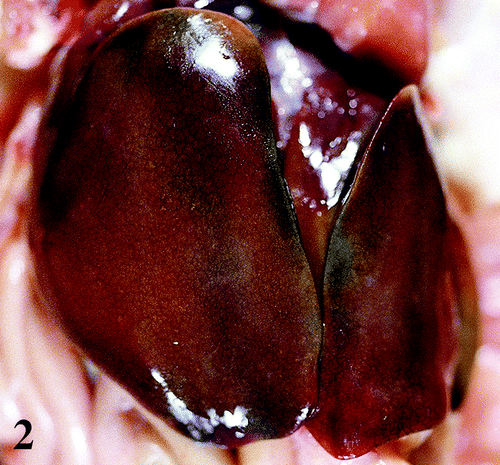
Table 1. Frequency and severity of gross lesions associated with M. synoviae in selected organs of broiler chickens
Histopathology
The relative frequency and severity of the microscopic lesions in selected organs are presented in .
Table 2. Relative frequency and severity of microscopic lesions associated with M. synoviae in selected organs of broiler chickens
Keel bursa
There was mild to severe synovitis characterized by mild to severe infiltration of lymphocytes and plasma cells, most prominent around blood vessels (). The endothelial cells lining the vessels were hypertrophied and some contained vacuolated cytoplasm. There was infiltration of lymphocytes and a few plasma cells in the vessel wall (). Additionally, there were moderate to severe accumulations of fibrin mixed with large numbers of heterophils and a few lymphocytes and macrophages. Lymphoid nodule formations and fibrosis on the surface of the synovium was also seen.
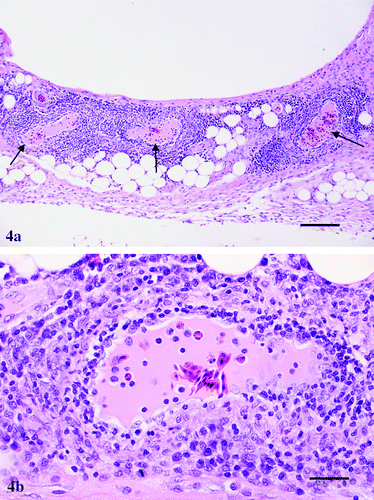
Figure 4. 4a: Keel bursa from a 47-day-old broiler chicken infected with M. synoviae showing severe perivascular cuffing (arrows) by mononuclear inflammatory cells. Haematoxylin and eosin, bar=200 μm. 4b:Higher magnification of one field from 4a showing in more detail prominent endothelial cells and infiltration by lymphocytes, a few plasma cells and macrophages in and around a blood vessel. Haematoxylin and eosin, bar=50 μm.
Joints
The synovium from the hock joints and a few intervertebral joints were thickened due to moderate to severe infiltrations of lymphocytes and plasma cells. There were a few birds similarly affected, but with mild fibrinoheterophilic inflammation.
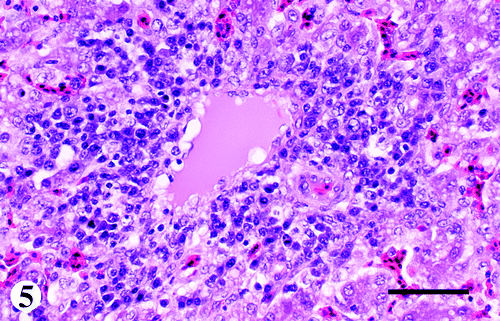
Liver
There were mild to severe infiltrations of lymphocytes and plasma cells in and around blood vessels of the portal triads and a few central veins (). Similar to the vessels in the keel bursa, the endothelial cells lining the hepatic vessels were also hypertrophied with vacuolated cytoplasm. Other changes consisted of increased cellularity in the sinusoids, lymphoid nodule formation and multifocal single-cell necrosis of hepatocytes. The Glisson's capsule was moderately to severely infiltrated with fibrin mixed with heterophils and a few lymphocytes.
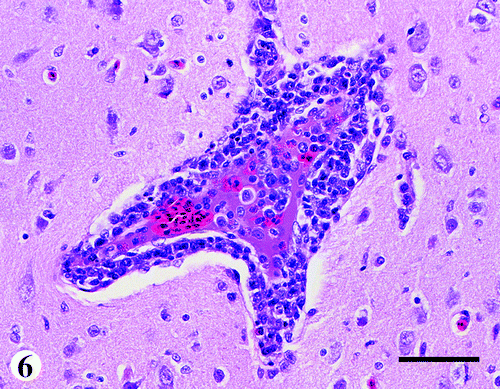
Brain
There was mild to moderate multifocal infiltration of lymphocytes and a few plasma cells in and around blood vessels of the neuropil (). The changes in the endothelial cells were similar to those of the vessels of the keel bursa and liver. Other changes consisted of a mild to moderate vasculitis and perivascular cuffing primarily by lymphocytes within the meninges.
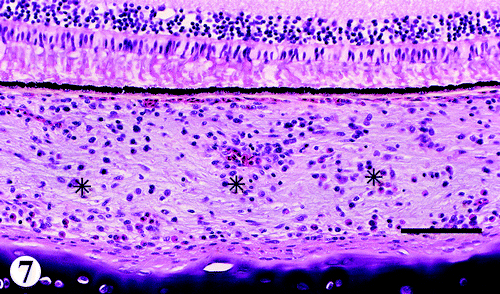
Eye
There were mild to moderate infiltrations of lymphocytes mixed with a few plasma cells, and occasional heterophils in the choroid (). Other changes consisted of similar infiltrates in the conjunctiva, iris, ciliary body and process, episclera, and optic nerves. Some of the muscles surrounding the sclera had a few lymphocytic foci.
Skeletal muscle
There were occasional vessels with mild to moderate fibrinoid necrosis of the tunica media and mild lymphocytic infiltrations. Otherwise, the muscles looked normal except for occasional foci of lymphocytes.
Other organs
There was mild to moderate infiltration of lymphocytes mixed with a few plasma cells and macrophages with lymphoid nodule formations in the mucosa of the turbinates, infraorbital sinuses, trachea, syrinx, bronchial mucosa, myocardium, air sac and pericardium, and in the interstitium of the kidney and glandular part of the proventriculus. In addition, the air sac, pericardium and skin in a few birds had severe fibrinosuppurative inflammation. The spleen had moderate to severe multifocal increase in the mononuclear phagocytic system cells, and vasculitis characterized by infiltration of lymphocytes in and around the blood vessels. There was mild to moderate lymphoid depletion in the bursa of Fabricius and thymus.
Bacteriology
M. synoviae was isolated from the air sac (3/11), keel bursa (5/21), trachea (3/14), joint (3/3), and liver (1/4). Escherichia coli were isolated from the air sac (5/12), pericardial sac (1/2), liver (3/4), keel bursa (9/21), and trachea (11/14). Other bacteria isolated included Staphylococcus spp. from the keel bursa (1/4) and liver (7/21); Ornithobacterium rhinotracheale from the trachea (3/14); β-haemolytic Streptococcus from the trachea (3/14); Bordetella avium from the trachea (2/14); and Gallibacterium anatis var. haemolyticum was isolated from the trachea (4/14).
RAPD analysis for M. synoviae
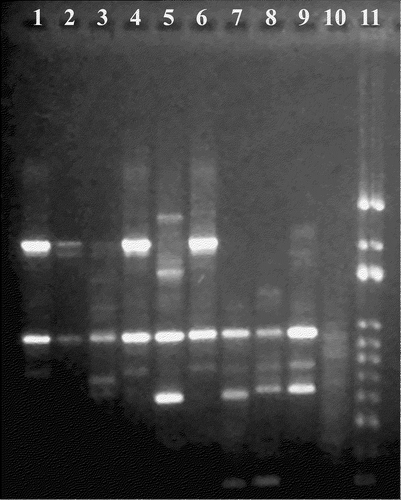
The DNA migration patterns of the six field isolates and three reference strains of M. synoviae are presented in . One isolate each from the broilers under study, the 33-day-old to 39-day-old broilers and the meat-type turkey, had similar migration patterns (, lanes 1, 4 and 6, respectively). Two isolates, one from the broiler chickens with keel bursitis and one from the 33-day-old to 39-day-old broilers (, lanes 2 and 3), appeared similar to the previous three isolates but were indeterminate. The M. synoviae isolate from the broiler-breeder was distinct from the other strains (, lane 5). All six field strains of M. synoviae had different migration pattern when compared with the three M. synoviae reference strains (, lanes 7, 8 and 9).
Figure 8. RAPD analysis of M. synoviae isolates. Lanes 1 and 2, isolates from 47-day-old broiler chickens; lanes 3 and 4, isolates from 33-day-old to 39-day-old broiler chickens; lane 5, isolate from a turkey; lane 6, isolate from a broiler breeder; lane 7, MS-H reference strain; lane 8, WVU 1853 reference strain; lane 9, F10-2AS reference strain; lane 10, negative control; lane 11, 100 base pair ladder.
Serology
In plate agglutination tests, 28/33 sera were positive and 3/33 were suspect for M. synoviae. In HI tests, 5/33 sera were positive and 18/33 were suspect for M. synoviae. In ELISA tests, 13/33 sera were positive for M. synoviae. All the sera were negative for M. gallisepticum. The titres for APMV-1, infectious bronchitis virus, and infectious bursal disease virus were compatible with vaccination titres.
Virology
Adenovirus was isolated from a trachea/lung pool and proventriculus. No other viruses including APMV-1 and infectious bronchitis virus were isolated from brain, proventriculus, trachea/lung or caecal tonsils.
Discussion
The gross and microscopic lesions observed in the broilers in this study would account for the clinical signs, increased mortality and condemnations at the processing plant. Keel bursitis and airsacculitis were the primary reasons for increased condemnation rates. The condemnation rate was much higher (up to 15%) in these broiler chickens compared with the 1.88% condemnation rate reported previously in the progeny of M. synoviae-positive breeders (King et al., Citation1973). M. synoviae infection was probably the primary cause of the clinical signs and lesions. M. synoviae infection was confirmed by its isolation from keel bursae, air sacs, tracheas, hock joints and one liver. Many sera were positive for M. synoviae by different serological tests, suggesting recent exposure to M. synoviae. Other bacteria, such as E. coli and O. rhinotracheale, may have contributed to the severity of the clinical signs, lesions and mortality in the chickens. Adenovirus was isolated from the trachea/lung pool and proventriculus. The significance of adenovirus in these broiler chickens could not be determined; adenoviruses are ubiquitous in poultry (McFerran & Adair, Citation2003) and no adenovirus inclusions were observed in any organ.
The systemic presentation of M. synoviae in broiler chickens with severe keel bursitis, hepatitis, splenitis, encephalitis, myocarditis, choroiditis and iridocyclitis, in addition to synovitis, sinusitis, tracheitis, bronchitis and airsacculitis, is unusual in field cases. Vasculitis, especially in the keel bursa, liver, spleen, brain, skeletal muscle, and choroid of the eye, was also present. Such systemic lesions occasionally with vasculitis have been produced by experimental infection with M. synoviae (Kerr & Olson, Citation1970; Kawakubo et al., Citation1980). Usually, M. synoviae infections in young chickens reared under field conditions are subclinical, but sometimes a clinical upper respiratory disease is observed, particularly when APMV-1 and/or infectious bronchitis virus are present (Kleven, Citation2003). In such cases, gross lesions, when present, are usually in the respiratory tract, primarily consisting of airsacculitis (Ghazikhanian et al., Citation1973; Olson, Citation1984; Kleven, Citation2003). The lymphoplasmacytic infiltration with lymphoid nodule formation and heterophilic infiltration found in several organs are common lesions associated with systemic M. synoviae infections. The mixed inflammatory infiltrations observed in these birds were similar to those previously reported (Sevoian et al., Citation1958; Kerr & Olson, Citation1970; Kleven et al., Citation1975; Fletcher et al., Citation1976; Kawakubo et al., Citation1980; Olson, Citation1984; Lockaby et al., Citation1998; Lockaby & Hoerr, Citation1999).
Vasculitis due to Mycoplasma spp. is not a common feature in chickens or turkeys, but has been reported in turkeys infected with M. synoviae as well as M. gallisepticum (Cordy & Adler, Citation1957, Citation1965; Chin et al., Citation1991a,Citationb). Lesions of vasculitis due to M. synoviae isolate WVU-1853 in 4-week-old to 8-week-old laying type chickens included mild to moderate fibrinoid necrosis of the tunica media and the infiltration of lymphocytes and a few plasma cells in the medium sized vessels of the heart, spleen and glomeruli of the kidney, and perivascular cuffing in the brain (Kawakubo et al., Citation1980). In contrast, vasculitis was not reported in broiler chickens following the experimental inoculation with six isolates of M. synoviae including an isolate, WVU-1853, of 1-day-old broiler chickens and examined between 7 and 42 days of age after inoculation (Lockaby et al., Citation1998). This difference could be related to the type of chickens used, differences in age of the birds at the time of inoculation and routes of inoculation.
The pathogenesis of vasculitis or systemic infections with certain M. synoviae isolates is not known. Factors such as variation in the pathogenicity and tissue tropism of the different strains of M. synoviae have been suggested (Kleven et al., Citation1975; Olson, Citation1984; Lockaby et al., Citation1998; Kang et al., Citation2002). Other factors, such as cytoadhesins, membrane proteins that mediate intimate attachment of the organisms to the host cells, effects of the organism on the host immune system, toxins, and stimulation of tissue cytokines that results in tissue damage, have been proposed but have not been proven (Lockaby et al., Citation1999; Kleven, Citation2003).
The most consistent lesions found in the birds examined in this study were keel bursitis followed by hepatitis. Hock joints were involved in only a few birds. This is the first report of M. synoviae associated with lesions in the eye and its components.
RAPD analysis showed that the field isolates from these broilers were different from the reference strains, WVU-1853, MS-H and F10-2AS. In previous studies, the reference isolate, WVU-1853, produced systemic lesions (Kawakubo et al., Citation1980; Kerr & Olson, Citation1970; Lockaby et al., Citation1998) similar to those seen in the broilers in this study including vasculitis in one study (Kawakubo et al., Citation1980). Interestingly, the isolates from the broilers were not similar to those from the breeder flock, but were similar to isolates from another broiler flock and a commercial turkey flock, both owned by the same company. One can infer that the isolate was spread horizontally through the company, rather than vertically from the breeders. In addition, it shows that various isolates of M. synoviae with different pathogenicity can be present within the same poultry company.
Translations of the abstract in French, German and Spanish are available on the Avian Pathology website.
The authors would like to thank Dr Stan Kleven for analysing the M. synoviae isolates by RAPD analysis and for his excellent suggestions. The authors would also like to thank Dr R. Crespo, Dr B. Charlton, Dr P. Woolcock and Dr M. McFarland and the staff, R. Gonzales, R. Kokka, M. Rezvani, L. Alsing, D. Ramirez, M. Imai, E. Emery, P. Lauck, V. Perez, H. Moriyama, C. Hufnagle and D. Neely, for their valuable technical assistance.
Additional information
Notes on contributors
Gabriel Sentíes-Cué Footnote#
Present address: Pathobiology and Population Medicine Research and Diagnostic Poultry Laboratory, Mississippi State University, PO Box 97813, Pearl, MS 39 288 USA.Notes
Present address: Pathobiology and Population Medicine Research and Diagnostic Poultry Laboratory, Mississippi State University, PO Box 97813, Pearl, MS 39 288 USA.
References
- Chin , RP , Meteyer , CU , Yamamoto , R , Shivaprasad , HL and Klein , PN . (1991a) . Isolation of Mycoplasma synoviae from the brains of commercial meat turkeys with meningeal vasculitis . Avian Diseases , 35 : 631 – 637 .
- Chin , RP , Daft , BM , Meteyer , CU and Yamamoto , R . (1991b) . Meningoencephalitis in commercial meat turkeys associated with Mycoplasma gallisepticum . Avian Diseases , 35 : 986 – 993 .
- Cordy , DE and Adler , HE . (1957) . The pathogenesis of the encephalitis in turkey poults produced by a neurotropic pleuropneumonia-like organisms . Avian Diseases , 1 : 235 – 245 .
- Cordy , DE and Adler , HE . (1965) . Brain and muscle lesions caused by Mycoplasma gallisepticum in turkey poults . American Journal of Veterinary Research , 26 : 186 – 189 .
- Fan , HH , Kleven , SH and Jackwood , MW . (1995) . Studies of intraspecies heterogeneity of Mycoplasma synoviae, M. meleagrides, and M. iowae with arbitrarily primed polymerase chain reaction . Avian Diseases , 39 : 766 – 777 .
- Fletcher , OJ , Anderson , DP and Kleven , SH . (1976) . Histology of air sac lesions induced in chickens by contact exposure to Mycoplasma synoviae . Veterinary Pathology , 13 : 303 – 314 .
- Ghazikhanian , G , Yamamoto , R and Cordy , DR . (1973) . Response of turkeys to experimental infection with Mycoplasma synoviae . Avian Diseases , 17 : 122 – 136 .
- Kang , MS , Gazdzinski , P and Kleven , SH . (2002) . Virulence of recent isolates of Mycoplasma synoviae in turkeys . Avian Diseases , 46 : 102 – 110 .
- Kawakubo , Y , Kume , K and Yohioka , M . (1980) . Histo- and immuno-pathological studies on experimental Mycoplasma synoviae infection of the chicken . Journal of Comparative Pathology , 90 : 457 – 467 .
- Kerr , KM and Olson , NO . (1967) . Cardiac pathology associated with viral and mycoplasma arthritis in chickens . Annals of the New York Academy of Sciences , 143 : 204 – 217 .
- Kerr , KM and Olson , NO . (1970) . Pathology of chickens inoculated experimentally or contact-infected with Mycoplasma synoviae . Avian Diseases , 14 : 291 – 320 .
- King , DD , Kleven , SH , Wenger , DM and Anderson , DP . (1973) . Field studies with Mycoplasma synoviae . Avian Diseases , 17 : 722 – 726 .
- Kleven SH (2003) Mycoplasma synoviae infection In Y.M. Saif (Ed.) Diseases of Poultry 11th edn Ames Iowa State University Press pp. 756–766
- Kleven , SH , Fletcher , OJ and Davis , RB . (1975) . Influence of strain of Mycoplasma synoviae and route of infection on development of synovitis or airsacculitis in broilers . Avian Diseases , 19 : 126 – 135 .
- Lockaby , SB and Hoerr , FJ . (1999) . Virulence of Mycoplasma synoviae in poultry: a review . World's Poultry Science Journal , 55 : 175 – 185 .
- Lockaby , SB , Hoerr , FJ , Lauerman , LH and Kleven , SH . (1998) . Pathogenicity of Mycoplasma synoviae in broiler chickens . Veterinary Pathology , 35 : 178 – 190 .
- Lockaby , SB , Hoerr , FJ , Lauerman , LH , Smith , BF , Samoylov , AM , Toivio-Kinnucan , M and Kleven , SH . (1999) . Factors associated with virulence of Mycoplasma synoviae . Avian Diseases , 43 : 251 – 267 .
- McFerran JB Adair BM (2003) Group I Adenovirus Infections In Y.M. Saif (Ed.) Diseases of Poultry 11th edn Ames Iowa State Press pp. 214–227
- Olson NO (1984) Mycoplasma synoviae infection In M.S. Hofstad (Ed.) Diseases of Poultry 8th edn Ames Iowa State University Press pp. 212–220
- Olson , NO and Kerr , KM . (1967) . The duration and distribution of synovitis-producing agents in chickens . Avian Diseases , 11 : 578 – 585 .
- Senne DA (1998) Virus propagation in embryonating eggs In D.E. Swayne (Ed.) A Laboratory Manual for the Isolation and Identification of Avian Pathogens 4th edn Kennett Square PA American Association of Avian Pathologists pp. 235–240
- Sevoian , M , Snoeyenbos , GH , Basch , HI and Reynolds , IM . (1958) . Infectious synovitis I. Clinical and pathological manifestations . Avian Diseases , 2 : 499 – 513 .
- Thayer SG Beard CW (1998) Serologic procedures In D.E. Swayne (Ed.) A Laboratory Manual for the Isolation and Identification of Avian Pathogens 4th edn Kennett Square PA American Association of Avian Pathologists pp. 255–266
- Timms , LM . (1978) . Mycoplasma synoviae: a review . The Veterinary Bulletin , 48 : 187 – 198 .
- Yogev , D , Levisohn , S , Kleven , SH , Halachmi , D and Razin , S . (1988) . Ribosomal RNA gene probes to detect intraspecies heterogeneity in Mycoplasma gallisepticum and M. synoviae . Avian Diseases , 32 : 220 – 231 .