Abstract
The effects of vitamin A, pentoxyfylline and methylprednisolone on experimentally induced amyloid arthropathy were investigated. In this study, 175 1-day-old brown layer chicks were used. Throughout the study Group II (vitamin A) received high doses of vitamin A (75 000 IU/kg), whereas Group I (negative control), Group III (positive control), Group IV (pentoxyfylline) and Group V (methylprednisolone) received normal levels of vitamin A in the diet. At the fifth week, the experimental Groups II, III, IV and V were injected with Freund's adjuvant intra-articularly to induce amyloid arthropathy. Group IV received pentoxyfylline and Group V received methylprednisolone (10 mg/kg, intramuscularly) once. Joint and blood samples were examined 13 weeks after the injections. The values in Groups I, II, III, IV and V, respectively, were as follows: amyloid arthropathy formation (%), 0, 100, 87, 76, 66; serum amyloid A (ng/ml), 166±17, 607±40, 423±39, 342±27, 293±22; serum retinol (μg/dl): 59.75±3.8, 42.72±3, 59.24±3.6, 102±9.1, 101.3±12.3; heterophil/lymphocyte ratio: 0.504, 0.75, 0.75, 0.087, 0.44. In conclusion, it was observed that vitamin A enhanced the development of amyloid arthropathy and there were positive associations between amyloidosis, increased levels of serum amyloid A and increased numbers of tissue infiltrating macrophages. Methylprednisolone had a more successful inhibitory effect on amyloid arthropathy than pentoxyfylline.
Il a été étudié les effets de la vitamine A, de la pentoxyfylline et de la méthylprednisolone sur l'arthropathie amyloïde induite expérimentalement. Pour cette étude, 175 futures pondeuses rousses âgées d'un jour ont été utilisées. Tout au long de l'étude, le groupe II (vitamine A) a reçu des doses élevées en vitamine A (75,000 IU/kg), alors que les groupes I (témoin négatif), III (témoin positif), IV (pentoxyfylline) et V (méthylprednisolone) recevaient des niveaux normaux de vitamine A dans l'alimentation. A la cinquième semaine, les groupes expérimentaux II, III, IV et V ont reçu par voie intra articulaire de l'adjuvant de Freund pour induire l'arthropathie amyloïde. Le groupe IV a reçu de la pentoxyfylline et le groupe V a reçu une fois de la methylprednisolone [10 mg/kg, par voie intramusculaire (i.m.) ]. Des échantillons d'articulation et de sang ont été étudiés 13 semaines après les injections. Les valeurs obtenues dans les groupes I, II, III, IV et V ont été respectivement les suivantes : - formation d'arthropathie amyloïde (en %) 0, 100, 87, 76, 66; - sérum amyloïde A (SAA) (ng/ml) 166±17, 607±40, 423±39, 342±27, 293±22; - les teneurs du sérum en rétinol (μg/dl): 59,75±3,8, 42,72±3, 59,24±3,6, 102±9,1, 101,3±12,3; - rapport hétérophiles/lymphocytes (H/L) 0,504, 0,75, 0,75, 0,087, 0,44.
En conclusion, il a été observé que la vitamine A augmentait le développement de l'arthropathie amyloïde et qu'il y avait des associations positives entre l'amyloïdose, les niveaux élevés du SAA et l'augmentation des nombres de tissus infiltrés par les macrophages. La méthylprednisolone a eu un effet plus satisfaisant sur l'arthropathie amyloïde que la pentoxyfylline.
Es wurden die Wirkungen von Vitamin A, Pentoxyfyllin und Methylprednisolon auf eine experimentell induzierte amyloide Gelenkserkrankung untersucht. In dieser Studie wurden 175 braune Legehennen-Eintagsküken verwendet. Während der Untersuchungen erhielt Gruppe II (Vitamin A) hohe Dosen von Vitamin A (75.000 IU/kg), wohingegen die Gruppen I (Negativkontrolle), III (Positivkontrolle,) IV (Pentoxyfyllin) und V (Methylprednisolon) normale Vitamin A-Gehalte im Futter hatten. In der fünften Woche wurde den Versuchsgruppen II, III, IV und V zur Auslösung einer amyloiden Arthropathy Freundsches Adjuvans intraartikular injiziert. Einmalig erhielten Gruppe IV Pentoxyfyllin und Gruppe V Methylprednisolon (10 mg/kg intramuskulär (i.m.)). 13 Wochen nach den Injektionen wurden Gelenks- und Blutproben untersucht. Die Werte in den Gruppen I, II, III, IV und V waren folgendermaßen: Ausbildung amyloider Gelenksveränderungen (%): 0, 100, 87, 76, 66; Serumamyloid A (SAA) (ng/ml): 166±17, 607±40, 423±39, 342±27, 293±27, 293±22; Serumretinol (g/dl: 59,75±3,8, 42,72±3, 59,24±3,6, 102±9,1, 101,3±12,3; Verhältnis von Heterophilen/Lymphozyten (H/L): 0.504, 0.75, 0.75, 0.087, 0.44.
Zusamenfassend kann festgestellt werden, dass Vitamin A die Entwicklung einer amyloiden Arthropathy verstärkte und dass es einen positiven Zusammenhang zwischen Amyloidose, erhöhter SSA-Werte und erhöhter Anzahl Gewebe infiltrierender Makrophagen gab. Methylprednisolon hatte einen stärkeren inhibitorischen Effekt auf die amyloide Arthropathy als Pentoxyfyllin.
Se investigaron los efectos de la vitamina A, la pentoxifilina y la metilprednisolona en una artropatía amiloidea inducida experimentalmente. En este estudio, se utilizaron 175 pollitas rubias de un día de edad. A lo largo del estudio, el Grupo II (vitamina A) recibió altas dosis de vitamina A (75,000 IU/kg), mientras que los Grupos I (control negativo), III (control positivo), IV (pentoxifilina) y V (metilprednisolona) recibieron niveles normales de vitamina A en la dieta. A la quinta semana, los grupos experimentales II, III, IV y V fueron inyectados con adyuvante de Freund's intra-articularmente para inducir artropatía amiloidea. El Grupo IV recibió pentoxifilina y el Grupo V recibió metilpredinisolona [10 mg/kg, intramuscularmente (i.m.) ] una vez. Se examinaron muestras de articulación y sangre a las 13 semanas. Los valores de los Grupos I, II, III, IV y V, respectivamente fueron los siguientes: desarrollo de artropatía amiloidea (%): 0, 100, 87, 76, 66; amiloide sérico A (SAA) (ng/ml): 166±17, 607±40, 423±39, 342±27, 293±22; retinol sérico (μg/dl): 59.75±3.8, 42.72±3, 59.24±3.6, 102±9.1, 101.3±12.3; proporción heterófilo/linfocito (H/L): 0.504, 0.75, 0.75, 0.087, 0.44. En conclusión, se observó que la vitamina A favorecía el desarrollo de artropatía amiloidea y hubo una asociación positiva entre amiloidosis, niveles de SAA incrementados e incremento de los números de los macrófagos infiltrando el tejido. La metilprednisolona tuvo un efecto inhibitorio mucho mayor que la pentoxifilina en la artropatía amiloidea.
Introduction
It has been widely recognized that amyloid arthropathy, characterized by the accumulation of amyloid in joints, is a common pathological disorder in certain kinds of birds (Landman, Citation1999) and the biochemical mechanism of the pathogenesis of amyloidosis in avians remains obscure (Landman et al., Citation1998b). It has been demonstrated that predisposing conditions such as chronic infections, inflammation or tumours cause an increase in the serum levels of serum amyloid A (SAA), a precursor protein of amyloid protein A of hepatic origin (Urieli-Shoval et al., Citation2000). This unstable precursor protein of amyloid proteins accumulates in the target tissues or organs and reorganizes into fibrillar form to cause amyloidosis by an unknown mechanism (Glenner, Citation1980; Landman et al., Citation1998b). Zschiesche & Linke (Citation1986) and Landman et al. (Citation1996) demonstrated that SAA is the precursor protein of type AA amyloidosis also in avians. It is thought that serine proteinases [U1]located on the surface of granular macrophages play a very important role in the reorganization of SAA into amyloid fibrils in tissues (Glenner, Citation1980; Landman et al., Citation1998b).
Amyloidosis can be defined as the deposition of soluble proteins or their fragments in the extracellular space of many different tissues and identified as a non-branching, 7 to 10 nm fibrillar form (Glenner, Citation1980).
Avian amyloidosis has been shown to affect certain kinds of birds causing quite a number of different diseases (Landman, Citation1999). Joint amyloidosis is one of the clinical problems associated with growth depression and lameness in brown layers (Landman et al., Citation1994). Landman et al. (Citation1998a) reported that Enterococcus faecalis was isolated from a case of joint amyloidosis that occurred in a field outbreak in chickens and experimentally induced E. faecalis amyloidosis that was reactive-type amyloid (AA amyloid).
It has been shown that the type AA amyloid-related precursor protein (SAA) of amyloid fibrils is increased by cytokines such as interleukin (IL)-1 (Ramadori et al., Citation1985), IL-6 (Marinkovic et al., Citation1989), tumour necrosis factor alpha (TNF-α), macrophage colony stimulating factor (M-CSF) (Betts et al., Citation1993). Retinoids have been shown to influence many aspects of immunity including the function of leucocytes and the expression of cytokines (TNF-α, IL-1, IL-2, IL-3 and IL-6) (Dillahay et al., Citation1988; Turpin et al., Citation1990; Göttgens & Green, Citation1995; Ross, Citation1999). Furthermore, Katz et al. (Citation1987) showed that retinoids stimulate both an increase in the number of macrophages in vivo and enhance their activity in vitro. Therefore anti-inflammatory agents such as colchicine and dimethylsulfoxide are used for amyloid treatment in humans (Kisilevsky et al., Citation1995; Soto et al., Citation1996) and mice (Inoue et al., Citation1996). However, there have been no reports concerned with the treatment of avian amyloidosis.
Pentoxyfylline, a well-known inhibitor of phosphodiesterase, is a trimethylated xanthine derivative, which triggers an increase in intracellular cyclic adenosine monophosphate, causing the dilatation of blood vessels and the improvement of microcirculation. It has been used to improve peripheral blood vessel disease for many years (Windmeier & Gressner, Citation1997; Schuppan et al., Citation2000). A rise in intracellular cAMP has been suggested as a pentoxyfylline-mediated inhibitory effect on the respiratory burst of neutrophils (Besler et al., Citation1986). Furthermore, pentoxyfylline has been shown to inhibit the synthesis of glycosaminoglycan, the production of fibronectin in cultured fibroblasts, the activity of collagenase and TNF-α (Berman & Duncan, Citation1989; Chang et al., Citation1993).
Methylprednisolone, an anti-inflammatory and immune suppressive agent, is a synthetic adrenocortical derivative, which inhibits TNF-α (Pitzalis et al., Citation1997; Xu et al., Citation1998), IL-2 (Wandinger et al., Citation1998), IL-6 (Stanton et al., Citation1999), IL-10 (Hodge et al., Citation1999) and the synthesis of glycosaminoglycans in rats (Laato et al., Citation1989).
Our previous study (Sevimli et al., Citation2004) showed that vitamin A increased the severity of E. faecalis-induced amyloid arthropathy. The aim of this study was therefore to determine the effects of high vitamin A added feeding on Freund's adjuvant-induced amyloid arthropathy and the effects of pentoxyfylline and methylprednisolone on amyloid arthropathy treatment.
Materials and Methods
Ethics
The experimental protocols were approved by the Animal Care and Use Committee at Uludag University and are in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals.
Animals and experimental design
One hundred and seventy-five 1-day-old brown layer chicks were used. The chicks had been vaccinated against avian influenza, infectious bronchitis and Marek's disease, Newcastle disease and Gumboro disease. The light schedule was 14 h light and 10 h dark. The chicks were allocated into five groups. Group I (negative control, n=35), Group III (positive control, n=35), Group IV (pentoxyfylline, n=35), Group V (methylprednisolone, n=35) were fed ad libitum with a commercial diet containing normal levels of vitamin A (5000 IU/kg) while Group II (vitamin A, n=35) were fed ad libitum with a commercial diet containing high levels of vitamin A (75 000 IU/kg). To induce amyloid arthropathy in Groups II, III, IV and V, the birds were injected with 0.25 ml complete Freund's adjuvant into the left inter-tarsal joint at the fifth week of the experiment as described by Landman et al. (1998), while Group I was injected intra-articularly with 0.9% NaCl (0.25 ml). On the same day, chicks in Group IV and Group V were. injected intramuscularly with 10 mg/kg pentoxyfylline and methylprednisolone, respectively, to induce immunosuppression. Four chickens in Group III and one chicken in Group IV died of pneumonia at the sixth week. All pullets were necropsied at 13 weeks post-injection.
Tissue sampling and processing
The whole inoculated hock joints (including the synovial membrane and joint capsule) collected during necropsy were fixed in 10% phosphate-buffered formaldehyde solution for 24 h and processed to paraffin, sectioned at 5 μm and stained with haematoxylin and eosin and Congo red stain (Lee & Luna, Citation1968). Immunohistochemistry was done to identify amyloid using the streptavidin–biotin–peroxidase method (True, Citation1990). Goat anti-mouse Amyloid A Ab-1 (mc1) antibody was used as supplied (Neomarkers-Freemont, USA) to demonstrate amyloid deposition in synovial membrane. AEC was used as chromogen in all tissue sections. Tissue sections were examined for amyloid occurrence rates and inflammatory cell infiltration rates.
The amount of amyloid deposition in synovial membrane was scored semiquantitatively in the Congo red and immunohistochemically stained slides (True, Citation1990). Congo red-stained slides were examined in polarized light for the characteristic green birefringence of amyloid while immunohistochemically stained slides were examined under light microscopy. Scores (– to +++) were given based on the amount of amyloid. The presence of amyloid was graded according to the intensity and dissemination of staining as negative (–), mild (+), moderate (++), severe (+++) under light microscopy with 10×magnification. The type of inflammatory cells was investigated in sections stained with haematoxylin and eosin under light microscopy at 20×magnification. The presence of heterophils, lymphocytes, plasmocytes and macrophages seen in the area with 20×magnification under a light microscope were also graded. The absence of cells was graded as negative (–); the presence of three to six cells graded as mild (+); seven to 10 cells graded as moderate (++); above 10 cells was graded as severe (+++).
Serological studies
Blood samples of 12 randomly selected birds per group were taken from the carotid artery into disodium ethylenediamine tetraacetic acid-coated tubes before necropsy and were used for SAA and serum retinol measurements.
SAA measurement
A commercial enzyme-linked immunosorbent assay kit (TP-802M; Tridelta, Maynooth Co. Kildare, Ireland) was used to detect SAA. In this study, a murine kit and its standards were used because a kit for chicken SAA was not available. However, the producer of the murine kit recommended the use of the murine kit since there is a cross-reactivity between chicken and mouse antibody (Tridelta). The Tridelta phase™ range SAA kit is a solid-phase sandwich enzyme-linked immunosorbent assay. A monoclonal antibody specific for SAA had been coated onto the wells of the microtitre strips provided.
Test reagents and samples were allowed to reach room temperature prior to use. The number of eight-well strips needed for the assay was determined. Fifty microlitres of diluted biotinylated anti-SAA were added into each well. The serum samples were vortexed and diluted 1:500 in 1×diluent buffer. Fifty microlitres ere added, in duplicate, of diluted sample into each well. The plates were covered with a dust cover. The plates were incubated for 1 h at 37°C. After incubation, aspiration was performed and the plates were washed four times with diluted wash buffer. After the last wash, the plates were dried on absorbent paper. One-hundred microlitres of streptavidin–peroxidase was added into each well. The plates were covered and incubated at room temperature in the dark for 30 min. The wells were aspirated and then washed four times. The plate was dried after the last wash and 100 microlitres tetramethyl benzidine substrate, which was provided with the kit, was added. The plates were covered and incubated in the dark at room temperature for 30 min. Fifty microlitres of the stop solution was added. The absorbance of each well was read at 450 nm using 630 nm as reference. The mean absorbance for each sample was calculated as standard. The absorbance of the standards was plotted against the standard concentration on semi-logarithmic graph paper.
Serum retinol measurement
The assessment of retinol levels in serum was made using a spectrophotometer (Mert, Citation1996).
Haematological study
The previously mentioned disodium ethylenediamine tetraacetic acid blood samples of 12 birds per group were examined for the heterophil/lymphocyte (H/L) ratio. H/L ratios were calculated on a blood smear stained with the May–Grünwald and Giemsa methods (Lee & Luna, Citation1968).
Statistical analysis
The values of the SAA and serum retinol levels were evaluated with variance analysis and Tukey tests, and the scores of the microscopic examinations were assessed with chi-square tests (SAS Institute Inc., Citation1991).
Results
Clinical findings
In all experimental groups, swelling in the inoculated left inter-tarsal joints resulting in lameness was observed on days 5 to 7 after the injections (p.i.) and these findings were more apparent in birds of Groups II and III than in Groups IV and V. No swelling of the inoculated joints or lameness was seen in the birds of Group I.
Necropsy findings
In all experimental groups, especially in Groups II and III, the inoculated left inter tarsal joints were swollen. Macroscopically, in the amyloid-positive pullets, the amyloid appeared as multifocal orange-coloured thickenings in the periarticular region.
Microscopical findings
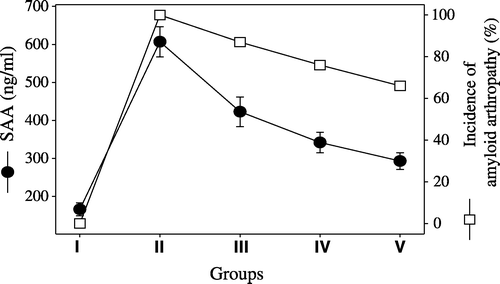
The findings of amyloid arthropathy and its severity in the joints are given in . The highest rate of amyloid arthropathy found was 100% in Group II, while the frequency of amyloid arthropathy was 87%, 76%, and 66% in Groups III, IV and V, respectively (). The differences between the five groups were significant (P<0.001). Amyloid arthropathy with the most severe amyloid deposition (+++) was also seen in Group II (54.28%), whereas the incidence of severe deposition (+++) was 32%, 29.41% and 23% in Groups III, IV and V, respectively ().
Figure 1. Amyloid arthropathy occurrence rate (%) according to congo red stain and immunohistochemistry by light microscopy in five groups. I, negative control group; II, vitamin A group; III, positive control group; IV, pentoxyflline group; V, methylprednisolone group.
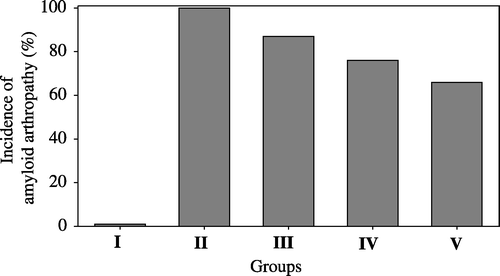
Table 1. The frequency and severity of amyloid arthropathy in chickens of five groupsa
The degree of differential cell infiltration in synovial membranes are presented in . Severe (+++) heterophil infiltration was seen in 87% of birds in Group III, followed by Group II with 77%, Group IV with 70% and Group V with 63%. The highest rate of severe macrophage infiltration was observed in 51% of birds in Group II, followed by Group IV with a rate of 41%, Group III with a rate of 39% and Group V with a rate of 6%. Severe (+++) lymphocyte and plasma cell infiltration was seen in 68% of birds in Group III and the lowest rate of severe (+++) lymphocyte and plasma cell infiltration was seen in Group IV where 44% of birds were affected. The comparison of the incidence of amyloid arthropathy with severe heterophil infiltration severe macrophage infiltration and severe lymphocyte and plasma cell infiltration are presented in –, respectively.
Figure 2. Occurrence of severe heterophil infiltration (•) and amyloid arthropathy (□) in joints in five groups. I, negative control group; II, vitamin A group; III, positive control group; IV, pentoxyflline group; V, methylprednisolone group.
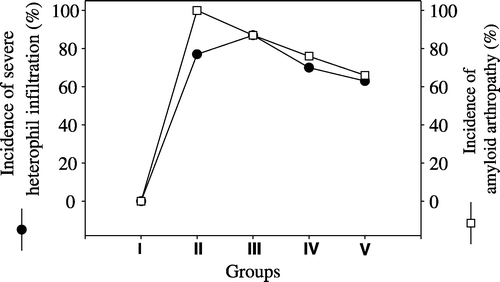
Figure 3. Occurrence of severe macrophage infiltrations (•) and amyloid arthropathy (□) in joints in five groups. I, negative control group; II, vitamin A group; III, positive control group; IV, pentoxyflline group; V, methylprednisolone group.
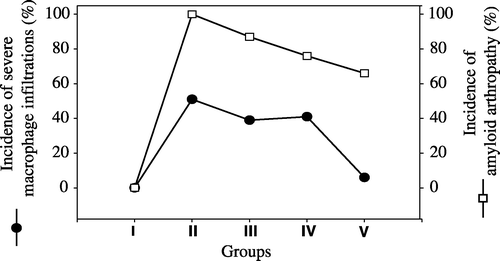
Figure 4. Lymphocytes and plasma cells infiltration rates (•) and amyloid arthropathy occurrence rates (□) in joints in five groups. I, negative control group; II, vitamin A group; III, positive control group; IV, pentoxyflline group; V, methylprednisolone group.
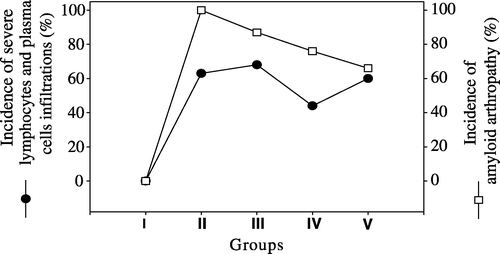
Table 2. Distribution of different inflammatory cell infiltration in synovial membranes and severity in five groups (%)a
Serological findings
SAA findings
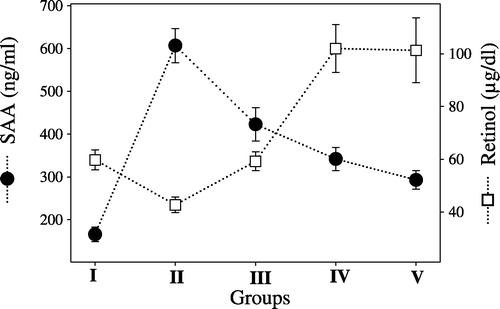
The SAA levels were increased in all groups when compared with the negative control group (166±17 ng/ml). The highest levels were observed in Group II (607±40 ng/ml), followed by Group III (423±39 ng/ml), Group IV (342±27 ng/ml) and Group V (293±22 ng/ml), respectively.
Serum retinol findings
A decrease in serum retinol levels was detected in Group II (42.72±3 μg/dl) when compared with Group I (59.75±3.8 μg/dl) and Group III (59.24±3.6 μg/dl), while an increase was detected in both Group IV (102± 9.1 μg/dl) and Group V (101.3±12.3 μg/dl)
The comparison of the incidence of amyloid arthropathy occurrence with SAA levels () and serum retinol levels () are presented in and .
Haematological findings
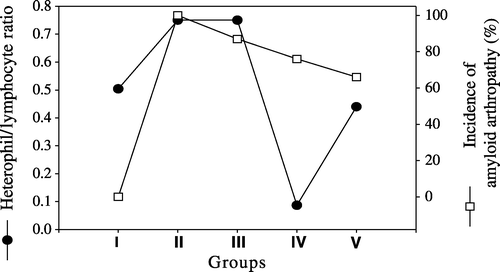
An increase in the H/L ratio was observed both in Groups II (0.75) and III (0.75) when compared with the negative control group (0.504), while a decrease in the H/L ratio was observed in Groups IV (0.087) and V (0.44). compares the incidence of amyloid arthropathy with the H/L ratio.
Discussion
There are publications about the possible role of retinol and its active metabolite retinoic acid on transthyretin-related and β-amyloid precursor-related amyloidosis (Lahiri & Nall, Citation1995; Yang et al., Citation1998; White & Kelly, Citation2001). On the other hand, there has been only one report examining the role of chronic hypervitaminosis A in type AA amylodosis of animals. The study examined six cats (Clark & Seawright, Citation1968).
In the present study, the most frequent and the most severe amyloid arthropathy was observed in the vitamin A-treated groups when compared with the positive control group (). This finding confirmed our previous report (Sevimli et al., Citation2004) that vitamin A increases the severity of E. faecalis-induced amyloid arthropathy in brown layer chickens.
It was also observed that the incidence and the severity of amyloid arthropathy was reduced in the pentoxyfilline-treated and methylprednisolone-treated groups when compared with the positive control group (). Methylprednisolone was found to be more successful in reducing the amyloid arthropathy. There are no previous reports on the use of pentoxyfilline or methylprednisolone for the prevention of amyloid formation.
It has been reported that an increase in serum levels of SAA precedes the formation of amyloidosis (Tape et al., Citation1988). Although SAA-dependent amyloidosis has been studied in a wide variety of mammalian species (Hol & Gruys, Citation1984), only a few studies have been conducted in chickens to date (Landman et al., Citation1996).
Our results indicated that the serum levels of SAA in experimental groups were significantly higher when compared with the negative control group (P<0.001) and this supports the suggestion that there is a positive correlation between the serum SAA levels and the formation of amyloid arthropathy. It has been demonstrated that retinoids and vitamin A induce cytokines such as IL-1 and TNF-α (Dillahay et al., Citation1988; Turpin et al., Citation1990). Cytokines including IL-1, IL-2, IL-6, TNF-α and M-CSF have been reported to induce hepatic or extrahepatic synthesis of SAA (Rivas et al., Citation1992; Rysava et al., Citation1992; Ray et al., Citation1999). Conversely, the synthesis of cytokines could be inhibited by anti-inflammatory agents. For example, pentoxyfylline has inhibitory effects on TNF-α and lymphokines (Haslet, Citation1998), whereas methylprednisolone has inhibitory effects on IL-1, IL-2, IL-4, IL-6, IL-10, interferon ? and interferon α, and TNF-α (Pitzalis et al., Citation1997; Wandinger et al., Citation1998; Xu et al., Citation1998; Hodge et al., Citation1999; Stanton et al., Citation1999; Ou et al., Citation2001).
In the present study, the most severe amyloid arthropathy and the highest SAA values were observed in the vitamin A-treated group. The severity of amyloid arthropathy was reduced and the increase in SAA, compared with the negative controls, was not marked in the pentoxyfylline-treated and methylprednisolone-treated groups (). The methylprednisolone-dependent decrease in serum SAA level was significantly greater than that of the pentoxyfylline-dependent decrease in serum SAA levels. This might suggest that a wide spectrum of cytokine inhibitors could be effective in preventing amyloidosis.
Some researchers suggest that neutrophils and macrophages play a very important role in the formation of amyloid fibrils (Skogen et al., Citation1980; Hawkins et al., Citation1993; Yamada et al., Citation1996; Zekerias et al., Citation2000). The present study confirmed that there was an association between the intensity and the type of leukocyte infiltration and the incidence and severity of amyloidosis. As shown in , the highest numbers of macrophages were found in the vitamin A-treated groups and the highest numbers of heterophils were found in the positive control group and vitamin A-treated group, respectively, while the lowest number of macrophages and heterophils were found in methylprednisolone-treated groups. These findings support the involvement of macrophages (Skogen et al., Citation1980) and neutrophils (Phillips et al., Citation1993) in fibrillogenesis.
The findings of the present study support the findings of Turpin et al. (Citation1990) who demonstrated that vitamin A increases the number and the activity of macrophages. Furthermore, this study also confirmed that methylprednisolone inhibits the mononuclear cell infiltration (Ou et al., Citation2001) and suppresses the migration of neutrophils (Pitzalis et al., Citation1997; Hodge et al., Citation1999).
There have been conflicting reports about the effects of pentoxyfylline on the migration of neutrophils into tissues (Besler et al., Citation1986; Boogaerts et al., Citation1990; Elferink et al., Citation1997; Hodge et al., Citation1999). In the present study, it was found that the number of heterophils was significantly lower in the pentoxyfylline-treated group than in the positive control group.
Thus, findings in this study were in agreement with the findings of Boogaerts et al. (Citation1990) and Elferink et al. (Citation1997), whereas they were not in agreement with the findings of Besler et al. (Citation1986) and Hodge et al. (Citation1999) regarding the effect of pentoxyfylline on heterophils.
When pentoxyfylline and methylprednisolone were compared, it was found that methyprednisolone significantly inhibits fibrillogenesis by lowering both heterophil and macrophage infiltration, while pentoxyfylline failed to prevent fibrillogenesis and migration of macrophages, although it inhibited heterophil infiltration into the tissues.
In this study, the role of lymphocyte and plasma cell infiltration into the joints was investigated but no correlation was found between the formation of amyloid and the presence of lymphocytes or plasma cells.
It is widely known that the number of heterophil leucocytes and monocytes increase in a bacterial infection and that these cells synthesize cytokines (Latimer et al., Citation1988; Andreasen et al., Citation1991; Kogut et al., Citation1994). In this study, the findings of high H/L ratio and its association with the formation of amyloid in both vitamin A and positive control groups supported the findings of Zekerias et al. (Citation2000), who found a positive correlation between the numbers of heterophils in peripheral blood and amyloid occurrence.
Some researchers observed that low plasma retinol concentrations were associated with elevated acute-phase protein concentration, especially with that of serum amyloid A (Thurnham et al., Citation2003). In this study, it was indicated that there was a negative association between the serum retinol levels and SAA levels and also amyloid deposition in tissues. Therefore, it is suggested that as tissue amyloid deposits form, vitamin A passes into the tissues and integrates into the amyloid. Landman et al. (Citation1994) suggested that coloration of amyloid in the joints is because of retinoids in the amyloid.
It has also been reported that pentoxyfylline (Berman & Duncan, Citation1989; Aulthouse et al., Citation1992; Chang et al., Citation1993) and methylprednisolone (Laato et al., Citation1989) have inhibitory effects on the synthesis of proteoglycans, glycosaminoglycans, collagen IV and fibronectin determined in amyloid (Skogen et al., Citation1980; Lyon et al., Citation1991; Gallo et al., Citation1994; Ancsin & Kisilevsky, Citation1997; Landman et al., Citation1998b).The role of pentoxyfylline and methylprednisolone in the prevention of amyloidosis should further be investigated.
The findings of this study and the reports by Aulthouse et al. (Citation1992) and Margis et al. (Citation1992) suggest that the effects of vitamin A on the synthesis of glycosaminglycan and fibronectin and its relationship with amyloidosis should further be investigated.
In conclusion, vitamin A increased the development and severity of Freund's adjuvant-stimulated amyloid arthropathy by increasing the levels of SAA, and there is a positive association between the severity of amyloidosis and the rate of macrophage and heterophil migration into the tissue. On the other hand, methylprednisolone and pentoxyfylline suppressed amyloidosis by decreasing serum SAA levels. Methylprednisolone suppressed amyloidosis better than pentoxyfylline.
Translations of the abstract in French, German and Spanish are available on the Avian Pathology website.
The project numbered 2001/42 was supported by Research Foundation of Uludag University.
References
- Ancsin , JB and Kisilevsky , R . (1997) . Characterization of high affinity binding between laminin and the acute phase protein, serum amyloid A . Journal of Biological Chemistry , 272 : 406 – 413 .
- Andreasen , CB , Latimer , KS , Harmon , BG , Glisson , JR , Golden , JM and Brown , J . (1991) . Heterophil function in healthy chickens and in chickens with experimentally induced staphylococcal tenosynovitis . Veterinary Pathology , 28 : 419 – 427 .
- Aulthouse , AL , Carubelli , CM , Dow , TM , Ziegelmayer , C and Beck , M . (1992) . Influence of retinol on human chondrocytes in agarose culture . The Anatomical Record , 1 : 52 – 59 .
- Berman , B and Duncan , MR . (1989) . Pentoxifylline inhibits normal human dermal fibroblast in vitro proliferation, collagen, glycosaminoglycan, and fibronectin production, and increases collagenase activity . Journal of Investigative Dermatology , 4 : 605 – 610 .
- Besler , H , Gilgal , R , Djaldetti , M and Zahavi , I . (1986) . Effects of pentoxifylline on the phagocytic activity, cAMP levels, and superoxide anion production by monocytes and polymorphonuclear cells . Journal of Leukocyte Biology , 40 : 747 – 754 .
- Betts , JC , Cheshire , JK , Akira , S , Kishimoto , T and Woo , P . (1993) . The role of NF-kappa B and NF-IL-6 transactivating factors in the synergistic activation of human serum amyloid A gene expression by IL-1 and IL-6. J . Biological Chemistry , 268 : 25624 – 25631 .
- Boogaerts , MA , Mabrain , S , Meeus , P , Van Hove , L and Verhoef , GEG . (1990) . In vitro modulation of normal and diseased human neutrophil function by pentoxifylline . Blut , 61 : 60 – 65 .
- Chang , CC , Chang , TC , Kao , SC , Kuo , YF and Chien , LF . (1993) . Pentoxifylline inhibits the proliferation and glycosaminoglycan synthesis of cultured fibroblasts derived from patients with Graves ophthalmopathy and pretibial myxoedema . Acta Endocrinologica (Copenh) , 4 : 322 – 327 .
- Clark , L and Seawright , AA . (1968) . Amyloidosis associated with chronic hypervitaminosis A in cats . Australian Veterinary Journal , 44 : 584
- Dillahay , DL , Walia , AS and Lamon , EW . (1988) . Effects of retinoids on macrophage function and IL-1 activity . Journal of Leukocyte Biology , 44 : 353 – 360 .
- Elferink , JGR , Tom Huizinga , WJ and Ben De Koster , M . (1997) . The effect of pentoxifylline on human neutrophil migration: a possible role for cyclic nucleotids . Biochemical Pharmacology , 54 : 475 – 480 .
- Gallo , G , Wisniewski , T , Choi-Miura , NH , Ghiso , S and Frangione , B . (1994) . Potential role of apolipoprotein-E in fibrillogenesis . American Journal of Pathology , 145 : 526 – 530 .
- Glenner , GG . (1980) . Amyloid deposits and amyloidosis. The β fibrilloses . New England Journal of Medicine , 302 : 1283 – 1292 .
- Göttgens , B and Green , AR . (1995) . Retinoic acid and the differentiation of lymphohaemapoietic stem cells . BioEssays , 17 : 187 – 189 .
- Haslet , PA . (1998) . Anticytokine approaches to the treatment of anorexia and cachexia . Seminars Oncology , 25 : 53 – 57 .
- Hawkins , PN , Richardson , S , Vigushin , DM , David , J , Kelsey , CR , Gray , RE , Hall , MA , Woo , P , Lavender , JP and Pepys , MB . (1993) . Serum amyloid P component scintigraphy turnover studies for diagnosis and quantitative monitoring of AA amyloidosis in juvenile rheumatoid arthritis . Arthritis and Rheumatism , 36 : 842 – 851 .
- Hodge , J , Hodge , G , Flower , R and Han , P . (1999) . Methylprednizolone up-regulates monocyte IL-10 production in stimulated whole blood . Scandinavian Journal of Immunology , 5 : 548 – 553 .
- Hol , PR and Gruys , E . (1984) . Amyloid A proteins in different species . Applied Pathology , 2 : 316 – 327 .
- Inoue , S , Hultin , PG , Szarek , WA and Kisilevsky , R . (1996) . Effect of poly vinylsulfonate on murine AA amyloid: a high-resolution ultrastructural study . Laboratory Investigation , 74 : 1081 – 1090 .
- Katz , DR , Dryzmala , M and Turton , JA . (1987) . Regulation of accessory cell function by retinoids in murine immune responses . British Journal of Experimental Pathology , 68 : 343 – 350 .
- Kisilevky , R , Lemieux , LS , Fraser , PE , Kong , X , Hultin , PG and Szarek , WA . (1995) . Arresting amyloidosis in vivo using small molecule anionic sulfonates or sulfates implications for Alzheimer's disease . Nature Medicine , 1 : 143 – 148 .
- Kogut , MH , McGruder , ED , Hargis , BM , Corrier , DE and DeLoach , JR . (1994) . Dynamics of avian inflammatory response to Salmonella-immune lymphokines: change in avian blood leucocyte population . Inflammation , 18 : 373 – 388 .
- Laato , M , Heino , J , Kahari , VM , Ninikoski , J and Gerdin , B . (1989) . Epidermal growth factor prevents methylprednisolone induced inhibition of wound healing . Journal of Surgery Research , 47 : 354 – 359 .
- Lahiri , DK and Nall , C . (1995) . Promoter activity of the gene encoding the beta-amyloid precursor protein is upregulated by growth factors, phorbol ester, retinoic acid and interleukin-1 . Molecular Brain Research , 32 : 233 – 240 .
- Landman , WJM . (1999) . Amyloid arthropathy in chickens . Veterinary Quarterly , 3 : 78 – 82 .
- Landman , WJM , Gruys , E and Dwars , RM . (1994) . A syndrome associated with growth depression and amyloid arthropathy in layers: a preliminary report . Avian Pathology , 23 : 461 – 470 .
- Landman , WJM , Sletten , K , Koch , CAM , Tooten , PCJ and Gruys , E . (1996) . Chicken joint amyloid protein is of the AA type I. Characterization of the amyloidprotein . Scandinavian Journal of Immunology , 43 : 210 – 218 .
- Landman , WJM , vd Bogaard , AE , Doornenbal , P , Tooten , PC , Elbers , AR and Gruys , E . (1998a) . The role of various agents in chicken amyloid arthropathy . Amyloid , 4 : 266 – 278 .
- Landman , WJM , Gruys , E and Gielkens , ALJ . (1998b) . Avian amyloidosis . Avian Pathology , 27 : 437 – 449 .
- Latimer , KS , Tang , KN , Goodwin , MA , Steffens , WL and Brown , J . (1988) . Leucocyte changes associated with acute inflammation in chickens . Avian Diseases , 32 : 760 – 772 .
- Lee G Luna HT (1968) Manual of Histologic Staining Methods of the Armed Forces Institute Of Pathology 3rd edn New York McGraw Hill Book Company pp. 235–236
- Lyon , AW , Narindrasorasak , S , Young , ID , Anastassiades , T , Couchman , JR , McCarthy , KJ and Ksilevsky , R . (1991) . Co-deposition of basement membrane components during the induction of murine splenic AA amyloid . Laboratory Investigation , 64 : 785 – 790 .
- Margis , R , Pinheiro-Margis , M , Silva , LC and Borojevic , R . (1992) . Effects of retinol on cell adherence and extracellular matrix synthesis in aliver myofibroblast or lipocyte cell line (GRX) . International Journal of Experimental Pathology , 2 : 125 – 135 .
- Marinkovic , S , Jahris , GP , Wong , GG and Baumann , H . (1989) . IL-6 modulates the synthesis of a specific set of acute plasma proteins in vivo . Journal of Immunology , 142 : 808 – 812 .
- Mert N (1996) Veteriner Klinik Biyokimya Bursa Ceylan Matbaacilik pp. 35–39
- Ou , ZL , Nakayama , K , Natori , Y , Doi , N and Saito , T . (2001) . Effective methylprednizolone dose in experimental crescentic glomerulonephritis . American Journal of Kidney Disease , 37 : 411 – 417 .
- Phillip , JS , Josep , MC , Carmela , RA , Orville , R , Tsuranobu , S and Martha , S . (1993) . Neutrophil proteases associated with amyloid fibrils . Biochemical and Biophysical Research Communications , 197 : 130 – 136 .
- Pitzalis , C , Sharrrack , B , Gray , IA , Lee , A and Hughes , RA . (1997) . Comparison of the effects of oral versus I.V methylprednisolone regimens on peripheral blood T lymphocyte adhesion molecule expression, T cell subsets distribution and TNF-α concentrations in multiple sclerosis . Journal of Neuroimmunology , 74 : 62 – 68 .
- Ramadori , G , Sipe , JD , Dinarello , CA , Mizel , SB and Colten , HR . (1985) . Pretranslational modulation of acute phase hepatic protein synthesis by murine recombinant IL-1 and purified human IL-1 . Journal of Experimental Medicine , 162 : 930 – 942 .
- Ray , A , Schatten , H and Rayb , K . (1999) . Activation of sp1 and its functional co-operation with serum amyloid A-activating sequence binding factor in synoviocyte cells trigger synergistic action of IL-1 and IL-6 in serum amyloid A gene expression . Journal of Biological Chemistry , 274 : 4300 – 4308 .
- Rivas , AL , Tihtle , L , Kimball , EJ , Scarlett , J and Quimby , FW . (1992) . A canine febrile disorder associated with elevated IL-6 . Clinical Immunology and Immunopathology , 1 : 36 – 45 .
- Ross AC (1999) Vitamin A and retinoids In M.E. Shils, J.A. Olson, M. Shike & A.C. Ross (Eds.) Modern Nutrition in Health and Disease 9th edn Baltimore MD Williams & Wilkins pp. 305–327
- Rysava , R , Merta , M. , Tesar , V , Jirsa , M and Zima , T . (1992) . Can serum amyloid A or macrophage colony stimulating factor serve as marker of amyloid formation process? . American Journal of Pathology , 141 : 525 – 529 .
- SAS Institute Inc. (1991) SAS/STAT User's Guide release 6.04 edn Cary NC SAS Institute Inc
- Schuppan , D , Koda , M , Bauer , M and Hahn , EG . (2000) . Fibrosis of liver, pancreas and intestine: common mechanisms and clear targets? . Acta Gastro-enterologica Belgica , 63 : 366 – 370 .
- Sevimli , A , Misirlioglu , D and Özakin , C . (2004) . The enhancing effect of Vitamin A on the occurrence of amyloid arthropathy in laying chickens infected with Enterococcus faecalis . Turkish Journal of Veterinary and Animal Sciences , 28 : 131 – 138 .
- Skogen , B , Thorsteinsson , L and Natvig , JB . (1980) . Degradation of protein SAA to an AA-like fragment by enzymes of monocytic origin . Scandinavian Journal of Immunology , 11 : 533 – 540 .
- Soto , C , Kindy , MS , Baumann , M and Frangione , B . (1996) . Inhibition of Alzheimer's amyloidosis by peptides that prevent β-sheet conformation . Biochemical and Biophysical Research Communications , 226 : 672 – 680 .
- Stanton , RP , Hobson , GM , Montgomery , BE , Moses , PA , Smith-Kirwin , SM and Funanage , VL . (1999) . Glucocorticoids decrease IL-6 levels and induce mineralization of cultured osteogenic cells from children with fibrous dysplasia . Journal of Bone and Mineral Research , 14 : 1104 – 1114 .
- Tape , C , Tan , R , Nesheim , M and Kisilevsky , R . (1988) . Direct evidence for circulating apoSAA as the precursor of tissue AA amyloid deposits . Scandinavian Journal of Immunology , 28 : 317 – 324 .
- Thurnham , DI , McCabe , GP , Northrop-Clewes , CA and Nestel , P . (2003) . Effects of subclinical infection on plasma retinol concentrations and assessment of prevalence of vitamin A deficiency: meta-analysis . The Lancet , 362 : 2052 – 2058 .
- True LD (Ed.) (1990) Principles of immunohistochemistry In Atlas of Diagnostic Immunohistopathology pp. 1.1–1.31 New York Gower Medical Publishing
- Turpin , J , Mehta , K and Blick , M . (1990) . Effect of retinoids on the release and gene expression of tumor necrosis factor-α in human peripheral blood monocytes . Journal of Leukocyte Biology , 48 : 444 – 450 .
- Urieli-Shoval , S , Linke , RP and Matzner , Y . (2000) . Expression and function of serum amyloid A, a major acute-phase protein, in normal and disease states . Current Opinion in Hematology , 7 : 64 – 69 .
- Wandinger , KP , Wessel , K , Trillenberg , P , Heindl , N and Kirchner , H . (1998) . Effect of high dose methylprednisolone administration on immune functions in multiple sclerosis patients . Acta Neurologica Scandinavica , 6 : 359 – 365 .
- White , JT and Kelly , JW . (2001) . Support for the multigenic hypothesis of amyloidosis: The binding stoichiometry of retinol-binding protein, vitamin A, and thyroid hormone influences transthretin amyloidogenicity in vitro . Proceedings of the National Academy of Sciences of the United States of America , 98 : 13019 – 13204 .
- Windmeier , C and Gressner , AM . (1997) . Pharmacological aspects of pentoxifylline with emphasis on its inhibitory actions on hepatic fibrogenesis . General Pharmacology , 29 : 181 – 196 .
- Xu , J , Fan , G , Chen , J , Wu , Y , Xu , XM and Hsu , CY . (1998) . Methylprednisolone inhibition of TNF-α expression and NF-KB activation after spinal cord injury in rats . Molecular Brain Research , 59 : 135 – 142 .
- Yamada , T , Liepnikzes , J , Benson , M , Kluve , M and Beckerman , B . (1996) . Accelerated amyloid deposition in mice treated with the aspartic protease inhibitor, pepstatin . Journal of Immunology , 157 : 901 – 907 .
- Yang , Y , Quitschke , WW and Brewer , GJ . (1998) . Upregulation of amyloid precursor protein gene promoter in rat primary hippocampal neurons by phorbol ester, IL-1 and retinoic acid, but not by reactive oxygen species . Molecular Brain Research , 60 : 40 – 49 .
- Zekerias , B , Landman , WJM , Tooten , PCT and Gruys , E . (2000) . Leucocyte responses in two breeds of layer chicken that differ in susceptibility to induced amyloid arthropathy . Veterinary Immunology and Immunopathology , 77 : 55 – 69 .
- Zschiesche , W and Linke , RP . (1986) . Zur Amyloidose der Zoovögel unter besonderer Berüctigung des Wassergeflügels . Erkränkungen der Zootiere , 28 : 301 – 306 .