Abstract
Pasteurella gallinarum has been considered an opportunistic pathogen rather than a primary pathogen for chickens. As P. gallinarum has been found to have a high genotypic diversity, one would expect a polyclonal distribution among isolates from different farms if this organism is a secondary invader. The aims of this investigation were to genetically characterize isolates obtained from outbreaks affecting several turkey farms to confirm the existence of the infection in turkeys and to investigate the genetic relationship between isolates from affected farms. A total of 17 isolates from 14 outbreaks of respiratory disease in Germany were subjected to extended phenotypic and genotypic characterization. All isolates were of the same phenotype, typical of P. gallinarum. Ribotyping of three isolates using either HpaII or HindIII showed that they had identical profiles and indicated that the isolates all originated from the same clone. Comparison with HpaII ribotypes from a previous study showed that the pattern was identical to that obtained with isolates from a Zimbabwean outbreak in chickens during 1999 to 2000. Restriction endonuclease analysis typing of 14 isolates from all 14 farms showed that they had identical profiles but these differed from those obtained with isolates from the Zimbabwean outbreak. Sequencing of the 16S rRNA gene and sequence comparisons with other Pasteurellaceae confirmed their classification as P. gallinarum. Identification of the same clone of P. gallinarum from 14 outbreaks of acute respiratory disease in turkeys within a time period of 2 months suggests a common source of infection, and that P. gallinarum probably played a primary role rather than a secondary role in the outbreaks.
Pasteurella gallinarum a été considéré comme un agent opportuniste plutôt qu'un agent pathogène primaire pour le poulet. Du fait qu'il a été montré que P. gallinarum avait une diversité génétique élevée, on pourrait s'attendre à une distribution polyclonale des souches des différents élevages, si cet organisme est un agent d'invasion secondaire. Les buts de cette recherche ont été de caractériser génétiquement les souches isolées dans des foyers affectant plusieurs élevages de dinde pour confirmer l'existence de l'infection chez les dindes et pour connaître la relation génétique entre les souches des élevages affectés. Dix-sept souches isolées de 14 foyers de maladie respiratoire en Allemagne ont fait 'objet d'une caractérisation phénotypique et génétique. Toutes les souches ont présenté le même phénotype typique de Pasteurella gallinarum. Le ribotypage de 3 souches utilisant soit HpaII soit HindIII a montré qu'elles présentaient des profils identiques. Ceci indique que les souches sont toutes issues d'un même clone. La comparaison des ribotypes HpaII à ceux d'une étude précédente montre que le profil est identique à celui des souches isolées d'un cas observé chez des poulets au Zimbabwe en 1999-2000. Le typage REA de 14 souches isolées dans les 14 élevages a montré que les profils étaient identiques mais qu'ils différaient de ceux des souches isolées du cas observé au Zimbabwe. Le séquençage du gène de l'ARNr 16S et les comparaisons des séquences avec les autres Pasteurellaceae ont confirmé qu'il s'agissait de P. gallinarum. L'identification du même clone de P. gallinarum isolé à partir des 14 cas de maladie respiratoire aiguë chez des dindes dans un intervalle de temps de 2 mois suggère une source commune d'infection et que P. gallinarum a joué probablement un rôle primaire plutôt que secondaire dans ces cas.
Pasteurella gallinarum wird eher als ein sekundär denn als ein primär pathogener Errreger für Hühner angesehen. Da bei P. gallinarum eine große genotypische Vielfalt festgestellt worden ist, würde man, wenn es sich um einen Sekündärkeim handelte, eine polyklonale Verteilung bei den Isolaten von verschiedenen Farmen erwarten. Ziele dieser Untersuchung waren die genetische Charakterisierung der Isolate von Krankheitsausbrüchen auf verschiedenen Putenfarmen, die Bestätigung des Vorkommens der Infektion bei Puten und die Untersuchung der genetischen Beziehungen zwischen den Isolaten aus den betroffenen Farmen. Insgesamt wurden bei 17 Isolaten aus 14 Ausbrüchen von Respirationserkrankungen in Deutschland umfangreiche phänotypische und genetische Charakterisierungen durchgeführt. Alle Isolate gehörten zu demselben für P. gallinarum typischen Phänotyp. Die Ribotypisierung von drei Isolaten unter Verwendung von HpaII oder HindIII zeigte, dass sie ein identisches Profil hatten, und wies darauf hin, dass alle Isolate von demselben Klon abstammten. Der Vergleich mit HpaII-Ribotypen aus einer vorherigen Studie ließ erkennen, dass das Muster identisch war mit demjenigen aus Isolaten von einem Ausbruch bei Hühnern in Zimbabwe in den Jahren 1999-2000. Die REA-Typisierung von 14 Isolaten aus allen 14 Farmen zeigte, dass ihr Muster identisch war, sich aber von dem der Isolate aus dem Ausbruch in Zimbabwe unterschieden. Die Sequenzierung des 16S rRNA-Gens und der Sequenzvergleich mit anderen Pasteurellaceae bestätigte ihre Klassifizierung als P. gallinarum. Die Identifizierung desgleichen P. gallinarum-Klons bei 14 Ausbrüchen einer akuten Respirationserkrankung in Puten in einem Zeitraum von zwei Monaten lässt auf eine gemeinsame Infektionsquelle schließen und legt nahe, dass P. gallinarum wahrscheinlich eher eine primäre als eine sekundäre Rolle im Krankheitsgeschehen gespielt hat.
Pasteurella gallinarum ha sido considerado en pollos como un agente oportunista más que como un patógeno primario. Debido a que se ha encontrado una gran diversidad genotípica de P. gallinarum, uno se esperaría encontrar una distribución policlonal entre los aislados provinentes de diferentes granjas, si este organismo es un agente secundario. El objetivo de este estudio fue caracterizar genéticamente los aislados obtenidos de brotes que afectaban varias granjas de pavos, para confirmar la existencia de infección y para investigar la relación genética entre aislados de granjas afectadas. Se realizó una caracterización fenotípica y genotípica extensa de un total de 17 aislados de 14 brotes de enfermedad respiratoria en Alemania. Todos los aislados fueron del mismo fenotipo, típico de Pasteurella gallinarum. El ribotipado de tres aislados usando tanto HpaII como HindIII, mostró que tenían un perfil idéntico e indicó que los tres aislados provenían del mismo clon. La comparación con ribotipos HpaII de un estudio previo mostró que el patrón era idéntico que el obtenido con los aislados de un brote en pollos en Zimbabwean durante 1999-2000. La tipificación con REA de 14 aislados de las 14 granjas mostró que tenían un perfil idéntico, pero se diferenciaban de los obtenidos en el brote de Zimbabwean. Secuenciando el rARN del gen 16S y comparando las secuencias con otras Pasteurellaceae, se confirmó su clasificación como P. gallinarum. La identificación del mismo clon de P. gallinarum de 14 brotes de enfermedad aguda respiratoria en pavos, dentro de un periodo de 2 meses, sugiere un foco de infección común, y que P. gallinarum probablemente juega un papel como agente primario en estos brotes.
Introduction
Schneider (Citation1948) was the first to draw attention to strains later classified as Pasteurella gallinarum (Hall et al., Citation1955). P. gallinarum in addition to a new taxon designated Pasteurella n. sp. were subsequently reported as important factors in the respiratory disease complex of chickens in southern California (Clark & Godfrey, Citation1960). The new taxon outlined by Clark and Godfrey was serologically similar to P. gallinarum in the agglutination test, but did not produce acid from maltose, trehalose or dextrin. Strains classified as taxon 4 by Bisgaard (Citation1982) and subsequently named Pasteurella langaaensis by Mutters et al. (Citation1985) seem to share the cultural and biochemical characteristics of strains previously described as P. n. sp. by Clark & Godfrey (Citation1960). Organisms obtained from upper respiratory tract infections of ducks, turkeys and pigeons, and tentatively designated taxon 14, were reported by Bisgaard & Mutters (Citation1986a). These organisms are difficult to separate from P. gallinarum, P. langaaensis and P. n. sp. unless extended phenotypic characterization is carried out. P. gallinarum, P. langaaensis, P. n. sp. and taxon 14 have traditionally been considered avian taxa (Bisgaard, Citation1993), while isolates of P. gallinarum from dogs, guinea pigs and humans have most probably been misidentified (Bisgaard & Mutters, Citation1986b; Boot & Bisgaard, Citation1995; Frederiksen & Tønning, Citation2001). Problems related to conventional identification of P. gallinarum have been discussed previously by Bisgaard & Mutters (Citation1986b).
High genotypic diversity has been reported among a broad collection of strains of P. gallinarum isolated from poultry from six countries and three continents (Christensen et al., Citation2002). These investigations also showed that rat isolates classified with P. gallinarum were not closely related to recognized species or species-like groups of Pasteurellaceae. True P. gallinarum seems to be associated with the order Galliformes only. Phylogenetic analysis of the avian taxa of Bisgaard by 16S rRNA gene sequence comparison showed that P. gallinarum, P. volantium, P. avium biovar 1, P. sp. A and [Haemophilus] paragallinarum might have evolved in close association with birds (Christensen et al., Citation2003). A close antigenic relationship was also found between these taxa by crossed immuno-electrophoresis by Schmid et al. (Citation1991), who speculated that the occurrence of different Pasteurella spp. with similar antigenic epitopes in certain animal hosts was the result of a selection of strains that are optimally adapted to these animal species.
Within the order Galliformes, P. gallinarum has so far been reported associated with lesions in Gallus domesticus, Meleagris gallopavo and Numida meleagris. However, only isolates from chickens and a single isolate from a turkey have been confirmed as P. gallinarum by genetic analysis (Christensen et al., Citation2002). A single isolate from a pheasant, which did not produce acid from maltose or dextrin, was subsequently reported to have 99.5% 16S rRNA gene sequence identity with P. gallinarum (Christensen et al., Citation2003).
Isolation of P. gallinarum from turkeys has only been reported in Israel (Mushin et al., Citation1977), the Czech Republic (Mráz et al., Citation1977) and Denmark (Bisgaard, Citation1982). A total of 15 sporadic outbreaks were reported in Israel over the same time period as a severe outbreak in four heavy breeding flocks of chickens (Bock et al., Citation1977). The affected birds were 7 to 34 weeks old, but no information on the epidemiology of the outbreaks was reported, and in most cases no other causative agents were detected.
The aims of this investigation were to characterize isolates from turkeys genetically, to verify and confirm the existence of P. gallinarum infections of turkeys, and to investigate to what extent the outbreaks were related.
Materials and Methods
Phenotypic analysis
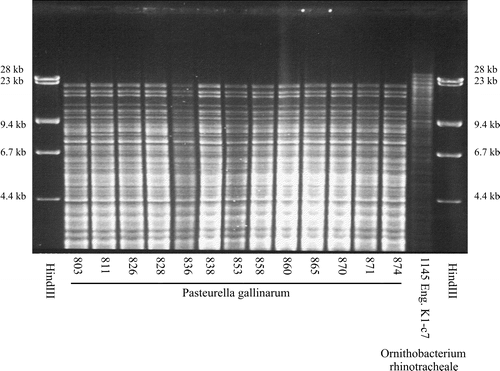
A total of 17 isolates from turkeys were phenotypically characterized as previously reported (Bisgaard et al., Citation1991). Isolates of P. gallinarum obtained from Zimbabwe (Christensen et al., Citation2002) and an isolate of Ornithobacterium rhinotracheale were included for comparison.
Restriction endonuclease analysis
Restriction endonuclease analysis (REA) was carried out on 14 of the 17 isolates using the method previously described by Christensen et al. (Citation1998). These isolates represented all 14 affected farms. HpaII was used for digestion of DNA.
Ribotyping and band pattern analysis
Ribotyping was performed on three isolates as described by Christensen et al. (Citation1993). Isolates were selected, based on the date of isolation, to cover the whole outbreak period. Briefly, 6 μg DNA was digested with HpaII or HindIII at 37°C for 2 h. The DNA fragments were separated by electrophoresis in a 0.8% agarose gel for 16 h at 1.6 V/cm. The gel was stained with ethidium bromide and viewed. The DNA was vacuum-blotted onto a nitrocellulose filter. The filter was incubated with a 16S-23S rRNA probe overnight at 56°C. Ribotype patterns were detected using the DIG wash and block buffer kit (Roche A/S, Hvidovre, Denmark) according to the manufacturer's instructions. Phage lambda DNA digested with HindIII was included as a size marker. Ribotype patterns were analysed using Gelcompar 4.0 (Applied Maths, Kortrijk, Belgium) using the unweighted pair-group method with arithmetic average.
Sequencing of 16S rRNA genes
Sequencing of the 16S rRNA gene of strain 803 was performed as previously described (Christensen et al., Citation2002). This strain was isolated from the first outbreak and was also one of those ribotyped. Searches for 16S rRNA sequences in public databases were performed using BLAST (Altschul et al., Citation1997). Determination of pair-wise similarity was performed using Bestfit (Wisconsin Sequence Analysis Package, Genetics Computer Group, Madison, Wisconsin, USA).
Results
Epidemiological observations
A total of 14 turkey farms belonging to two different integrations and located within a radius of approximately 10 km were suddenly affected by acute respiratory disease within a period of 2 months. Flock size varied between 9000 and 28 000 birds. All turkeys affected were of the BUT Big 6 line and originated from the two different integrations. One of the two hatcheries involved in 13 affected farms also provided 1-day-old turkeys to approximately 50 other farms outside the affected area, none of which were affected. Within the affected area, horizontal transmission of Mycoplasma gallisepticum and Mycoplasma synoviae had been observed previously. For this reason six flocks had been vaccinated against M. gallisepticum using either the Poulvac MG (Fort Dodge) (two farms) or MG Nobilis (Intervet) vaccine (four farms) according to the manufacturers’ recommendations, and four farms using the Intervet vaccine also vaccinated against avian influenza (H6) using an inactivated autogenous vaccine. Due to the risk of horizontal transmission of diseases within the area all but three farms received turkeys that had been reared for the first 4 to 5 weeks at brooding farms 60 to 200 km outside the area. Flocks started within the area were generally affected with respiratory disease at an age of about 6 weeks, while flocks reared outside the area were affected 2 to 4 weeks after arrival. All farms were operated on an all-in all-out basis in semi-confined houses (semi-open sides covered by wire nets). The turkeys were reared on wood shavings or straw. However, two pairs of farms each shared staff.
Vaccination against Newcastle disease was obligatory and all flocks were routinely checked for antibodies against M. gallisepticum, M. synoviae, Newcastle disease and avian influenza before slaughtering at 16 weeks (females) or 21 weeks (males) of age. Turkey rhinotracheitis virus infections were endemic in the region (before, during and after the outbreak) and vaccination was not carried out. Only one subsequent flock was affected with P. gallinarum.
Postmortem examinations
Mortality rates varied. The highest mortality rates were 18% and 26%. A total mortality rate of 3% to 5% was normal during rearing. A foamy airsacculitis accompanied by bronchopneumonia and tracheitis was observed. Multiple organs, muscles and the skin were congested.
Bacteriology
During the acute phase, pure cultures of P. gallinarum-like organisms were isolated from the trachea, lung or airsacs. A total of 17 isolates were subjected to extended phenotypic characterization, the results of which allowed classification as P. gallinarum (Christensen et al., Citation2002; Mutters et al., Citation2004). All strains shared the same phenotype in the 80 characteristics investigated.
REA typing
The 14 strains compared by REA typing shared the same profile (). The strain of O. rhinotracheale included for comparison had a completely different profile.
Ribotype patterns
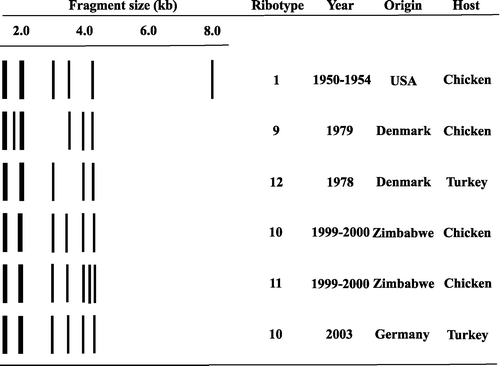
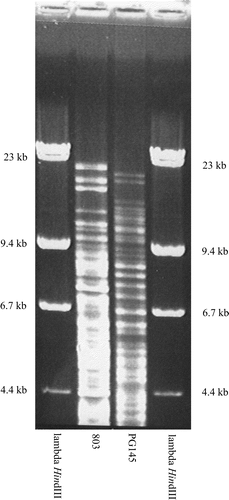
Three isolates, 724, 803 and 988, obtained early, mid and late in the outbreak were selected for ribotyping. All three isolates had identical ribotypes with HpaII (RT10) (). Identical profiles were also obtained using HindIII (data not shown). HpaII digestion allowed comparison with isolates of P. gallinarum analysed in a previous study (Christensen et al., Citation2002) and for this reason the numbering of ribotypes from the previous paper was used. Surprisingly, the three isolates had the same ribotype (RT10) as 15 isolates from chickens in Zimbabwe suffering from sinusitis, conjunctivitis, arthritis, supra-orbital and wattle swelling, and/or wattle abscesses (Mohan et al., Citation2000; Christensen et al., Citation2002). Two of these isolates were included in comparisons of the REA type (PG145) and 16S rRNA sequences (PG173). The REA types of the German (803) and Zimbabwean (PG145) isolates sharing a ribotype were distinct (). Three other related ribotypes had one or two band differences from this type. One of these was obtained with an isolate from Zimbabwe (RT11) and the other two with isolates from Denmark (RT9 and RT12). One of the Danish isolates (CCUG18394; RT12) was isolated from a turkey with sinusitis and septicaemia in 1978 (Christensen et al., Citation2002).
16S rRNA gene analysis
Analysis of strain 803 by 16S rRNA sequencing found that it had 99.5% and 99.3% identity with the type strains of P. volantium (accession number M75059) and P. gallinarum (accession number M75070), respectively. P. gallinarum and P. volantium cannot be separated by 16S rRNA sequencing as there is insufficient difference between the sequences (Christensen et al., Citation2003). The 16S rRNA gene of strain 803 had 99.4% identity with one of the isolates from the Zimbabwean outbreak (PG 173), and 99.3% identity with strain B69/96/3, which was isolated from a pheasant (Christensen et al., Citation2003). The accession number for the 16S rRNA sequence of strain 803 is AY598819.
Discussion
P. gallinarum has traditionally been considered an opportunistic pathogen rather than a primary pathogen of chickens (Hall et al., Citation1955; Mushin et al., Citation1977; Yadav et al., Citation1977; Tjahjowati et al., Citation1995). Naturally occurring outbreaks are reported to be associated with mycoplasma or virus infections of the upper respiratory tract (Clark & Godfrey, Citation1960; Bock et al., Citation1977; Droual et al., Citation1992a). The lesions reported are as diverse as those reported for Pasteurella multocida, and include conjunctivitis, abscesses in the head and wattles, sinusitis, tracheitis, airsacculitis, hepatitis, endocarditis, salpingitis, oophoritis, peritonitis and synovitis (Christensen & Bisgaard, Citation2000; Christensen et al., Citation2002).
This study examined isolates from turkeys with a sudden onset of respiratory disease accompanied by high mortality affecting most farms within a radius of about 10 km. During the acute phase of the outbreak, P. gallinarum was isolated in pure culture from turkeys on all affected farms. No other significant pathogens were demonstrated. However, the impact of mycoplasmas, influenza virus or other respiratory pathogens cannot be ruled out, even though some flocks had been vaccinated. The same clone, as defined by both REA and ribotyping, was demonstrated on all 14 farms, suggesting a primary rather than a secondary role in the outbreaks. Unfortunately the number of isolates available was rather limited. However, isolates from all farms were included and three farms were represented by two isolates, including one from the single farm on which a subsequent flock was affected. These observations suggest that the outbreak clone remained stable for at least 6 months. These findings confirm recent observations by Christensen et al. (Citation2002) and Shivaprasad & Droual (Citation2002). Surprisingly, the outbreak clone of P. gallinarum had the same ribotype as isolates from an outbreak in chickens in Zimbabwe (Christensen et al., Citation2002). Similarly, eight strains of P. multocida subsp. multocida isolated from five different host species in two different countries had identical ribotypes, as did 10 strains of P. multocida subsp. septica isolated from at least four different host species in four different countries, emphasizing that this method targets gene clusters, not the whole genome (Petersen et al., Citation2001). This is probably also the explanation for strains 803 and PG173 sharing ribotypes even though there was 0.6% difference in their 16S rRNA sequences. Clonal outbreaks as defined by REA typing have also been demonstrated for P. multocida (Blackall et al., Citation1995; Christensen et al., Citation1998; Muhairwa et al., Citation2000).
The natural reservoir of P. gallinarum remains to be investigated. With the exception of a single isolate from a duck kept in contact with chickens (Muhairwa et al., Citation2001), P. gallinarum has not been isolated from the upper respiratory tract of healthy birds. Of the taxa within the Pasteurellaceae associated with birds, the strongest host associations include those between P. gallinarum and [H.] paragallinarum and the Galliformes, and those between taxa 33 and 34 of Bisgaard and the Psittaciformes (Christensen et al., Citation2003, Citation2004). A stratified cross-sectional study of the prevalence and transmission of haemolytic Gallibacterium species, which also belong to the Pasteurellaceae, showed that these organisms were widely distributed within Danish commercial chicken production systems, and that prevalences were greatly influenced by the production system and the level of biosecurity. No evidence of vertical transmission was observed (Bojesen et al., Citation2003), confirming our experiences that members of the Pasteurellaceae are not vertically transmitted. A single hatchery was used by 13 of the 14 affected farms in the outbreak, including the first farm affected, but as the majority of the clients of this hatchery did not report similar respiratory problems, it seems unlikely that the infection was introduced by vertical transmission. Introduction from the environment due to lack of biosecurity and subsequent spread from farm to farm seems more likely. The affected area was an optimal biotope for pheasants and other Galliformes, which may have been the reservoir (Christensen et al., Citation2003).
A different clone of P. multocida was found in each of four outbreaks of fowl cholera on a duck farm in Denmark over a 2-year period, indicating that elimination of Pasteurellaceae from infected farms with low biosecurity is possible (Muhairwa et al., Citation2000). Although a single relapse was observed in subsequent flocks, proper cleaning and disinfection and a subsequent focus on biosecurity also eliminated P. gallinarum from these turkey farms.
Although the population structure and virulence factors of P. gallinarum have not yet been investigated, the demonstration of clonal outbreaks suggest the existence of common virulence factors(s). Experimental investigations of the virulence of P. gallinarum have been inconclusive (Droual et al., Citation1992b), indicating that virulence may vary between strains (Shivaprasad & Droual, Citation2002). Fundamental work on virulence factors of P. gallinarum associated with strains obtained from clonal outbreaks is seriously needed.
Translations of the abstract in French, German and Spanish are available on the Avian Pathology website.
Tony Bønnelycke and Anna Sandmann are thanked for technical assistance. The study was financed by the Danish Agricultural and Veterinary Research Council, grant No. 9702797.
References
- Altschul , SF , Madden , TL , Schaffer , AA , Zhang , J , Zhang , Z , Miller , W and Lipman , DJ . (1997) . Gapped BLAST and PSI-BLAST: a new generation of protein database search programs . Nucleic Acids Research , 25 : 3389 – 3402 .
- Bisgaard , M . (1982) . Isolation and characterization of some previously unreported taxa from poultry with phenotypical characters related to Actinobacillus and Pasteurella species . Acta Pathologica Microbiologica Immunologica Scandinavica , B90 : 59 – 67 .
- Bisgaard , M . (1993) . Ecology and significance of Pasteurellaceae in animals . Zentralblatt für Bakteriologie , 279 : 7 – 26 .
- Bisgaard , M and Mutters , R . (1986a) . A new facultatively anaerobic, Gram-negative, fermentative rod obtained from different pathological lesions in poultry and tentatively designated taxon 14 . Avian Pathology , 15 : 117 – 127 .
- Bisgaard , M and Mutters , R . (1986b) . Characterization of some previously unclassified “Pasteurella” spp. obtained from the oral cavity of dogs and cats and description of a new species tentatively classified with the family Pasteurellaceae Pohl 1981 and provisionally called taxon 16 . Acta Pathologica Microbiologica Immunologica Scandinavica , B94 : 177 – 184 .
- Bisgaard , M , Houghton , SB , Mutters , R and Stenzel , A . (1991) . Reclassification of German, British and Dutch isolates of so-called Pasteurella multocida obtained from pneumonic calf lungs . Veterinary Microbiology , 26 : 115 – 124 .
- Blackall , PJ , Pahoff , JL , Marks , D , Fegan , N and Morrow , CJ . (1995) . Characterisation of Pasteurella multocida isolated from fowl cholera outbreaks on turkey farms . Australian Veterinary Journal , 72 : 135 – 138 .
- Bock , RR , Samberg , Y and Mushin , R . (1977) . An outbreak of a disease in poultry associated with Pasteurella gallinarum . Refuah-Vetrinarith , 34 : 99 – 103 .
- Bojesen , AM , Nielsen , SS and Bisgaard , M . (2003) . Prevalence and transmission of haemolytic Gallibacterium species in chicken production systems with different biosecurity levels . Avian Pathology , 32 : 503 – 510 .
- Boot , R and Bisgaard , M . (1995) . Reclassification of 30 Pasteurellaceae strains isolated from rodents . Laboratory Animals , 29 : 314 – 319 .
- Christensen , H , Dziva , F , Olsen , JE and Bisgaard , M . (2002) . Genotypical heterogeneity of Pasteurella gallinarum as shown by ribotyping and 16S rRNA sequencing . Avian Pathology , 31 : 603 – 610 .
- Christensen , H , Foster , G , Christensen , JP , Pennycott , T , Olsen , JE and Bisgaard , M . (2003) . Phylogenetic analysis by 16S rDNA gene sequence comparison of avian taxa of Bisgaard and characterization and description of two new taxa of Pasteurellaceae . Journal of Applied Microbiology , 95 : 354 – 363 .
- Christensen , H , Bisgaard , M , Aalbæk , B and Olsen , JE . (2004) . Reclassification of Bisgaard taxon 33, with proposal of Volucribacter psittacicida gen. nov., sp. nov. and Volucribacter amazonae sp. nov. as new members of Pasteurellaceae . International Journal of Systematic and Evolutionary Microbiology , 54 : 813 – 818 .
- Christensen , JP and Bisgaard , M . (2000) . Fowl cholera . Revue scientifique technique Office internationale Epizootique , 19 : 626 – 637 .
- Christensen , JP , Olsen , JE and Bisgaard , M . (1993) . Ribotypes of Salmonella enterica serovar Gallinarum biovars gallinarum and pullorum . Avian Pathology , 22 : 725 – 738 .
- Christensen , JP , Dietz , HH and Bisgaard , M . (1998) . Phenotypic and genotypic characters of isolates of Pasteurella multocida obtained from back-yard poultry and two outbreaks of avian cholera in the avifauna in Denmark . Avian Pathology , 27 : 373 – 381 .
- Clark , DS and Godfrey , JF . (1960) . Atypical Pasteurella infections in chickens . Avian Diseases , 4 : 280 – 290 .
- Droual , R , Shivaprasad , HL , Meteyer , CU , Shapiro , DP and Walker , RL . (1992a) . Severe mortality in broiler chickens associated with Mycoplasmna synoviae and Pasteurella gallinarum . Avian Diseases , 36 : 803 – 807 .
- Droual , R , Walker , RL , Shivaprasad , HL , Jeffrey , JS , Meteyer , CU , Chin , RP and Shapiro , DP . (1992b) . An atypical strain of Pasteurella gallinarum: pathogenic, phenotypic, and genotypic characteristics . Avian Diseases , 36 : 693 – 699 .
- Frederiksen , W and Tønning , B . (2001) . Possible misidentification of Haemophilus aphrophilus as Pasteurella gallinarum . Clinical Infectious Diseases , 32 : 987 – 989 .
- Hall , WJ , Heddleston , KL , Legenhausen , DH and Hughes , RW . (1955) . Studies on Pasteurellosis. I. A new species of Pasteurella encountered in chronic fowl cholera . American Journal of Veterinary Research , 16 : 598 – 604 .
- Mohan , K , Dziva , F and Chitauro , D . (2000) . Pasteurella gallinarum: Zimbabwean experience of a versatile pathogen . Onderstepoort Journal of Veterinary Research , 67 : 301 – 305 .
- Mráz , O , Jelen , P and Boháček , P . (1977) . On species characteristics of Pasteurella gallinarum Hall et al., 1955 . Acta Veterinaria Brno , 46 : 135 – 147 .
- Muhairwa , AP , Christensen , JP and Bisgaard , M . (2000) . Investigations on the carrier rate of Pasteurella multocida in healthy commercial poultry flocks and flocks affected by fowl cholera . Avian Pathology , 29 : 133 – 142 .
- Muhairwa , AP , Mtambo , MMA , Christensen , JP and Bisgaard , M . (2001) . Occurrence of Pasteurella multocida and related species in village free ranging chickens and their animal contacts in Tanzania . Veterinary Microbiology , 78 : 139 – 153 .
- Mushin , R , Bock , R and Abrams , M . (1977) . Studies on Pasteurella gallinarum . Avian Pathology , 6 : 415 – 423 .
- Mutters , R , Ihm , P , Pohl , S , Frederiksen , W and Mannheim , W . (1985) . Reclassification of the genus Pasteurella Trevisan 1887 on the basis of deoxyribonucleic acid homology, with proposals for the new species Pasteurella dagmatis, Pasteurella canis, Pasteurella stomatis, Pasteurella anatis, and Pasteurella langaa . International Journal of Systematic Bacteriology , 35 : 309 – 322 .
- Mutters R Christensen H Bisgaard M (2004) Genus Pasteurella Trevisan 1887, 94, AL Nom. cons. Opin. 13, Jud Comm. 1954. InR. Garrity(Ed.) Bergey's Manual of Systematic Bacteriology 2 2nd edn New York Springer (in press)
- Petersen , KD , Christensen , H , Bisgaard , M and Olsen , JE . (2001) . Genetic diversity of Pasteurella multocida fowl cholera isolates as demonstrated by ribotyping and 16S rRNA and partial atpD sequence comparisons . Microbiology , 147 : 2739 – 2748 .
- Schmid , H , Hartung , M and Hellmann , E . (1991) . Crossed immunoelectrophoresis applied to representative strains from 11 different Pasteurella species under taxonomic aspects . Zentralblatt für Bakteriologie , 275 : 16 – 27 .
- Schneider , L . (1948) . Additional data to the etiology of pasteurellosis with special reference to different species of hosts . Acta Veterinaria Hungarica , 1 : 31 – 42 .
- Shivaprasad , HL and Droual , R . (2002) . Pathology of an atypical strain of Pasteurella gallinarum infection in chickens . Avian Pathology , 31 : 399 – 406 .
- Tjahjowati , G , Orr , JP , Chirino-Trejo , M and Mills , JHL . (1995) . Experimental reproduction of endocarditis with Pasteurella gallinarum in mature Leghorn Chickens . Avian Diseases , 39 : 489 – 498 .
- Yadav , MP , Sharma , VD and Sethi , MS . (1977) . An outbreak of fowl cholera due to Pasteurella gallinarum in Uttar Pradesh (India) . Avian Diseases , 21 : 323 – 325 .