Abstract
Splenocytes from chickens infected with low-passage stocks of Marek's disease virus (MDV) RB-1B, a very virulent (vv) strain and vv+ RK-1 were used to compare the efficacy of chick kidney cells (CKC), chicken embryo fibroblasts (CEF) and chicken embryo kidney cells (CEKC) for virus isolation. CKC were superior to CEF and CEKC. MDV foci were present at 4 days post infection in CKC but not until 6 days post infection in CEF or CEKC. Virus yield was higher in CKC than in CEF or CEKC at 6 days post infection. Passage of RB-1B in CKC yielded a significantly higher virus increase than with CEF or CEKC. The same was true for RK-1 comparing CKC with CEKC. Interestingly, RK-1-infected CEF were negative or had very low number of foci in passage 1, but virus yield increased 500-fold to 600-fold on passage in CKC, CEF, and CEKC. Recommendations on procedures for successful virus isolation are provided.
Les splénocytes de poulet infectés par du virus de la maladie de Marek (MDV) correspondant aux premiers passages d'une souche très virulente (vv) RB-1B et d'une souche hypervirulente vv+RK-1, ont été utilisés pour comparer l'efficacité des cellules de rein de poussin (CKC), des fibroblastes d'embryon de poulet (CEF) et des cellules embryonnaires de reins de poulet (CECK). Les CKC ont été supérieurs aux CEF et aux CECK. Les foyers de MDV ont été mis en évidence le 4è jour après l'infection (dpi) pour les CKC mais pas avant le 6è dpi pour les CEF et CECK. La récolte de virus a été supérieure avec les CKC qu'avec les CEF ou CECK au 6è dpi. Le passage de RB-1B sur CKC a donné des quantités significativement supérieure en virus par rapport au passage sur CEF ou CECK. Les mêmes résultats ont été obtenus avec RK-1 en comparant CKC et CECK. D'une manière intéressante, les CEF infectés par RK-1 ont été négatifs ou présentaient des foyers en nombre très faible au premier passage, mais la quantité de virus récoltée a augmenté de 500 à 600 fois en passant sur CKC, CEF et CECK. Les recommandations sur les procédures de l'isolement de virus avec succès sont fournies.
Für den Vergleich der Eignung von Hühnernierenzellen (CKC), Hühnerembryofibroblasten (CEF) und Hühnerembryonierenzellen (CEKC) zur Virusisolierung wurden Milzzellen von Hühnern, die mit einer niedrigen Passage des hochvirulenten (vv) Stammes RB-1B des Virus der Marekschen Krankheit (MDV) infiziert worden waren, und der vv+ RK-1-Stamm verwendet. CKC waren den CEF und den CEKC überlegen. In CKC traten MDV-Plaques am 4. Tag nach der Infektion (dpi) auf, in CEF und CEKC jedoch nicht vor dem 6 dpi. Die Passagierung von RB-1B führte in CKC zu einem signifikant höheren Virustiteranstieg als in CEF oder CEKC. Das gleiche galt für RK-1 beim Vergleich von CKC und CEKC. Interessanterweise waren RK-1- infizierte CEF in der 1. Passage negativ oder wiesen nur eine sehr geringe Zahl von Plaques auf, aber der Virusgehalt stieg nach Passagierung in CKC, CEF und CEKC um das 500- bis 600-fache an. Die Anzüchtungsanforderungen für eine erfolgreiche Virusisolierung werden beschrieben.
Se utilizaron esplenocitos de pollos infectados con estoc de pocos pases de virus de Marek (MDV) RB-1B, una cepa muy virulenta (vv) y vv+ RK-1 para comparar la eficacia de células de riñón de pollo (CKC), fibroblastos de embrión de pollo (CEF) y células de riñón de embrión de pollo (CEKC) para el aislamiento del virus. Las CKC fueron superiores a las CEF y CEKC. Se observaron focos de MDV a los 4 días post infección (dpi) en CKC, pero no hasta 6 dpi en CEF o CEKC. Los rendimientos víricos fueron superiores en CKC respecto a CEF o CEKC a los 6 dpi. Pases de RB-1B en CKC produjeron un incremento del virus significativamente más elevado que con CEF o CEKC. Interesantemente, CEF infectados con RK-1 fueron negativos o presentaron un bajo número de focos en el pase 1, pero el virus incrementó 500 o 600 veces en pases en CKC, CEF y CEKC. Se proponen algunas recomendaciones sobre los procedimientos para poder aislar satisfactoriamente este virus.
Introduction
Marek's disease virus (MDV), the causative agent of Marek's disease (MD), was first isolated in chick kidney cell (CKC) cultures by Churchill & Biggs (Citation1967) and shortly afterwards in duck embryo fibroblasts (DEF) (Nazerian et al., Citation1968; Solomon et al., Citation1968). In both culture systems, characteristic cytopathic effects (CPE) developed and incomplete herpesvirus particles were detected in the nuclei of infected cells. Calnek & Madin (Citation1969) indicated that inoculation of CKC at 24 to 48 h with monolayers 70% to 80% confluent yielded higher virus isolation rates using JMCT (a MD-derived tumour transplant) cells than with monolayers that were 30% to 40% complete. The use of chicken embryonal fibroblasts (CEF) yielded conflicting results for virus isolation. Kottaridis et al. (Citation1968) reported the development of CPE after the third passage in CEF, and inoculation of these cells into chickens induced MD. However, Witter et al. (Citation1968) reported that CEF were refractory to MDV infection. Chicken embryo liver cell, chicken embryo lung cell, and chicken embryo kidney cell (CEKC) cultures did not show CPE after inoculation with blood from JM-infected chickens, but chickens inoculated with these cells developed MD. Calnek (Citation1967) also concluded that different types of chicken embryonal cells were not able to replicate MDV. Schat (Citation1985) mentioned unpublished data indicating that replication of MDV in CEKC was abortive and that virus would be lost after several passages in CEKC. Unfortunately, the negative virus isolation results using different chicken embryonal cells were mostly referred to as unpublished data or were obtained prior to the isolation of MDV and therefore lacked positive controls.
These results clearly suggest that CKC or DEF are the optimal cell cultures to isolate MDV (Sharma, Citation1998). The genetic susceptibility of the SPF chickens used for the preparation of CKC does not influence virus replication rates (Spencer, Citation1969). However, CEKC and CEF are frequently used (De Laney et al., Citation1995, Citation1998; Lee et al., Citation1999), often resulting in low or very low virus isolation rates. In the summing-up of the 6th International Marek's Disease Meeting in 2000, Calnek stated “I found it disheartening that some of the techniques used for virus isolation were so woefully inefficient. For instance, the use of CEK rather than CK cultures could certainly account for the failure to detect viraemia in many of the birds” (Calnek & Morgan, Citation2001, p. 332). Similarly, during the Molecular Workshop on Marek's Disease in Cyprus in 2002 it was mentioned that virus isolation often failed when samples were obtained from MD-positive chickens that were suspected to be infected with “hypervirulent” MDV strains in Europe. The question was raised of whether recent isolates had changed and that virus isolation of these strains had become more difficult. This paper presents comparative studies on isolation of MDV in CKC, CEF and CEKC from chickens infected with very virulent (vv) RB-1B, vv+ RK-1 MDV strains, and viruses of undetermined virulence from recent field cases. DEF were not included in this study because there are very few specific pathogen free (SPF) duck flocks available.
Materials and Methods
Cell cultures
Primary CKC, CEF, and CEKC were prepared from 14-day-old chicks, 10-day-old embryos, and 19-day-old to 20-day-old embryos, respectively, following standard procedures (Schat & Purchase, Citation1998). Cells were seeded in 35 mm dishes and incubated at 38.5 to 39°C in 5% CO2 to establish nearly confluent monolayers at 24 h. Growth medium consisted of M199 supplemented with 10% tryptose-phosphate broth (TPB), 0.63% of a 10% NaHCO3 solution, 1% of Antibiotic-Antimycotic Mixture 100X (Gibco, Grand Island, New York, USA), and 4% foetal bovine serum (FBS). For CEF and CEKC, this medium was replaced after 24 h by the same medium but supplemented with only 0.2% FBS. The maintenance medium for CKC consisted of an equal mixture of Ham's F10 and 199 supplemented with 7.5% TPB, 0.63% of a 10% NaHCO3 solution, 1% of Antibiotic-Antimycotic Mixture 100X, and 0.2% FBS. Embryonated eggs and chicks were obtained from SPF N2a (Weinstock & Schat, Citation1987) flocks maintained by the Department of Microbiology and Immunology at Cornell University.
Virus strains
The vv MDV strain RB-1B (Schat et al., Citation1982) passage (p) 5 was passed twice (experiment 1) or three times (experiment 2) in SPF chickens. The vv+ RK-1 strain (Calnek et al., Citation1998) p9 was passed once (experiment 1) or twice (experiment 3) in SPF chickens. Spleen cells from individual chickens were harvested 7 to 14 days post infection (d.p.i.), red blood cells were removed by centrifugation over Ficoll-Paque (Amersham Biosciences, Sweden), and lymphocytes were stored in liquid nitrogen using medium containing dimethyl sulphoxide (Schat & Purchase, Citation1998). Dr Patricia Wakenell (College of Veterinary Medicine, University of California at Davis, California, USA) kindly provided CKC infected with p2 of an unidentified MDV isolate obtained from tumour material from a Silkie hen. Tumour material (NC) from a recent outbreak of MD was obtained from Dr Andrea Miles (College of Veterinary Medicine, North Carolina State University, Raleigh, North Carolina, USA). The latter two samples were added to determine the efficacy to isolate MDV from field material.
Monoclonal antibodies and indirect immunofluorescence assays
Monoclonal antibodies (mAb) specific for serotype 1, serotype 2, and serotype 3 (Lee et al., Citation1983) were kindly provided by Dr Lucy Lee (ADOL, East Lansing, Michigan, USA). CEF grown on glass coverslips infected with p5 of the Silkie chicken were harvested and fixed in acetone, when CPE was visible. CEF containing p3 of the NC isolate were placed in 18 wells of a glass slide, air-dried, and acetone fixed. The fixed cells were incubated for 30 min with the appropriate dilutions of the three mAbs, washed, incubated for 30 min with fluorescin isothiocyanate-labelled goat anti-mouse IgG H and L chain, washed, and examined for fluorescent cells.
Experimental design
Primary cell cultures were inoculated at 24 h after seeding when the monolayers were between 90% and 100% confluent. Media were changed at 3 and 5 d.p.i. and foci were enumerated at 4 and 6 d.p.i. All cultures were trypsinized at 6 d.p.i. and the cells were inoculated onto new primary cultures as outlined in the following for each individual experiment.
Experiment 1
Four 35 mm Petri dishes with CKC, CEKC, and CEF were inoculated with 0.5×106 or 0.25×106 splenocytes from a RB-1B-infected chicken or 0.3×106 splenocytes from a RK-1 infected chicken. At 6 d.p.i., each CKC and CEKC culture was divided over two CKC and two CEKC cultures and the foci were enumerated at 5 d.p.i. Selected cultures of p2 were trypsinized, cells were divided over two new CKC and CEKC, and foci were counted at 5 d.p.i. CEF infected with splenocytes were not used for additional passage. Foci were enumerated at 5 d.p.i.
Experiment 2
Four CKC, CEKC, and CEF cultures/bird were inoculated with 0.5×106 splenocytes from three individual RB-1B-infected chickens and with the NC tumour material. At 6 days, cells were trypsinized and each CKC and CEKC culture from 2 RB-1B-infected chickens and the NC isolate were divided over two CKC and two CEKC. CEF cultures from two RB-1B-infected birds and the NC isolate were also trypsinized, and the cells from each culture were divided over two CKC and two CEF cultures. Foci in all p2 cultures were enumerated at 5 d.p.i.
Experiment 3
Splenocytes (0.5×106) from three RK-1-infected chickens and p2 of the Silkie hen isolate were inoculated onto four CKC, CEF, and CEKC cultures/bird. At 6 d.p.i., each dish of each cell type was trypsinized and 50% of the cell yield of each dish was used to inoculate two dishes with the same cell type. The remaining cells of each dish were used to infect two dishes of one of the other two cell types. Foci were counted at 5 d.p.i.
Statistical analysis
The numbers of foci for each bird were expressed as the mean number of foci/0.5×106 splenocytes in p1. The number of foci for each cell type in p2 and p3 were expressed as the estimated total number of foci if four dishes with that cell type had been inoculated by multiplying the total number of foci in two dishes with a factor 2. The numbers of estimated foci for each cell type were compared for statistical differences using the two-tailed Student's t test.
Results
Primary isolation of RB-1B and RK-1 strains
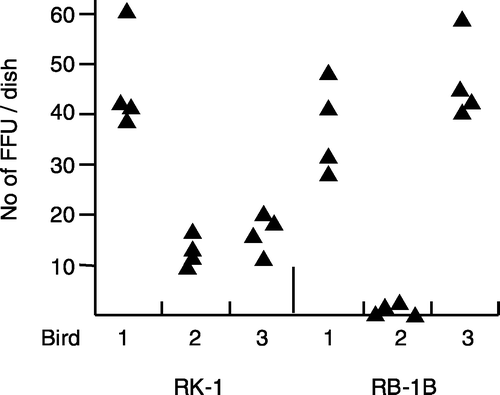
The results for the isolation of RB-1B and RK-1 are summarized in (experiments 1 to 3), respectively. At 4 d.p.i., CPE was detected in CKC but, with one exception, not in CEF and CEKC. At 6 d.p.i., virus was isolated in CKC from all birds, but only 50% of the birds were positive in CEF or CEKC. The number of focus-forming units (FFU)/0.5×106 splenocytes was always lower in CEF and CEKC than in CKC. For each bird four dishes of each cell type were inoculated and there was little variation in the number of foci/dish, as illustrated in for experiments 2 and 3 in CKC.
Figure 1. Number of FFU of MDV isolated in CKC, CEKC, and CEF cultures inoculated with 0.5×106 splenocytes/culture infected with RB-1B (1a) or RK-1 (1b). The FFU represent the average for four cultures/bird. Foci were enumerated at 4 d.p.i. (open bars) and 6 d.p.i. (closed bars). The absence of a bar indicates that foci were not present.
Primary isolation of virus from field samples
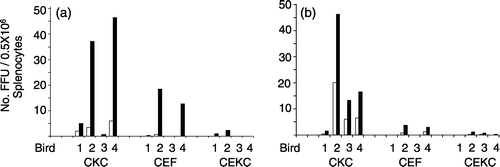
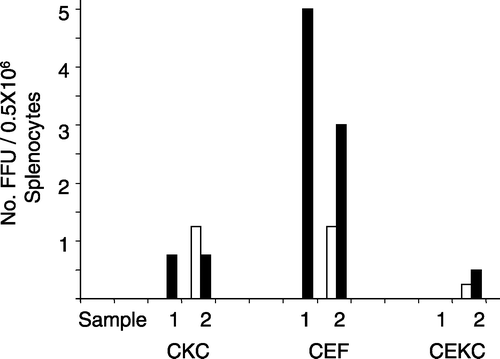
Two field samples were tested and in both cases the number of foci was higher in CEF than in CKC or CEKC (). However, the foci included HVT, which was confirmed by immunofluorescence assays. The isolation of HVT was not unexpected because these birds had been vaccinated with HVT.
Figure 3. Number of FFU isolated in CKC, CEKC, and CEF cultures inoculated with 0.5×106 cells/culture of tumour material (p2 in CKC) from a Silkie hen (sample 1) and NC tumour cells (sample 2). The FFU represent the average for four cultures/sample. Foci were enumerated at 4 d.p.i. (open bars) and 6 d.p.i. (closed bars). The absence of a bar indicates the absence of foci.
Passage of RB-1B and RK-1
In order to prepare stocks of virus representing the original isolate it will be important to increase titres of new isolates with the smallest number of passages. To determine whether there are differences between cell types in increasing titres, the first passage of RB-1B-infected and RK-1-infected CKC, CEF and CEKC were trypsinized and the cells were used to inoculate two 35 mm dishes with CKC, CEF, and/or CEKC. In experiment 1, CKC and CEKC-isolated RB-1B and RK-1 were passed to these two cell types. Passage of CKC-isolated virus to CKC and CEKC increased the titre, while passage of CEKC-isolated virus resulted in low virus yields. Similar results were obtained when RB-1B p2 in CEKC was used to generate p3 in CKC and CEKC (). The p2 CEKC were obtained from CEKC infected with p1 RB-1B in CKC, because inoculation of CEKC with p1 RB-1B in CEKC did not yield enough foci to warrant making a passage. In experiments 2 and 3, there was a significant increase in the number of foci when virus-infected CKC were inoculated onto CKC, but when these cells were inoculated onto CEF or CEKC the yield remained similar to the input value or slightly decreased (). RB-1B isolated in CEKC could be passed to CKC cultures with a significant increase in virus yield (), confirming the data of experiment 1. However, passage of RK-1 CEKC did not increase the virus yield significantly in any of the three cell types (). The passage of CEF-isolated RB-1B to CEF and CKC resulted in a marked increase in FFU yield in CKC and a lower yield in CEF, especially in the bird that was negative for virus isolation (). CEF was not a good cell type for primary isolation of RK-1 with 0 or very few foci for individual birds in contrast to isolation on CKC (). However, passage of the RK-1-inoculated CEF to CEF, CKC, and CEKC yielded on average between 500 and 700 FFU, with the highest number in CKC, but the differences between CKC, CEF, and CEKC were not statistically significant (). This yield is significantly higher than when RK-1 was isolated in CKC and passed to CKC (compare and ).
Figure 4. Relative increase of the number of MDV FFU in p2. 4a: p1 RB-1B-infected CKC were trypsinized and the cells were passed to CKC or CEKC (experiment 2). 4b: p1 RK-1-infected CKC were trypsinized and the cells were passed to CKC, CEF, or CEKC (experiment 3). The values are based on the average counts of FFU for three individual bird samples and are expressed as the relative number of FFU in p2 compared with the number of FFU in p1. The average number of FFU for the three birds in p1 was set at 1. The open and closed symbols in (b) are representing the same birds in p1, but cells were passed to CKC and CEKC (open symbols) or CKC and CEF (closed symbols). Note that the scales for (a) and (b) are different. Significant differences: * P<0.05 and *** P<0.001 using Student's t test; vertical bars indicate the standard deviation.
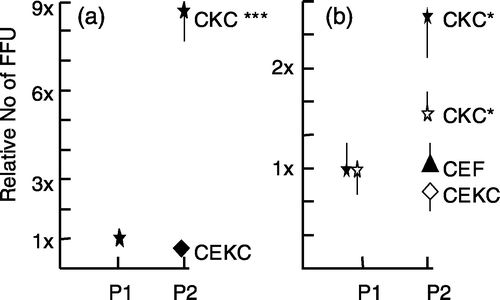
Figure 5. Increase in the number of MDV FFU in p2 using MDV-infected CEKC. 5a: p1 RB-1B-infected CEKC were trypsinized and the cells were passed to CEKC or CKC (experiment 2). 5b: p1 RK-1-infected CEKC were trypsinized and the cells were passed to CEKC, CEF, or CKC (experiment 3). The values represent the average number of FFU based on four dishes/bird for two individual bird samples (#1=open symbols and #2=closed symbols (a) or the average value for three individual birds (b). The open and closed symbols in (b) are representing the same birds in p1, but cells were passed to CEKC and CEF (open symbols) or CEKC and CKC (closed symbols). Significant differences: ** P<0.01 using Student's t test; vertical bars indicate the standard deviation.
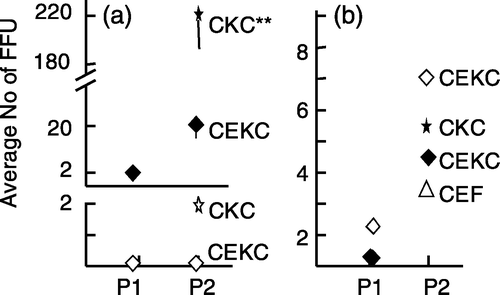
Figure 6. Increase in the number of MDV FFU in p2 using MDV-infected CEF. 6a: p1 RB-1B-infected CEF were trypsinized and the cells were passed to CEF or CKC (experiment 2). 6b: p1 RK-1-infected CEF were trypsinized and the cells were passed to CKC, CEF, or CEKC (experiment 3). The values represent the average number of FFU based on four dishes for two individual bird samples (#1=open symbols and #2=closed symbols (a) or the average value for three individual birds (b). The open and closed symbols in (b) are representing the same birds in p1 but cells were passed to CEF and CEKC (open symbols) or CEF and CKC (closed symbols). Significant differences: * P<0.05 using Student's t test; vertical bars indicate the standard deviation.
Table 1. Isolation and passage of MDV strains RB-1B and RK-1 in CKC and CEKC cultures
Discussion
The results confirm that CKC are superior for isolation of serotype 1 MDV compared with CEF and CEKC. The latter are not acceptable for virus isolation as was suggested previously by Schat (Citation1985), and although virus can be isolated in CEKC, passage of CEKC-isolated virus onto CEKC resulted in minimal increases to actual decreases in virus yield. CEF are also not recommended for virus isolation because titres are lower than in CKC and foci could not be detected at 4 d.p.i. in contrast to CKC. This is especially important if vaccine viruses are present in the inoculum, because HVT but also CVI988 and serotype 2 strains may replicate faster than, and outgrow, wild-type viruses if foci can only be identified at 6 d.p.i. Moreover, HVT, serotype 2 viruses, and CVI988 vaccines can be readily isolated on CEF. Identification of foci in CKC at 4 d.p.i. will facilitate the selection of individual foci of wild-type virus using morphological characteristics such as small clusters of round cells (Schat, Citation1985; Witter & Schat, Citation2003). These foci can be harvested from CKC using Eppendorf pipettes while the cultures are observed through a tissue culture microscope. The harvested cells can be collected in tubes containing a small amount of trypsin-versene (Schat & Purchase, Citation1998) to disperse the cells and inoculated onto new CKC cultures. This approach will enrich for the virus present in the harvested cells, but this approach does not constitute biological cloning (see later).
The finding that RK-1 was present in a large number of CEF after inoculation without causing CPE was unexpected and the reasons for this observation are not clear. Moreover, inoculation of CKC, CEF, and CEKC with the first passage in CEF yielded equally high titres. It is not clear whether this is specific to RK-1 or that other vv+ strains will show the same pattern.
Recommendations for virus isolation
The following procedure for virus isolation is recommended. Prepare CKC from 14-day-old chickens or DEF from 12-day-old duck embryos as described by Schat & Purchase (Citation1998). The use of SPF chickens or SPF duck eggs is essential to reduce the risk of introducing extraneous viruses. Because there is no egg transmission of MDV, placement of 1-day-old SPF chickens in isolators is sufficient to provide a source of kidneys. However, if isolators are not available, it may be possible to maintain a source of SPF chickens free of MDV and other agents using a clean room and proper management rules, but it will be essential to check the CKC cultures for possible contamination. Spleen cells, tumour cells, or peripheral blood cells are all good sources for virus isolation, but removal of red blood cells is strongly recommended to facilitate the detection of foci. The number of lymphocytes to be inoculated depends on the size of the culture dish. Between 0.5×106 and 106 cells is recommended for dishes with a diameter of 35 to 60 mm. Higher numbers of lymphocytes may inhibit the formation of foci and decrease the virus yield per 106 lymphocytes (Calnek et al., Citation1982). Media changes 2 and 4 d.p.i. will be sufficient and allow the rescue of virus from latently infected lymphocytes. If whole blood is used for virus isolation, media changes and extensive washing with phosphate-buffered saline is recommended at 1 d.p.i. Inoculation of three to four dishes with a single sample from a chicken will be enough to isolate virus if MDV is present, even if present at low levels. Selection and passage of individual foci at 4 d.p.i. will strongly reduce the chances of contamination with vaccine viruses or wild-type serotype 2 viruses. Once virus is isolated it is recommended to expand the virus by inoculating SPF chickens with p2 or p3 and to harvest splenocytes from these birds between 7 and 14 d.p.i. These cells can be used to reisolate virus and obtain standardized stocks of low passage for experimental purposes. This approach will yield an “unlimited” source of very low-passage material. It is also recommended to produce biological clones by inoculation of CKC with cell-free virus followed by selection of a single focus in cultures with a small number of foci (Calnek, Citation1973). Cell-free virus can be obtained from feather follicle epithelium (Calnek et al., Citation1970a) harvested between 14 and 21 d.p.i. from chickens inoculated with p2 or p3 using SPGA as a diluent (Calnek et al., Citation1970b). The addition of 5 to 10 mM ethylenediamine tetraacetic acid to the medium will increase the virus yield (Adldinger & Calnek, Citation1971). Cloned virus can be amplified in birds once more and cell-culture-propagated virus with adequate titres can be obtained in three to four passages in CKC starting with cloned virus in splenocytes.
Translations of the abstract in French, German and Spanish are available on the Avian Pathology website.
The author thanks Ms P. H. O'Connell for technical support. This work was supported in part by the USDA Regional Research NE-1016.
References
- Adldinger , HK and Calnek , BW . (1971) . An improved in vitro assay for cell-free Marek's disease virus . Archiv für die gesamte Virusforschung , 34 : 391 – 395 .
- Calnek BW (1967) Proceedings of the 39th Northeastern Conference on Avian Diseases In CD Northeastern Conference on Avian Diseases 75th Anniversary, 2003 p. 29 Stony Brook NY USA
- Calnek , BW . (1973) . Influence of age at exposure on the pathogenesis of Marek's disease . Journal of the National Cancer Institute , 51 : 929 – 939 .
- Calnek , BW and Madin , SH . (1969) . Characteristics of in vitro infection of chicken kidney cell cultures with a herpesvirus from Marek's disease . American Journal of Veterinary Research , 30 : 1389 – 1402 .
- Calnek BW Morgan R (2001) Summing up of the Marek's disease symposium In K.A. Schat, R.M. Morgan, M.S. Parcells & J.L. Spencer (Eds.) Current Progress on Marek's Disease Research Kennett Square PA American Association of Avian Pathologists pp. 331–341
- Calnek , BW , Adldinger , HK and Kahn , DE . (1970a) . Feather follicle epithelium: a source of enveloped and infectious cell-free herpesvirus from Marek's disease . Avian Diseases , 14 : 219 – 233 .
- Calnek , BW , Hitchner , SB and Adldinger , HK . (1970b) . Lyophilization of cell-free Marek's disease herpesvirus and a herpesvirus from turkeys . Applied Microbiology , 20 : 723 – 726 .
- Calnek , BW , Shek , WR , Schat , KA and Fabricant , J . (1982) . Dose-dependent inhibition of virus rescue from lymphocytes latently infected with turkey herpesvirus or Marek's disease virus . Avian Diseases , 26 : 321 – 331 .
- Calnek , BW , Harris , RW , Buscaglia , C , Schat , KA and Lucio , B . (1998) . Relationship between the immunosuppressive potential and the pathotype of Marek's disease virus isolates . Avian Diseases , 42 : 124 – 132 .
- Churchill , AE and Biggs , PM . (1967) . Agent of Marek's disease in tissue culture . Nature , 215 : 528 – 530 .
- De Laney , DB , Jones , AE , Zerbes , M and Tannock , GA . (1995) . Isolation of serotype 1 Marek's disease viruses from vaccinated Australian flocks . Veterinary Microbiology , 46 : 213 – 219 .
- De Laney , DB , Morrow , CJ , Read , KM and Tannock , GA . (1998) . The development and evaluation of two tissues culture grown Marek's disease challenge viruses . Avian Pathology , 27 : 472 – 477 .
- Kottaridis , SD , Luginbuhl , RE and Fredrickson , TN . (1968) . Marek's disease. II. Propagation of the Connecticut-A isolate in cell culture . Avian Diseases , 12 : 246 – 258 .
- Lee , LF , Liu , X and Witter , RL . (1983) . Monoclonal antibodies with specificity for three different serotypes of Marek's disease viruses in chickens . Journal of Immunology , 130 : 1003 – 1006 .
- Lee , SI , Ohashi , K , Morimura , T , Sugimoto , C and Onuma , M . (1999) . Re-isolation of Marek's disease virus from T cell subsets of vaccinated and non-vaccinated chickens . Archives of Virology , 144 : 45 – 54 .
- Nazerian , K , Solomon , JJ , Witter , RL and Burmester , BR . (1968) . Studies on the etiology of Marek's disease. II. Finding of a herpesvirus in cell culture . Proceedings of the Society of Experimental Biology and Medicine , 127 : 177 – 182 .
- Schat KA (1985) Characteristics of the virus In L.N. Payne (Ed.) Marek's Disease Boston Martinus Nijhoff pp. 77–112
- Schat KA Purchase HG (1998) Cell-culture methods In D.E. Swayne, J.R. Glisson, M.W. Jackwood, J.E. Pearson & W.M. Reed (Eds.) A Laboratory Manual for the Isolation and Identification of Avian Pathogens 4th edn Kennett Square PA American Association of Avian Pathologists pp. 223–234
- Schat , KA , Calnek , BW and Fabricant , J . (1982) . Characterisation of two highly oncogenic strains of Marek's disease virus . Avian Pathology , 11 : 593 – 605 .
- Sharma JM (1998) Marek's disease In D.E. Swayne, J.R. Glisson, M.W. Jackwood, J.E. Pearson & W.M. Reed (Eds.) A Laboratory Manual for the Isolation and Identification of Avian Pathogens 4th edn Kennett Square PA American Association of Avian Pathologists pp. 116–124
- Solomon , JJ , Witter , RL , Nazerian , K and Burmester , BR . (1968) . Studies on the etiology of Marek's disease. I. Propagation of the agent in cell culture . Proceedings of the Society of Experimental Biology and Medicine , 127 : 173 – 177 .
- Spencer , JL . (1969) . Marek's disease herpesvirus: in vivo and in vitro infection of kidney cells of different genetic strains of chickens . Avian Diseases , 13 : 753 – 761 .
- Weinstock D Schat KA (1987) Virus specific syngeneic killing of reticuloendotheliosis virus transformed cell line target cells by spleen cells In W.T. Weber & D.L. Ewert (Eds.) Progress in Clinical and Biological Research 238 New York Alan R. Liss pp. 253–264
- Witter RL Schat KA (2003) Marek's disease In Y.M. Saif, H.J. Barnes, A.M. Fadly, J. R. Glisson, L.R. McDougald & D.E. Swayne (Eds.) Diseases of Poultry 11th edn Ames Iowa State Press pp. 407–465
- Witter , RL , Burgoyne , GH and Solomon , JJ . (1968) . Preliminary studies on cell cultures infected with Marek's disease agent . Avian Diseases , 12 : 169 – 185 .