Abstract
Absorption of fluid by the small intestine of 4-week-old to 12-week-old farmed pheasants and partridges has been studied using an inverted sac technique. The mean rate of absorption was 54±4 (mean±standard error of the mean) μl/g dry tissue/min in pheasants and 49±3 μl/g dry tissue/min in partridges. Use of inhibitors and ion substitution suggested transepithelial transport driven by baso-lateral Na+/K+ pumps, in combination with mucosal Na+-coupled transporters, including Cl−-coupled transporters. Absorption was more than halved to 17±2 μl/g dry tissue/min (P<0.001) in birds that were very heavily infected with Spironucleus spp. in their small intestine and showing a syndrome of diarrhoea, depression and loss of weight to severe emaciation. Birds carrying light to moderate levels of infection with Spironucleus had very variable rates of absorption that were statistically similar to the controls. Doubling the glucose concentration in the buffer to 40 mM significantly enhanced absorption.
Absorption de liquide au niveau de l'intestin grêle chez le gibier à plume en bonne santé et chez d'autre infecté par Spironucleus spp.
L'absorption de liquide au niveau de l'intestin grêle chez des faisans et des perdrix d’élevage âgés de 4 à 12 semaines a été étudiée par la technique du sac inversé. Le taux moyen d'absorption a été de 54±4 (moyenne±e.s).μl (g. tissu sec/min)−1 chez les faisans et de 49±3 chez les perdrix. L'utilisation d'inhibiteurs et la substitution d'ions a suggéré un transport transépithélial assuré par des pompes Na+/K+ basolatérales, en association avec des transporteurs muqueux couplés au Na+, incluant des transporteurs couplés au Cl-. L'absorption a été de 17±2 μl.g.min−1 (P<0.001) donc réduite de plus de la moitié de chez les animaux qui étaient très fortement infectés par Spironucleus spp. au niveau de l'intestin grêle et qui présentaient un syndrome diarrhéique, de la dépression et une perte de poids, voire une émaciation sévère. Les animaux faiblement ou moyennement infectés par Spironucleus ont présenté des taux variables d'absorption qui étaient statistiquement similaires à ceux des témoins. Le doublement de la concentration en glucose du tampon à 40 mM a augmenté significativement l'absorption.
Flüssigkeitsabsorption im Dünndarm von gesunden und mit Spironucleus spp. infiziertem Federwild
Die Flüssigkeitsabsorption im Dünndarm von 4–12 Wochen alten, auf einer Farm gehaltenen Fasanen und Rebhühnern wurde mit Hilfe einer umgestülpten Sacktechnik untersucht. Die mittlere Absorptionsrate betrug 54±4 (Mittelwert±Standardabweichung) μl (g Trocken- gewebe/min−1) in Fasanen und 49±3 in Rebhühnern. Die Anwendung von Inhibitoren und Ionensubstitution ließ auf einen transepithelialen Transport schließen, der durch basolaterale Na+/K+-Pumpen in Kombination mit Schleimhaut verbundenen Na+- und Cl--Tranportern betrieben wird. Bei Tieren, deren Dünndärme hochgradig mit Spironucleus spp. infiziert worden waren, war die Absorption mehr als halbiert auf 17±2 μl.g.min−1 (p<0,001). Sie zeigten ein Syndrom mit Diarrhoe, Apathie und Gewichtsverlust bis hin zur Kachexie. Tiere mit gering- bis mittelgradiger Spironucleus-Infektion hatten sehr unterschiedliche Absorptionsraten, die sich statistisch nicht von denen der Kontrolltiere unterschieden. Die Verdopplung der Glukosekonzentration im Puffer auf 40mM erhöhte die Absorption signifikant.
Absorción de fluido en el intestino delgado de aves de caza sanas y de infectadas con Spironucleus spp.
Se ha investigado la absorción de fluido en el intestino delgado de faisanes y perdices de granja, de 4 a 12 semanas de edad, mediante una técnica de saco invertido. La media de la proporción de absorción fue de 54±4 (media±s.e.m) μl (g. materia seca/min)−1 en faisanes y 49±3 en perdices. El uso de inhibidores y la sustitución iónica sugirieron un transporte transepitelial a través de las bombas basolaterales de Na+/K+, en combinación con transportadores ligados a Na+, incluyendo transportadores ligados a Cl−. La absorción fue más de la mitad hasta 17±2 μl.g.min−1 (P < 0.001) en aves que presentaban una infección manifiesta con Spironucleus spp. en el intestino delgado y un síndrome de diarrea, depresión y pérdida de peso hasta llegar a un estado de emaciación grave. Las aves que presentaban niveles de discretos a moderados de infección por Spironucleus presentaron proporciones variables de absorción que fueron estadísticamente similares a las de los controles. Al doblar la concentración de glucosa en el tampón hasta 40 mM mejoró notablemente a absorción.
Introduction
The small intestine of mammals is a major site of absorption, both of solutes and water. The low intracellular Na+ and negative intracellular potential difference maintained through primary active transport by serosal Na+/K+ pumps are used to drive transepithelial transport. A number of mucosal transport systems, including the Na+Cl− cotransporter and Na+-glucose cotransporter, are involved. The osmotic effect of solute absorption provides the requisite force for water uptake (via the standing gradient mechanism, first proposed by Diamond and Bossart [Citation1967]). Intestinal pathology can perturb normal solute (and thus water) transport, and may result in malabsorption, diarrhoea or malnutrition.
Less is known about the absorptive function, and underlying cellular mechanisms, of the avian small intestine. Some of the components found in mammalian intestinal epithelium have also been described in the domestic fowl. Among these are various ion channels and peptide transporters, including a Na+/H+ exchanger (Musch et al., Citation1992; Donowitz et al., 1998a, b), a Na+-dependent glucose transporter (Garriga et al., Citation1999) and a Na+-dependent amino-acid transporter (Gous et al., Citation1977) on the mucosal cell membrane, together with a Na+/K+ pump on the serosal side (Sklan & Noy, Citation2000). To date, absorption in the intestines of the game birds pheasants and partridges has not been studied.
Various pathogens of the avian small intestine, including rotavirus and Cryptosporidium spp., are associated with malabsorption and diarrhoea. Both infections disrupt villus structure, causing epithelial detachment and villus atrophy, thus reducing the absorptive surface area and hindering normal absorptive processes (Hoerr et al., Citation1986; Yason & Schat, Citation1987). In mammals, rotavirus has been shown to result in secretory diarrhoea, via stimulation of Cl− secretion, an effect mediated via an enterotoxin (Morris & Estes, Citation2001). In a similar way, cholera toxin, via elevation of intracellular cAMP levels, also causes extreme secretory diarrhoea (Morris & Estes, Citation2001). The protozoan parasite Hexamita or Spironucleus species (Protozoa: Diplomonadida: Hexamitidae) has been described as a cause of disease in hosts that include turkeys, pheasants, partridges, pigeons, fish and mice. In birds the parasite was first described in turkeys as Hexamita meleagridis, but taxonomic descriptions of all the diplomonads have been very limited. Electron microscopic studies in fish and mice have moved their species from the genus Hexamita to the genus Spironucleus (Burgerolle et al., Citation1973, Citation1980; Poynton et al., Citation2004) and preliminary studies suggest the same for the parasite in pheasants and partridges (unpublished observations). As several species of Spironucleus infecting different fishes now have been described (Poynton & Sterud, Citation2002), it is premature to conclude that the Spironucleus described in European pheasants and partridges is synonymous with H. meleagridis in turkeys. H. meleagridis was considered a very important cause of acute disease in turkeys in countries in North America and elsewhere. Now it is described only occasionally in turkeys in North America (McDougald, Citation1998a, Citation1998b). Conversely, Spironucleus now is described frequently in some countries in Europe in game birds that show signs similar to those described in turkeys (Swarbrick, Citation1990; McDougald, 1998). Clinical signs include watery diarrhoea, depression, and rapid weight loss, and small intestinal contents can be very watery and occasionally the intestinal wall bulbous. Affected birds show considerable morbidity and variable mortality from 1% to 50% or more but, to date, the mechanism of disease in these birds has not been established.
To investigate this problem, we examined absorption in the small intestine of pheasants (Phasianus colchicus) and red-legged partridges (Alectoris rufa) using the everted sac methodology (Wilson & Wiseman, Citation1954). Various transport inhibitors and solute substitutions were used to define the components of transport in normal intestine. Finally, transport in tissues from healthy birds was compared with those from birds infected with Spironucleus.
Materials and Methods
Birds
Birds, 4 to 12 weeks of age, were presented by gamekeepers for diagnosis or confirmation of diagnosis of disease due to Spironucleus or to diagnose the absence of parasitic infection from their flock. They were killed by cervical dislocation and the small intestine was removed immediately. Overall, 685 tissues from 214 birds (115 uninfected and 99 infected) from 37 farms are included in the results (where two or more pieces of intestine from the same bird were examined using the same solution, a mean of the two to four results has been used in the presented results). Depending on age, birds came from rearing pens (mainly moveable huts but a few permanent huts, all with outside grass runs) or from wooded release pens. Flocks varied from 200 to 40 000 in size. Some of the flocks had earlier been affected by rotavirus or coccidiosis (>90% included a coccidiostat in the feed). However, disease was commonly not described in the batch of birds used in these experiments and no birds used were described as suffering other disease in the previous few weeks.
Diagnosis of Spironucleus spp
Mucosal smears were taken from the curvature of the duodenum and three approximately equidistant sites in the jejunum and ileum, and from the caecum, and were examined grossly and under light microscopy for the presence of parasites (coccidia, Spironucleus, Trichomonas, helminths, etc.). In a proportion of birds low numbers of Trichomonas and light coccidial infections were present in the small intestine. Birds with heavier infections of these and other parasites were excluded. In previous years, examinations for other pathogenic viral or bacterial infections had proved negative. Previously, some 80% of birds heavily infected with Spironucleus also revealed high levels of non-lactose fermenting Escherichia coli (Lloyd, unpublished observations). Culture of a limited number of heavily infected birds in the present study revealed <50% infected with heavy infections of E. coli. The severity of infection with Spironucleus, when present, was estimated subjectively by counting the number of parasites per 20×objective field (850 μm2 diameter), and were graded 1 to 6, where grade 1 indicates one parasite per field (in at least one field in at least one of the intestinal smears), grade 2 indicates >1 to 5 in each of several fields, grade 3 indicates >5 to 20 in all fields, grade 4 indicates >20 to 100 in all fields, grade 5 indicates >100 to 500 in all fields, and grade 6 indicates >500 with many in some crypts in the grade 6+ birds. Overall intensity was calculated by adding the grades in the four small intestinal segments examined. Birds were judged clinically on the presence of diarrhoea (normal or with watery fluid in the intestine, and variable liquidity and enlargement of the caeca) and on muscle weight (thin or severely emaciated with “knife-bone keeled” breast) and on the level of their activity. The birds were then grouped as Group I (healthy, grade 0), Group II (grades 1 to 5 in one or more segments, usually with some diarrhoea, active, possibly thin but never “knife-bone”) and Group III (very heavily infected, being grade 6 in at least two segments of the small intestine with diarrhoea and/or thin to “knife-bone” breast and in poor health). Group II included some birds that had been in a flock affected for some days and under treatment with metronidazole. Birds from Groups II and III sometimes came from the same farm.
Tissue preparation
Everted sacs were prepared following a method adapted from Wilson & Wiseman (Citation1954). Three or four pieces of intestine, each about 4 to 6 cm long, were removed immediately post mortem. The first piece was taken from the jejunum beginning some 5 cm behind the duodenum, the second two or three pieces from the jejunum/ileum ending near the tip of the caecum. The pieces were placed in buffer and all subsequent procedures were conducted at 37°C (unless otherwise indicated)—tissues behaved more consistently when kept warm in this way. Intestinal cylinders were everted using Hartmann's aural forceps. The everted tissue was tied twice to the end of a 1 ml syringe (grooved twice for secure attachment of the ligature) using Ethicon Mersilk 3.5 metric (Johnson and Johnson, Belgium) and tied off at the other end to form a sac. The serosal side (inside) of the sac was filled with buffer and the sac immersed in buffer or other test solution.
Buffer and inhibitors
All chemicals were purchased from Sigma Chemical Co. (Poole, UK).
Buffers
The standard buffered saline, designed to approximate pheasant plasma (Lloyd et al., submitted), comprised 132 mM NaCl, 4 mM KCI, 4 mM Na2PO4, 1 mM MgCl2, 20 mM glucose, 1 mM glycine, 25 mM NaHCO3 and 1 mM CaCl2, with osmolarity 337±5 mOsm/kg and pH 7.4 when bubbled with 5% CO2/95% O2 at 37°C. For solute substitution experiments, the following alternative salines were made. Sodium-free, NaCl was replaced with choline chloride (132 mM); KCl and Na2PO4 with KH2PO4 (4 mM), NaHCO3 was still present in the buffer but Na+ levels were <20% of the standard buffer. Glucose-free, glucose was replaced with mannitol (20 mM). Glycine-free, glycine was omitted. Chloride-free, NaCl, KCI and CaCl2 were replaced with NO3 salts; MgCl2 with MgSO4. The buffer also was used with glucose concentrations varying from 5 to 120 mM. Four commercial electrolyte solutions (three sold for pets or ruminants and one for domestic poultry) were used instead of game bird buffer in some experiments.
Inhibitors
A stock solution of ouabain (10 mM) was made in distilled water and used at a final concentration of 0.1 mM. Tissues were bathed in saline containing ouabain throughout. Stock solutions of furosemide and amiloride (both 100 mM) were made in dimethylsulphoxide and diluted to a final concentration of 1 mM or 0.1 mM, respectively. Stocks of phloretin (100 mM) were made in 95% ethanol, and were used at a final concentration of 1 mM. Tissues were bathed in saline containing inhibitor throughout.
Transport measurement
Each everted sac was suspended in a separate bottle containing the appropriate saline (± transport inhibitors) and bubbled throughout with 5% CO2/95% O2. The tissue plus syringe was blotted and weighed at the start of the experiment and every 10 min thereafter. Fluid absorption was taken as the gain in weight, where a gain of 1 g was taken to be absorption of l ml fluid. The dry weight of the sac, cut at the ligatures, was measured after drying in a 100°C oven overnight, and absorption is given in microlitres per gram of dry tissue per minute.
Statistics
Results are presented as the mean±standard error of the mean of n different tissues or birds’ intestines. Comparisons were made using Student's paired or unpaired t test or Pearson's correlation coefficient, as appropriate.
Results
Fluid transport in healthy intestine
After preparing the everted sacs, most tissues (>95%) showed rapid rates of fluid absorption (transport from mucosa to serosa). Some tissues did not absorb; these often were difficult to evert so were probably due to tissue damage during preparation and were omitted. About 1% were stable for less than 30 min but the rate of absorption was usually maintained for >30 min, and, in some cases, for >2 h. Results include only tissues with stable absorption rates for at least 30 min. No examples of secretion were observed in healthy birds. The mean rate of absorption in healthy tissues from pheasants was 54±4 μl/g/min (n=48 birds), and that from partridges was 49±3 μl/g/min (n=16 birds). There was no significant difference in the rate of absorption between the two groups of birds. No systematic differences were observed between sacs from proximal or distal small intestine, and there were no obvious differences with age. The effect of incubation at 41°C, the average temperature of birds, was investigated in a smaller number of tissues (n=6). In these cases, absorption was initially high but declined rapidly within 30 min, probably because, at higher temperatures, in the absence of a blood supply, diffusion was insufficient to maintain normal metabolism.
Effect of solute substitution and inhibitors
In order to define better the mechanism of absorption, various manoeuvres that inhibit transport in mammalian and chicken small intestine were examined. These included treatment with inhibitors (ouabain for Na+/K+ pump, amiloride for Na+ channels and Na+/H+ exchange, furosemide for Cl−-coupled processes, phloretin for glucose transport). Results are presented in . Inhibition by all was >70%. Solute substitution (choline for Na+, NO3 − for Cl−, mannitol for glucose) produced inhibitions were >50%. All inhibitions were significant except for exposure to furosemide (>0.05 to <0.09), in this case probably because of variable control values (in all three experiments, inhibitions >50%). Removal of glycine was without effect.
Table 1. Effect of inhibitors on fluid absorption in pheasant small intestine
Effect of infection with Spironucleus spp
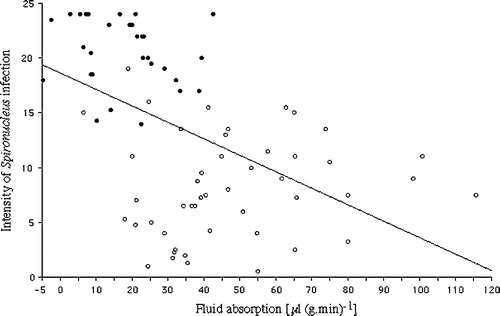
Absorption rates in intestines from healthy birds were compared with those from pheasants and partridges infected with Spironucleus. There were no significant differences in the rates of absorption between pheasants and partridges with comparable levels of Spironucleus infection so their results have been pooled. When all the Spironucleus-infected birds (n=79) were compared with the uninfected birds (n=64) absorption was significantly lower (35±3 μl/g/min, P<0.001) than in uninfected birds (53±2 μl/g/min). Despite this there was only a moderate correlation between declining absorption and increasing levels of infection (r=0.492, P<0.01) (). The intestines fell into two groups, however. Tissues from Group III birds (heavily infected grade 6 in at least two intestinal segments, depressed and thin to “knife bone”) showed mean absorption of 17±2 (n=32), significantly lower than control uninfected birds (P<0.001). Tissues from Group II birds (with lower severities of infection) had variable rates of absorption () of 47±4 (n=47), not significantly different from the controls. A loss of weight during incubation among the segments was occasionally seen in Group III birds.
Figure 1. Fluid absorption by pheasant and partridge small intestinal sacs incubated in normal game bird buffer compared with the intensity of infection with Spironucleus (r=0.492, P<0.01). ○, Group II birds lightly to moderately infected with Spironucleus. •, Group III birds heavily infected with Spironucleus with grade 6 infection in at least two segments of the intestine and clinically ill.
Effect of varied glucose concentrations and electrolyte solutions
Varying concentrations of glucose were examined in a group of birds that included normal birds and birds only lightly infected with Spironucleus. Doubling the concentration of glucose (DBG=40 mM) in the normal game bird buffer significantly increased absorption (), although further increases were detrimental probably due to osmotic effects while levels below 15 mM significantly lowered the rate of absorption. Adjusting Na in the DBG solution to maintain molarity or increasing glycine (four times) did not seem to have any additive effects over DBG, although too few samples were examined for statistical analysis. DBG solutions also significantly increased the rate of absorption in intestinal tissues from heavily infected birds (). In addition, when intestinal sacs from healthy or very heavily infected Group III birds were incubated first in normal buffer or buffer without glucose (for 10 to 30 min) and then transferred to double glucose buffer, the rates of absorption were enhanced although, in the case of healthy birds incubated first in no glucose buffer, not to the rates seen if they had been incubated in glucose buffer throughout. The results are presented in and the pattern of absorption in two birds is shown in . Halving the Na+ concentration and using a hypotonic solution (80% of normal buffer) reduced absorption by 42% and 49%, respectively, when examined in a small number of birds. Rates of absorption were lowered in all four proprietary electrolyte solutions examined. Different reasons may account for this in the different electrolyte solutions: high glucose (≥120 mM), low Na+ and high K+ (about 85 mM and 27 mM, respectively), or extremely low overall molarity.
Figure 2. Effect of glucose concentration in the game bird buffer on fluid absorption by pheasant and partridge small intestinal sacs. Significant differences to normal buffer: Significant differences: A P<0.001, C P<0.01, D P<0.025.
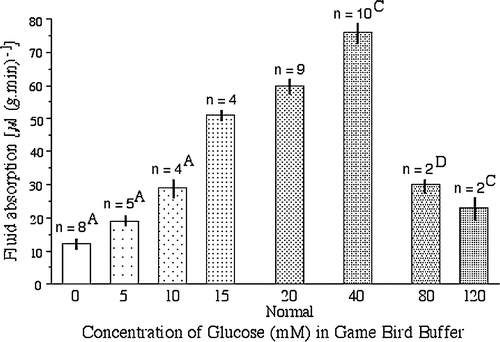
Figure 3. Effect of increasing glucose concentrations in buffer from 0 or 20 mM to 20 or 40 mM after 30 to 40 min of incubation. Top, rate of absorption in a Group I healthy bird, Bottom, rate of absorption in a Group III heavily infected bird.
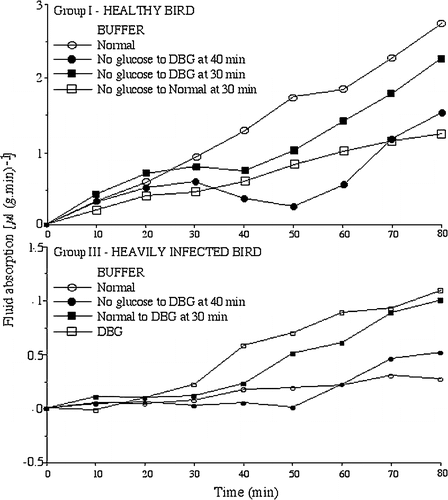
Table 2. Effect of doubling the glucose concentration in the normal game bird buffer on absorption of fluid by game bird intestines
Table 3. Effect on fluid absorption in game bird small intestine of incubation in buffer containing lower levels of glucose followed by buffer containing double glucose (40 mM)
Discussion
This paper represents the first mechanistic study on fluid absorption in the small intestine of pheasants and partridges. While small intestinal absorption in mammals has been studied in detail, and although there is some information on transport in intestine from domestic fowls, little is known about game birds. We chose to use everted sacs, because although the methodology is comparatively simple it provides valuable information on the overall transport function of the epithelium under study (Barthe et al., Citation1999). The rate of absorption in tissues from healthy pheasants and partridges was 54±4 and 49±3 μl/g/min, respectively. Absorption was largely abolished by exposure to ouabain, and was strongly inhibited by amiloride, phloretin and furosemide. Ion (Na+ and Cl−) and glucose substitutions inhibited absorption by about 50%. These features are consistent with transepithelial transport driven by basolateral Na+/K+ pumps, in combination with mucosal Na+-coupled transporters, including Cl−-coupled transporters and glucose transporters, similar to the processes found in mammalian small intestine.
Finally, we present the first evidence that that absorption was more than halved in pheasants and partridges carrying heavy infections with Spironucleus. Infections with Spironucleus have been related to a syndrome of diarrhoea, ill-thrift, emaciation (“knife bone” breast) and variable mortality in game birds. Histologically, however, there is no evidence of intestinal pathology. Thus villous atrophy, ulceration or inflammation are all absent (Hussain, Citation2001; Lloyd, unpublished observations). The presence of high levels of Spironucleus was associated with considerable reduction in intestinal absorption in tissues from both pheasants and partridges. By contrast, the presence of smaller numbers of protozoa produced variable transport rates that were not significantly different from healthy birds’ intestines. Secretion was only rarely observed. The observed reduction in the efficiency of small intestinal absorption could result in diarrhoea. The current findings now suggest that the parasite may reduce absorption through interfering with normal processes. In addition, since they are free swimming, during preparation of the everted sacs most parasites will be removed from contact with the epithelium. Possibly the presence of live Spironucleus spp. in large numbers on the villi and in crypts is required for full effect (e.g. through release of enterotoxins, or consumption of glucose). The beneficial effects of increased glucose (40 mM) could supply energy but also glucose is known to enhance Na+ co-transport and therefore water absorption. Further definition of the transporters involved requires application of more invasive or reductive techniques such as Ussing chamber technology, radiotracer fluxes, ion-sensitive microelectrodes, fluorescent probes or molecular methodology. This would enable formulation of oral preparations to maximize absorption and thereby reduce the deleterious effects of infection.
Translations of the abstract in French, German and Spanish are available on the Avian Pathology website.
Acknowledgments
This work was supported by the Game Conservancy Trust. The authors would like to thank the gamekeepers and Peter Dalton's Game Consultancy.
References
- Barthe , L , Woodley , J and Houin , G . (1999) . Gastrointestinal absorption of drugs: methods and studies . Fundementals in Clinical Pharmacology , 13 : 154 – 168 .
- Burgerolle , G , Joyon , L and Oktem , N . (1973) . Contribution a l’étude cytologique et phylétique des diplozaires (Zoomastigophorea, Diplozoa, Dangeard 1910). II. Étude ultrastructurale du genre Spironucleus (Lavier 1936) . Protistologica , IX : 405 – 502 .
- Burgerolle , G , Kunsstyr , L , Senaud , J and Friedhoff , KT . (1980) . Fine structure of trophozoites and cysts of the pathogenic diplomonad Spironucleus muris . Zeitschrift fur Parasitenkunde , 62 : 47 – 61 .
- Diamond , JM and Bossart , WH . (1967) . Standing gradient osmotic flow. A mechanism for coupling water and solute transport in epithelia . Journal of General Physiology , 50 : 2061 – 2083 .
- Donowitz , M , de la Horra , C , Calonge , ML , Wood , IS , Dyer , J , Gribble , SM , de Medina , FS , Tse , CM , Shirazi-Beechey , SP and Ilundain , AA . (1998) . In birds, NHE2 is major brush-border Na+/H+ exchanger in colon and is increased by a low-NaCl diet . American Journal of Physiology , 274 : R1659 – R1669 .
- Garriga , C , Rovira , N , Moreto , M and Planas , JM . (1999) . Expression of Na+-D-glucose cotransporter in brush-border membrane of the chicken intestine . American Journal of Physiology , 276 : R627 – R631 .
- Gous , RM , Lindner , WA , Stielau , WJ and Dreosti , IE . (1977) . Sodium dependence and counterflow of some amino acids in chick intestine . Poultry Science , 56 : 793 – 796 .
- Hoerr , FJ , Current , WL and Haynes , TB . (1986) . Fatal cryptosporidiosis in quail . Avian Diseases , 30 : 421 – 425 .
- Hussain , AZ . (2001) . Morphometric evaluation of the small intestines and caeca of pheasants infected with Hexamita and Trichomonas spp . Veterinary Record , 148 : 484 – 485 .
- McDougald , LR . (1998a) . Intestinal protozoa important to poultry . Poultry Science , 77 : 1156 – 1158 .
- McDougald LR (1998b) Hexamitiasis In S.E. Aiello (Ed.) The Merck Veterinary Manual pp. 1897–1898 Whitehouse Station Merck & Co
- Morris , AP and Estes , MK . (2001) . Microbes and microbial toxins: paradigms for microbial–mucosal interactions. VIII. Pathological consequences of rotavirus infection and its enterotoxin . American Journal of Gastroenterology and Liver Pathology , 281 : G303 – G310 .
- Musch MW Bookstein C Arvans DL Cragoe EJ Jr Rao MC Chang EB (1992) Characterization of chicken intestinal brush border membrane Na/H exchange Comparative Biochemistry and Physiology. Comparative Biochemistry 103 439 444
- Poynton , SL and Sterud , E . (2002) . Guidelines for species descriptions of diplomonad flagellates from fish . Journal of Fish Diseases , 25 : 15 – 31 .
- Poynton , SL , Fard , MR , Jenkins , J and Ferguson , HW . (2004) . Ultrastructure of Spironucleus salmonis n. comb. (formerly Octomitus salmonis sensu Moore 1922, Davis, 1926, and Hexamita salmonis sensu Ferguson, 1979), with a guide to Sprionucleus species . Diseases of Aquatic Organisms , 60 : 49 – 64 .
- Sklan , D and Noy , Y . (2000) . Hydrolysis and absorption in the small intestines of posthatch chicks . Poultry Science , 79 : 1306 – 1310 .
- Swarbrick , O . (1990) . Hexamitiasis and an emaciation syndrome in pheasant poults: clinical aspects and differential diagnosis . Veterinary Record , 126 : 265 – 267 .
- Wilson , TH and Wiseman , G . (1954) . The use of sacs of everted small intestine for the study of transference of substances from the mucosal to the serosal surface . Journal of Physiology , 123 : 116 – 125 .
- Yason , CV and Schat , KA . (1987) . Pathogenesis of rotavirus infection in various age groups of chickens and turkeys: clinical signs and virology . American Journal of Veterinary Research , 48 : 977 – 983 .