Abstract
Several field isolates of fowlpoxvirus (FPV) from Burkina Faso, West Africa, were isolated and partly evaluated by molecular analysis. In addition, the in ovo antiviral activity against FPV of a gall extract from Guiera senegalensis was determined. Three viral isolates were obtained from suspected fowlpox cases after passage in embryonating chicken eggs and their poxviral identity confirmed by electron microscopy. All isolates were found to be pathogenic for chicks and all grew well in cell culture. Polymerase chain reaction and sequencing of amplicons revealed sequences identical with those of other FPV strains. The most studied isolate was then employed for use in an antiviral assay. An aqueous acetone extract from the galls of G. senegalensis was found to inhibit both virus-induced pock formation and to reduce viral titre in embryonating chicken eggs. The suggested mechanism of action is the activation of the alternative complement pathway and the inhibition of FPV-induced cholesterogenesis in ovo by constituents of the gall extract.
Plusieurs souches de virus de la variole aviaire (FPV) isolées au Burkina Faso, en Afrique de l'Ouest, ont été partiellement étudiées par une analyse moléculaire. En plus, l'activité antivirale, vis-à-vis de la FPV, d'un extrait de galle du Guiera senegalensis a été déterminée in ovo. Trois souches virales ont été obtenues à partir de suspicions de FP après passages sur œufs embryonnés de poule et leur identité poxvirale a été confirmée en microscopie électronique. Toutes les souches se sont révélées pathogènes pour les poussins et toutes ont bien poussé sur cultures cellulaires. La PCR et le séquençage des amplicons ont révélé des séquences identiques à ceux d'autres souches de FVP. La souche la plus étudiée a ensuite été utilisée dans un essai d'activité antivirale. Un extrait acétone aqueux de galle du G. senegalensis s'est révélé inhiber la formation de pustule induite par le virus et réduire le titre du virus sur œufs embryonnés de poule. Le mécanisme suggéré de l'action des constituants de l'extrait de Galle est l'activation de la voie alterne du complément et l'inhibition de la cholestérogenèse induite par le FPV in ovo.
Verschiedene Feldisolate des Geflügelpockenvirus (FPV) aus Burkina Faso, Wesrafrika, wurden isoliert und teilweise mit molekularen Untersuchungsmethoden charakterisiert. Außerdem wurde die antivirale Aktivität eines Galleextrakts aus Guiera senegalensis gegen FPV in ovo ermittelt. Drei Virusstämme wurden von FP-Verdachtsfällen nach Anzüchtung in embryonierten Hühnereiern isoliert und durch elektronenmikroskopische Untersuchungen als Pockenviren identifiziert. Alle Isolate waren pathogen für Hühner und vermehrten sich in der Zellkultur gut. Die PCR und die Sequenzierung von Amplifikaten ließ Sequenzen erkennen, die mit denjenigen von anderen FPV identisch waren. Das am besten untersuchte Isolat wurde dann für den Einsatz in einem antiviralen Test verwendet. Ein wässeriges Acetonextract aus der Galle von G. senegalensis war fähig sowohl die virusinduzierte Pockenformation zu hemmen als auch den Virustiter in embyonierten Hühnereiern zu reduzieren. Es wird vermutet, dass die Wirkungsweise auf der Aktivierung einer alternativen Komplementreihenfolge und der Hemmung der FPV-induzierten in ovo-Cholesterogenese durch Bestandteile des Galleextrakts beruht.
Varios aislamientos de campo del virus de viruela (FPV) de Burkina Faso, oeste de África, fueron aislados y parcialmente evaluados mediante análisis moleculares. Además, se determinó la actividad antiviral in ovo frente a FPV de extracto de bilis de Guiera senegalensis. Se obtuvieron tres aislados virales de casos sospechosos de FP, tras realizar un pase en huevos embrionados y la identificación viral fue confirmada por microscopia electrónica. Todos los aislamientos resultaron patógenos en pollos y crecieron bien en cultivo celular. La técnica de PCR y la secuenciación de los amplicones revelaron secuencias idénticas con otras cepas de FPV. El aislado más estudiado fue el empleado posteriormente en una prueba antiviral. Un extracto acuoso y con acetona de bilis de G senegalensis resultó inhibir la formación de placas inducidas por el virus y redujo el título viral en huevos de pollo embrionados. El mecanismo de acción sugerido es la activación de la vía alternativa del complemento y la inhibición de la colesterogénesis inducida por FPV in ovo por constituyentes del extracto de bilis.
Introduction
Fowlpox (FP) is a common disease of poultry that is readily controlled by vaccination. Disease outbreaks still occur among flocks despite previous vaccinations (Garcìa et al., Citation2003). Apart from Newcastle disease, FP is the most important cause of mortality in free-ranging chickens, which constitute the major component of the poultry industry in several developing countries (Bonfoh et al., Citation1997; Mopate et al., Citation1997). Globally, improvements in village chicken production are increasingly being considered (Kitalyi, Citation1998). While many efforts are being made to control Newcastle disease, little consideration is given to controlling FP despite its relatively high incidence. In Burkina Faso, situated in West Africa, a need for suitable control programmes is, however, recognized, but their success will be reliant on an adequate scientific knowledge of the local fowlpoxvirus (FPV) strains present and will require their isolation, identification and characterization. An alternative to vaccine-based control could be the therapeutic use of medicinal plants.
Galls from Guiera senegalensis, a popular medicinal plant in West Africa, are being used as an ethnoveterinary treatment for FP in chickens. In the past, these galls were also used to treat smallpox and measles in man (Nacoulma, Citation1996). Galls are abnormal growths or hypertrophic lesions of plant tissues following exposure to chemical or mechanical irritants, or due to the effects of certain hormones. Parasitic fungi, bacteria, nematodes worms, insects and mites are known to release chemical irritants (Ramade, Citation1993). In the case of G. senegalensis, several insect eggs and larvae were found, some of which developed into butterflies (unpublished observation).
Nine galloylquinic acids have been isolated from different parts including the galls of G. senegalensis (Bouchet et al., Citation2000) and have shown antiviral (Mahmood et al., Citation1993) as well as antioxidant (Bouchet et al., Citation1998) activity. We have previously shown that an aqueous acetone extract from the galls of G. senegalensis inhibited both FPV-induced cytopathic effects (CPE) and plaque formation in a chicken embryo skin (CES) cell culture (Lamien et al., Citation2005).
We report on the first described isolation, identification and preliminary molecular analysis of pathogenic FPV from Burkina Faso as well as the in ovo antiviral effect of an aqueous acetone extract from galls of G. senegalensis on a local FPV isolate. This work forms part of our ongoing research on isolating and characterizing antiviral compounds against FPV. Combating FP forms part of the process of improving free-range chicken production and hence food security in developing countries. Screening for FPV inhibitors could also be of value in obtaining antiviral agents against, for example, smallpoxvirus, which has re-emerged as a threat for use as a biowarfare agent (Breman & Henderson, Citation1998).
Materials and Methods
Isolation and characterization of local FPV isolates
Samples
Village chickens with suspected FP lesions were collected from July to December 2002 during an outbreak, from flocks where high mortality was observed. The first sample, FPV/BFK/Ouaga 2002/1, was collected in July 2002 while the other two, FPV/BFK/Ouaga 2002/2 and FPV/BFK/Ouaga 2002/3, were collected from two chickens from the same flock during October 2002.
Sample preparation
The lesions from each bird were ground separately using a chilled mortar and pestle in the presence of 5 ml phosphate-buffered saline (PBS) and 1 g sterile medium grain carborundum. The mixture was clarified by low-speed centrifugation at 700 g for 10 min. The supernatant was collected and antibiotics added to a final concentration of 100 units/ml penicillin G, 100 μg/ml streptomycin, 250 ηg/ml amphotericin B and the suspension was kept at room temperature for 1 h before inoculation.
Egg inoculation.
Approximately 0.1 ml of each suspension was inoculated separately onto the chorioallantoic membranes (CAMs) of 10-day-old to 12-day-old developing chicken embryos (Hitchner, Citation1980; Payment & Trudel, Citation1989) in eggs laid by unvaccinated hens. Following inoculation, the embryos were incubated at 37°C and examined daily for signs of mortality. At day 5 post inoculation CAMs were examined for lesions, and when these were seen further passages were performed.
Electron microscopy.
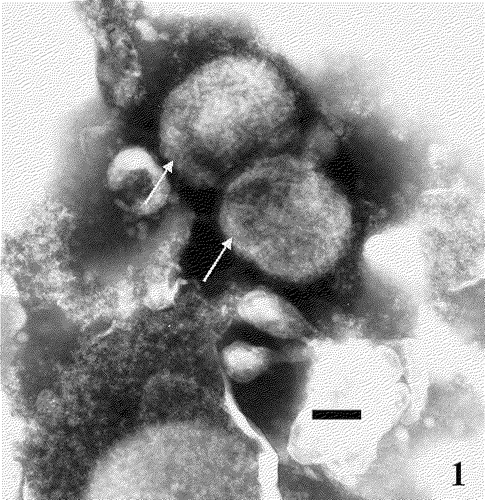
CAM lesions obtained following egg inoculation were examined by electron microscopy using negative staining with phosphotungstic acid. Briefly, a suspension of ground infected CAMs in PBS was centrifuged at 700 g for 10 min and the supernatant then centrifuged at 44 000 g for 60 min. The pellet was resuspended in distilled water. A drop was deposited on a slide and diluted with 1% foetal calf serum and the viruses allowed to adsorb onto a carbon coated electron microscopy grid for 5 min. The grid were washed with distilled water and stained with 2% phosphotungstic acid for 2 min, and then observed using a transmission electron microscope (JEOL JEM 1200 EX MKI, Tokyo, Japan).
Experimental infection.
In an attempt to reproduce the disease, 1-day-old chickens from an unvaccinated flock were inoculated by wing-web scarification (Tripathy & Hanson, Citation1980) with Burkina Fasan field isolates, using three birds per sample. Chicks were observed every 2 to 3 days for lesions and symptoms. The inoculum consisted of a CAM homogenate (10% w/v in PBS) from the third passage of FPV/BFK/Ouaga 2002/1 and the first passage of FPV/BFK/Ouaga 2002/2 and FPV/BFK/Ouaga 2002/3.
Cell culture inoculation
Primary CES cell cultures were prepared from 11-day-old specific pathogen free embryos by an adaptation of the method of Silim et al. (Citation1981). Secondary CES cells were obtained by dispersing the primary cells. The CAM lesions were prepared as described, using a 0.45 μm filter. The virus suspension (0.5 ml) was adsorbed on confluent CES monolayers in 25 cm2 flasks for 2 h before the maintenance medium was added. For subsequent passaging, the inoculum was prepared from FPV infected cells that had been lysed by three freeze–thaw cycles at days 4 to 7 post infection.
DNA extraction
After marked CPE were observed, the cells infected with FPV/BFK/Ouaga 2002/1, FPV/BFK/Ouaga 2002/2 and FPV/BFK/Ouaga 2002/3 were frozen and thawed three times. After clarification of the lysate by low-speed centrifugation at 2200g in a benchtop centrifuge for 10 min, the virus-containing supernatant was used for DNA extraction using a standard phenol:chloroform extraction (Sambrook et al., Citation1989) followed by ethanol precipitation. DNA was also extracted from mock infected CES and used as the negative control.
Polymerase chain reaction (PCR) amplification and sequencing of PCR products
The in-house-designed primer sequences were: primer 1, FT2 5′-AGG GGT ACC GCG TAT TGC ACC GCA TCC-3′ (forward); primer 2, FT3 5′-AAG GGA TCC CGA GGT CAT CAT TTA CAG CAG G-3′ (reverse).
Primers were designed to amplify the region from nucleotides 87 595 to 88 783 in the FPV genome (accession number AF 198100), so as to generate a 1.2 kb fragment. PCR was carried out in a total volume of 25 μl, containing 2.5 μl of 10× Buffer (TaKaRa), 2 μl dNTPs (2.5 mM each), 1 μl FT2 (5 pmol/μl), 1 μl FT3 (5 pmol/μl), 0.2 μl Ex Taq (5 U/μl) (TaKaRa) and 1 μl extracted DNA from each sample. No extracted DNA was included in the negative control. The temperature profile consisted of an initial denaturation at 94°C for 3 min, and 30 cycles of denaturation at 94°C for 45 sec, annealing at 55°C for 30 sec and elongation at 72°C for 1 min. PCR products (5 μl) were analysed on a 1% agarose gel. λDNA digested with Pst1 was used as molecular size marker during electrophoresis. The 1.2 kb amplicon from each sample was purified using a QIA Quick gel extraction protocol (Qiagen, Germany).
Sequencing was performed using the ABI Prism Dye Terminator Cycle Sequencing Ready Reaction Kit (Applied Biosystems) according to the manufacturer's instructions in an ABI 377™ automated sequencer.
In ovo antiviral effect of a G. senegalensis gall extract
Plant collection and extraction. Galls from G. senegalensis J.F. Gmel. (Combretaceae) were carefully collected to minimize plant damage, in the Ouagadougou region of Burkina Faso in 2003. A voucher specimen was deposited at the herbarium of the University of Ouagadougou (Lamien 01) and identified and verified as such by Prof. Jeanne Millogo. Each gall was carefully cleaned and the enclosed insect eggs and larvae removed. Galls were then dried at room temperature and pulverized into a thin powder using a blender. Powdered galls (50 g) were extracted at room temperature using 80% aqueous acetone (500 ml) with mechanical agitation for 48 h. Acetone was removed under reduced pressure and the aqueous solution lyophilized yielding a green powder (10.5 g).
Virus and chicken eggs
Fertilized chicken eggs from the poultry facility at the Laboratoire National d'Elevage (Ouagadougou) were used. This flock had not been immunized against FP and were not known to have been infected by FPV or any other poultry pathogens.
The FPV/BKF/Ouaga 2002/1 FPV isolate was used, which had been passaged four times on the CAMs of embryonating eggs followed by five passages on secondary CES cells. After the fifth passage in CES cells, harvested virus was freeze-dried. Prior to the antiviral assay, the freeze-dried virus was resuspended in PBS and inoculated onto CAMs of embryonating eggs. Infected CAMs were then collected and ground in the presence of PBS (10% w/v suspension). After centrifugation, the supernatant was collected and the virus titrated in embryonating chicken eggs as described (Reed & Muench, Citation1938).
Toxicity of plant extract
Toxicity and antiviral assays were performed using a stock solution of the extract (50 mg/ml) in DMSO. A 10-fold serial dilution (25 mg/ml to 25 μg/ml) of the G. senegalensis gall extract was prepared in PBS and inoculated onto the CAMs of 12-day-old embryonating eggs. The embryos were harvested after a 5-day incubation period at 37°C in a humidified incubator. Embryo survival was recorded and the CAMs examined macroscopically for morphological changes. The maximal non-toxic concentration was defined as the maximal concentration of extract where all the embryos survived without inducing macroscopic changes to the CAM.
Antiviral assay
A 10-fold serial dilution (500, 50, 5 and 0.5 μg/ml) of the aqueous acetone extract from G. senegalensis galls was prepared in PBS. Each dilution was mixed with an equal volume of an FPV suspension (2.5×105 EID50/ml) and inoculated immediately (0.15 ml) onto CAMs of 10-day-old to 12-day-old embryonating chicken eggs. These were then incubated at 37°C for 5 days. Negative controls consisted of eggs inoculated with virus suspension in PBS alone. After incubation, the CAMs were collected and a 50% (w/v) suspension was prepared in PBS. A viral titration was performed for each dilution of the extract showing the highest inhibition as well as for the negative control, as described elsewhere (Reed & Muench, Citation1938). The results were expressed as the mean of two independent experiments, using separately prepared extracts.
Results
FPV isolation and characterization
Virus isolation
Lesions from naturally infected chickens were used to inoculate eggs. Morphological changes ranging from individual pock lesions to marked thickening of the CAMs were observed within 5 days after inoculation of all three samples. The homogenate of the infected CAM was subjected to a second CAM passage. After a 5-day incubation period, typical pox lesions were observed with all three isolates. More pronounced pocks were seen when using the FPV/BFK/Ouaga 2002/3 isolate. In all cases the white opaque pock formation was seen after the second passage, although no embryonic deaths were observed with all three isolates.
Electron microscopy
Typical FPV particles, negatively stained with phosphotungstic acid (), were identified in the CAM lesions from infected chick embryos in each sample.
Experimental infection
All the birds developed lesions at the inoculation site within 8 days after inoculation with the different field isolates. Secondary lesions appeared at several sites such as the area around the beak, the eyelids, and legs after 11 days with the FPV/BKF/Ouaga 2002/1 isolate, while only the eyes and beak region were affected after 13 days with the FPV/BKF/Ouaga 2002/2 and FPV/BKF/Ouaga 2002/3 isolates. All three chicks inoculated with FPV/BKF/Ouaga 2002/1 died within 30 days because of the severity of the lesions. Only one chick each died within 30 days after inoculation with FPV/BKF/Ouaga 2002/2 and the FPV/BKF/Ouaga 2002/3 isolate, respectively. In addition, 5-week-old chicks that were infected with the FPV/BKF/Ouaga 2002/1 isolate by scarification showed marked secondary lesions within 15 days and severe disease within 30 days (4/5 chicks died).
Adaptation to cell culture
FPV/BFK/Ouaga 2002/2 and FPV/BFK/Ouaga 2002/3 produced CPE, characterized by granulation, clumping and rounding of cells, within 5 days of the first passage in CES cells. Typical FPV-associated CPE, characterized by the detachment of most of the rounded cells, was observed on day 4 of the second passage. FPV/BFK/Ouaga 2002/1 produced CPE only after three passages. Typical FPV-induced CPE was observed with FPV/BFK/Ouaga 2002/1 on day 3 of passage 4 on CES cells, which became more pronounced by day 7. In a plaque assay performed in six-well plates, the isolate FPV/BFK/Ouaga 2002/3 failed to produce plaque in liquid overlay. The isolate produced a diffuse CPE in secondary CES. FPV/BFK/Ouaga 2002/1 produced different sizes of plaques that were not well defined until after 8 days while FPV/BFK/Ouaga 2002/2 produced well-defined but differently sized plaques within 4 days.
PCR amplification and sequencing of the amplicon
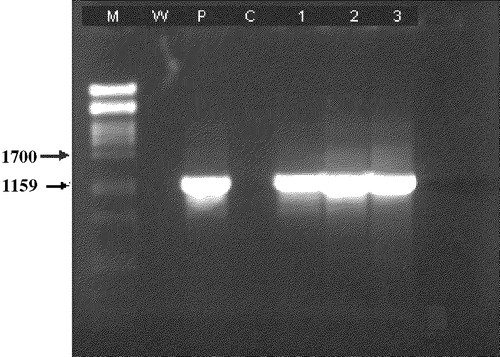
A 1.2 kb DNA fragment was amplified from the FPV DNA template () obtained from all three samples. DNA from uninfected cells was used as the negative control while DNA from cells infected with a FPV vaccine (Onderstepoort Biological Products, South Africa) was used as the positive control (). The amplicons from each test sample were then sequenced and the partial sequences obtained were identified by BLAST analysis to be that of FPV (NBCI Gene Bank). Identical sequence similarity was obtained with the corresponding region of the FPV HP 440 strain (accession number AJ 223385), the FPV complete genome (accession number AF 198100) and the FPV thymidine kinase gene (accession number M16617).
Antiviral activity of a G. senegalensis gall extract against FPV
Toxicity
The aqueous acetone extract of galls of G. senegalensis were inoculated onto the CAMs of 12-day-old chicken embryos and incubated for 5 days to evaluate their toxicity. No toxic effect could be detected in chicken embryos at doses of up to 250 μg/ml (). Even at a dose of 2500 μg/ml, 87% of the embryos were still alive without noticeable changes on the CAMs. In contrast, only 25% of the embryos survived at a dose of 25 000 μg/ml, with surviving embryos showing changes mainly in the zone on the CAM where the extract was inoculated. The maximal non-toxic concentration was taken to be 250 μg/ml.
Table 1. The toxic effects of increasing doses of a G. senegalensis extract for chicken embryos
Antiviral activity of a G. senegalensis gall extract
Reduced numbers of pocks were seen when using the G. senegalensis extract at 250 μg/ml while more clearly defined pocks with cell infiltrations were seen at 25 μg/ml, in comparison with infected untreated control (). The harvested CAMs that had been exposed to the extract (25 or 250 μg/ml) as well as the negative control (0 μg/ml) were collected in each experiment, and the virus yield determined by titration () as previously described. A 79-fold reduction (1.9 log reduction) of the virus titre was observed at a concentration of 25 μg/ml, while at 250 μg/ml a 791-fold reduction (2.9 log reduction) was seen, in comparison with the untreated infected control.
Figure 3. Effect of G. senegalensis gall extracts on FPV growth on CAMs of 12-day-old chicken embryos. The effects of different concentrations used are indicated (arrows) at: (a) 0 μg/ml, a zone of diffuse lesions; (b) 25 μg/ml, well-formed pocks; (c) 250 μg/ml, considerably reduced number of pocks.
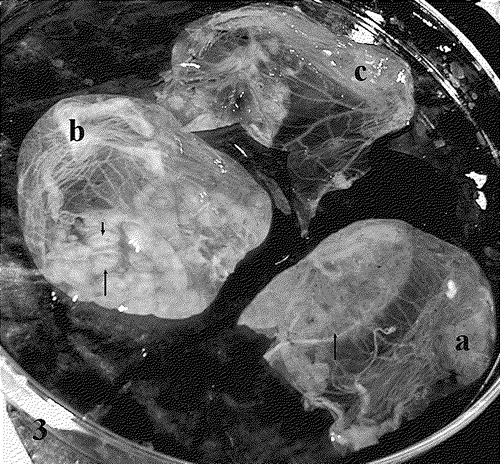
Table 2. Antiviral activity of different doses of a G. senegalensis gall extract
Discussion
FP is frequently reported in free-range chicken in Burkina Faso as well as other developing countries. Although FP is well known to indigenous Burkina Fasan farmers, the isolation and characterization of Burkina Fasan FPV strains has not been reported. We isolated virus from clinical specimens and showed the formation of pock lesions on the CAMs of infected eggs. The presence of poxviruses was confirmed by electron microscopy observation.
Pathogenicity was retained when these isolates were passaged on CAMs and then inoculated into 1-day-old and 5-week-old chicks. These viruses were successfully adapted to secondary CES cells, which developed typical CPE after three to four passages.
A 1.2 kb fragment of the FPV genome was successfully amplified by PCR, and a partial sequence was obtained that was identical with sequences available in the NBCI Gene Bank. Heterogeneity could thus not be established but restriction fragment length polymorphism analysis of the genome, among others, could help in determining this. We also wished to evaluate the anti-poxvirus effect of an extract from G. senegalensis galls, for ethnoveterinary use. The local FPV isolate FPV/BKF/Ouaga 2002/1, which had been most extensively passaged in chicken embryo and cell culture, was used. The maximal non-toxic concentration of the aqueous acetone extract of the galls tolerated by chicken embryos was shown to be 250 μg/ml. Gall extracts tested at various non-toxic concentrations showed strong antiviral activity against the local FPV isolate. Infected CAMs from untreated controls showed diffuse pock lesions while very clear pocks were seen at 25 μg/ml with a strong reduction in pock numbers observed at 250 μg/ml. Pock morphology has been shown to alter with increasing severity of FPV infection, changing from discrete to disseminate pock lesions (Ohta et al., Citation1986). In a study on the influence of complement on FPV infection, Ohta et al. (Citation1986) showed that in chicken embryos with immature immune systems, treatment with cobra venom factor that induces complement depletion causes changes in pock morphology, from clearly defined to diffuse lesions, with an increase in viral content in the pock and an inhibition of cell infiltration at the pock site. We therefore assumed that the change in pock morphology observed at 25 μg/ml is an indication of a reduction of viral infectivity due to the plant extract. This was confirmed by viral titration that indicated a 79-fold titre reduction in comparison with a non-treated infected control. The strong reduction in pock numbers and viral titre at 250 μg/ml confirmed that the plant extract had antiviral activity. The effect of the plant extract on pock morphology is opposite to that described for cobra venom factor (Ohta et al., Citation1986). FPV infections have been shown to activate the alternative complement pathway in infected cells, leading to a reduction in virus (Ohta et al., Citation1983). One possibility is the stimulation of the alternative complement pathway in the chick embryo by components of the extract. This hypothesis is strongly supported by the finding that extracts of G. senegalensis could provide protection against Naja nigricollis venom, which is known to induce tissue necrosis due to its phospholipase content and also to deplete complement (Abubakar et al., Citation2000).
Another antiviral mechanism could be the inhibition of cholesterol biosynthesis by the G. senegalensis gall extract, since the envelope of FPV has been shown to contain cholesterol. It is known that the virus causes the accumulation of cholesteryl esters in chorioallantoic membranes, as well as the stimulation of enzymes for cholesterol biosynthesis (Buttke & Gafford, Citation1982). Abe et al. (Citation2000a,Citationb,Citationc) have demonstrated that several galloyl derivatives are potent and selective inhibitors of squalene epoxidase, another rate-limiting enzyme for cholesterol biosynthesis. Several natural and synthetic galloyl esters including those isolated from green tea (Camellia sinensis (L.) O. Kuntze) (Abe et al., Citation2000a,Citationb,Citationc) and rhubarb (Rheum palmatum L.) have been shown to possess a strong inhibitory effects on squalene epoxidase. The presence of the galloyl moiety is regarded as being important for this activity. It was also suggested that these esters possess potent enzymatic inhibition because of their scavenging activity of oxygen radicals (Abe et al., Citation2000a,Citationb,Citationc). Of relevance is the finding that the galls of G. senegalensis have been shown to contain galloyl quinic esters (Bouchet et al., Citation1998, Citation2000) with strong radical scavenging activity (Bouchet et al., Citation1998). We have screened the free radical scavenging activity of the extract showing antiviral activity using the DPPH method (Velazquez et al., Citation2003), and obtained an IC50 (55% inhibitory concentration) of 0.13 μg/ml, thereby confirming the presence of strong radical scavenging activity in the crude extract (unpublished data). The standards used in this assay were gallic acid (0.57 μg/ml) and quercetin (0.87 μg/ml).
In summary, we obtained local isolates of FPV and then provided a possible scientific basis for the ethnoveterinary use of galls of G. senegalensis to combat FPV infection in chickens. Further studies are needed to genotypically compare local with foreign FPV isolates. We are currently developing assays to further understand the mechanism of FPV inhibition by G. senegalensis gall extracts and to isolate the active principles present.
Translations of the abstract in French, German and Spanish are available on the Avian Pathology website.
This work was supported by the International Atomic Energy Agency technical cooperation project BKF/5002. The authors are grateful to the head of the Laboratoire National d'Elevage and all the staff for their support. They also thank Dr G. H. Gerdes, Virology Division, Onderstepoort Veterinary Institute for identifying the fowlpoxviruses by electron microscopy identification.
References
- Abe , I , Seki , T and Noguchi , H . (2000a) . Potent and selective inhibition of squalene epoxidase by synthetic galloyl esters . Biochemical and Biophysical Research Communications , 270 : 137 – 140 .
- Abe , I , Seki , T , Noguchi , H and Kashiwada , Y . (2000b) . Galloyl esters from rhubarb are potent inhibitors of squalene epoxidase a key enzyme in cholesterol biosynthesis . Planta Medica , 66 : 753 – 756 .
- Abe , I , Seki , T , Umehara , K , Miyase , T , Noguchi , H , Sakakibara , J and Ono , T . (2000c) . Green tea polyphenols: novel and potent inhibitors of squalene epoxidase . Biochemical and Biophysical Research Communications , 268 : 767 – 771 .
- Abubakar , MS , Sule , MI , Pateh , UU , Abdurahman , EM , Haruna , AK and Jahun , BM . (2000) . In vitro snake venom detoxifying action of the leaf extract of Guiera senegalensis . Journal of Ethnopharmacology , 69 : 253 – 257 .
- Bonfoh B Ankers P Pfister K Pangui LJ Toguebaye BS (1997) Répertoire de quelques contraintes de l'aviculture villageoise en Gambie et propositions de solutions pour son amélioration In Proceedings of the INFPD Workshop pp. 135–147 M'Bour Senegal
- Bouchet , N , Barrier , L and Fauconneau , B . (1998) . Radical scavenging activity and antioxidant properties of tannins from Guiera senegalensis (Combretaceae) . Phytotherapy Research , 12 : 159 – 162 .
- Bouchet , N , Levesque , J and Pousset , JL . (2000) . HPLC isolation, identification and quantification of tannins from Guiera senegalensis . Phytochemical Analysis , 11 : 52 – 56 .
- Breman , JG and Henderson , DA . (1998) . Poxvirus dilemmas—monkeypox, smallpox, and biologic terrorism . The New England Journal of Medicine , 339 : 556 – 559 .
- Buttke , TM and Gafford , LG . (1982) . Effect of fowlpox virus on lipid metabolism in cultured chicken embryo cells . Journal of Virology , 43 : 749 – 752 .
- Garcìa , M , Narang , N , Reed , WM and Fadly , AM . (2003) . Molecular characterization of reticuloendotheliosis virus insertions in the genome of field and vaccine strains of fowl poxvirus . Avian Diseases , 47 : 343 – 354 .
- Hitchner SB (1980) Virus propagation in embryonating eggs In S.B. Hitchner, G.H. Domermuth, H.G. Purchase & J.E. Williams (Eds.) Isolation and Identification of Avian Pathogens 2nd edn New York American Association of Avian Pathologists pp. 120–121
- Kitalyi AJ (1998) Village Chicken Production Systems in Rural Africa: Household Food Security and Gender Issues FAO Animal production and health paper 142 Rome Information Division, Food and Agriculture Organization of the United Nations
- Lamien , CE , Meda , A , Mans , J , Romito , M , Nacoulma , OG and Viljoen , GJ . (2005) . Inhibition of fowlpox virus by an aqueous acetone extract from galls of Guiera senegalensis J. F. Gmel (Combretaceae) . Journal of Ethnopharmacology , 96 : 249 – 253 .
- Mahmood , N , Moore , PS , De Tommasi , N , De Simone , F , Colman , S , Hay , AJ and Pizza , C . (1993) . Inhibition of HIV infection by caffeoylquinic acid derivatives . Antiviral Chemistry & Chemotherapy , 4 : 235 – 240 .
- Mopate LY Hendrikx P Imadine M (1997) Contraintes sanitaires des (poulets) dans la région du centre-est du Tchad In Proceedings of the INFPD Workshop pp. 103–110 M'Bour Senegal
- Nacoulma OG (1996) Plantes médicinales et pratiques médicales traditionnelles au Burkina Faso—Cas du plateau central Tome 2 Thèse de Doctorat d'Etat ès Sciences Naturelles, Université de Ouagadougou
- Ohta , H , Kai , C , Yoshikawa , Y and Yamanouchi , K . (1983) . Activation of chicken complement alternative pathway by fowlpox virus-infected cells . Infection and Immunity , 42 : 721 – 727 .
- Ohta , H , Yoshikawa , Y , Kai , C , Yamanouchi , K , Taniguchi , H , Komine , KI , Ishijima , Y and Okada , H . (1986) . Effect of complement depletion by cobra venom factor on fowlpox virus infection in chickens and chicken embryos . Journal of Virology , 57 : 670 – 673 .
- Payment P Trudel M (1989) Isolement et identification des virus In P. Payment & M. Trudel (Eds.) Manuel de techniques virologiques Québec Presse de l'université du Québec pp. 21–44
- Ramade F (1993) Dictionnaire encyclopédique de l’écologie et des sciences de l'environnement Paris Ediscience Internationale
- Reed , LJ and Muench , H . (1938) . A simple method of estimating fifty per cent endpoints . The American Journal of Hygiene , 27 : 493 – 497 .
- Sambrook J Fritsch EF Maniatis T (1989) Purification of nucleic acids In Molecular Cloning. A Laboratory Manual 2nd edn New York Cold Spring Harbor Laboratory Press pp. E3–E4
- Silim , A , El Azhary , ASY and Roy , RS . (1981) . A simple technique for preparation of chicken-embryo-skin cells cultures . Avian Diseases , 26 : 182 – 185 .
- Tripathy DN Hanson LE (1980) Avian pox In S.B. Hitchner, G.H. Domermuth, H.G. Purchase & J.E. Williams (Eds.) Isolation and Identification of Avian Pathogens 2nd edn New York American Association of Avian Pathologists pp. 109–111
- Velazquez , E , Tournier , HA , Mordujovich de Buschiazzo , P , Saavedra , G and Schinella , GR . (2003) . Antioxidant activity of Paraguayan plant extracts . Fitoterapia , 74 : 91 – 97 .