Abstract
This study describes experimental infections in 4-week-old chickens inoculated intravenously with approximately 108 colony-forming units Streptococcus gallinaceus strain CCUG 42692T (C13156) or Enterococcus hirae strain DSM 20160 (C17410). Birds were necropsied following death and obvious clinical signs of disease or were euthanized weekly after infection for up to 4 weeks. At necropsy, lesions included splenomegaly, hepatomegaly, valvular and/or mural endocarditis. Cardiac lesions included focal necrotizing myocarditis and/or yellow–white vegetative valvular endocarditis or greyish proliferations associated with the mitral valves in 35% (6/20) and 79% (19/24) of birds infected with S. gallinaceus and in 20% (4/20) and 55% (12/22) of birds infected with E. hirae via the brachial and jugular veins, respectively. S. gallinaceus was reisolated from heart valves in 45% (9/20) and 75% (18/24) and E. hirae in 35% (7/20) and 73% (16/22) after inoculation via brachial and jugular veins, respectively. Both challenge strains were also isolated from liver, spleen, bone marrow and hock joints. A significant difference between the infections with the two strains was seen only with reisolation of E. hirae from hock joints (P<0.007). Significant differences were apparent between the two inoculation routes only with E. hirae, where infection via the jugular vein was associated with higher culture positive isolations from the heart (P=0.029), bone marrow (P=0.002) and hock joints (P<0.001) compared with the brachial vein. Birds injected with sterile phosphate-buffered saline were negative for culture of the challenge strains and no lesions were observed in these controls. The results confirm that both S. gallinaceus and E. hirae can cause endocarditis in experimentally infected chickens.
Reproduction de l’état septique et d'une endocardite chez le poulet suite à une infection expérimentale par Streptococcus gallinaceus et Enterococcus hirae
Cette étude décrit des infections expérimentales chez des poulets âgés de quatre semaines inoculés par voie intraveineuse avec approximativement 108 colonies formant unité (CFU) de la souche CCUG 42692T (C13156) de S. gallinaceus, ou de la souche DSM 20160 (C17410) d’E. hirae. Les animaux morts ou présentant des symptômes évidents de maladie ont été autopsiés, d'autres ont été euthanasiés toutes les semaines après l'infection jusqu’à la 4ème semaine. A l'autopsie les lésions comprenaient une splénomégalie, une hépatomégalie, une endocardite valvulaire et/ou pariétale. Les lésions cardiaques comprenaient une myocardite nécrosante et/ou une endocardite valvulaire végétative blanc jaunâtre ou des proliférations grisâtres associées aux valves mitrales chez respectivement 35% (6/20) et 79% (19/24) des animaux inoculés dans la veine brachiale et la veine jugulaire avec S. gallinaceus ainsi que chez 20% (4/20) et 55% (12/22) des animaux inoculés de la même façon avec E. hirae. S. gallinaceus a été réisolé des valves du cœur chez respectivement 45% (9/20) et 75% (18/24) des animaux inoculés dans la veine brachiale et la veine jugulaire, et E. hirae l'a été chez 35% (7/20) et 73% (16/22) dans les mêmes conditions. Les deux souches d’épreuves ont été réisolées du foie, de la rate de la moelle osseuse et de l'articulation du genou. Une différence significative entre les infections par les deux souches n'a été observée que lors du réisolement de E. hirae à partir de l'articulation du genou (p<0.007). En ce qui concerne les deux voies d'inoculation, des différences significatives ont été mises en évidence pour la souche E. hirae. Dans ce cas, les isolements positifs, par culture, ont été supérieures à partir du cœur (p=0.029), de la moelle osseuse (p=0.002) et de l'articulation du genou (p<0.001) chez les animaux inoculés dans la veine jugulaire comparés à ceux inoculés dans la veine brachiale. Les essais d'isolement des souches d’épreuve à partir des animaux inoculés avec la solution tampon phosphate ont été négatifs et aucune lésion n'a été observée chez ces animaux témoins. Les résultats confirment que les deux agents S. gallinaceus et E. hirae peuvent entraîner une endocardite chez les poulets expérimentalement infectés.
Reprouktion von Sepsis und Endokarditis durch eine experimentelle Infektion von Hühnern mit Streptococcus gallinaceus und Enterococcus hirae
Hier wird eine Studie zu experimentellen Infektionen von vierwöchigen Hühnern beschrieben, die intravenös mit ungefähr 108 Kolonie bildenden Einheiten (CFU) des Streptococcus gallinaceus (S. gallinaceus) -Stamm CCUG 42692T (C13156) oder Enterococcus hirae (E. hirae)-Stamm DSM20160 (C17410) infiziert wurden. Sektionen wurden entweder im Falle eines Todes nach deutlichen klinischen Krankheitssymptomen oder nach Euthanasie in wöchentlichen Abständen bis vier Wochen nach der Infektion durchgeführt. Als pathomorphologische Veränderungen wurden Splenomegalie, Hepatomegalie sowie Endocarditis valvularis und/oder parietalis festgestellt. Die kardialen Läsionen, die fokale nekrotisierende Myokarditis und/oder gelblich-weiße vegetative Klappenendokarditis oder gräuliche Proliferationen auf den Mitralklappen umfassten, traten bei 35% (6/20) bzw. 79% (19/24) der mit S. gallinaceus infizierten Tiere und bei 20% (4/20) bzw. 55% (12/22) der mit E. hirae infizierten Tiere auf, die entweder via Brachial- oder Jugularvene inokuliert worden waren. S. gallinaceus wurde aus den Herzklappen zu 45% (9/20) bzw. 75% (18/24) und E. hirae zu 35% (7/20) bzw. 73% (16/22) nach Inokulation in die Brachial- bzw. Jugularvene reisoliert. Beide Inokulationsstämme wurden auch aus Leber, Milz, Knochenmark und Fersengelenken isoliert. Zwischen den beiden Infektionsstämmen wurde ein signifikanter Unterschied nur bei der Reisolierungsrate von E. hirae aus den Fersengelenken festgestellt (p<0,007). Signifikante Unterschiede zwischen den beiden Inokulationsrouten waren nur bei E. hirae erkennbar, wobei die Infektion via Jugularvene im Vergleich zur Brachialveneninokulation mit höheren Reisolierungsraten aus Herz (p=0,029), Knochenmark (p=0,002) und Fersengelenken (p<0,001) verbunden war. Tiere, die mit sterilem Phophatpuffer injiziert worden waren, erwiesen sich in der für die Inokulationsstämme spezifischen Kultur als negativ und Läsionen wurden in diesen Kontrolltieren ebenfalls nicht beobachtet. Diese Ergebnisse bestätigen, dass sowohl S. gallinaceus als auch E. hirae in experimentell infizierten Hühnern Endokarditis hervorrufen kann.
Reproducción de septicemia y endocarditis mediante infección experimental de pollos con Streptococcus gallinaceus y Enterococcus hirae
En este estudio se describe una infección experimental en pollos de cuatro semanas de edad inoculados vía intravenosa con aproximadamente 108 unidades formadoras de colonias (UFC) de S. gallinaceus cepa CCUG 42692T (C13156) o E. hirae cepa DSM 20160 (C17410). Las aves fueron necropsiadas tras mostrar signos clínicos obvios y muerte o fueron sacrificadas semanalmente tras la infección hasta las cuatro semanas. A la necropsia se observaron lesiones de esplenomegalia, hepatomegalia, y endocarditis valvular y mural. Las lesiones cardíacas consistieron en miocarditis necrotizante focal y endocarditis valvular con proliferaciones de color blanquecino o amarillento o proliferaciones grisáceas asociadas a las válvulas mitrales en 35% (6/20) y 79% (19/24) de las aves infectadas con S. gallinaceus y 20% (4/20) y 55% (12/22) de las aves infectadas con E. hirae via vena braquial y yugular, respectivamente. S. gallinaceus fue reaislado de las válvulas cardíacas en un 45% (9/20) y 75% (18/24) y E. hirae en un 35% (7/20) y 73% (16/22) tras la inoculación vía vena braquial y yugular, respectivamente. Ambas cepas fueron aisladas de hígado, bazo, medula ósea y articulaciones de la rodilla. Se observó diferencias significativas entre las infecciones con las dos cepas únicamente en el reaislamiento de E. hirae a partir de las articulaciones de la rodilla (p<0.007). Unicamente se observaron diferencias significativas entre las dos vías de inoculación en el caso de E. hirae, en el que la infección vía vena yugular se asoció con mayor número de cultivos positivos de corazón (p=0.029), médula ósea (p=0.002) y articulaciones de la rodilla (p<0.001) comparado con la vena braquial. Las aves inoculadas con tampón fosfato salino estéril resultaron negativas al cultivo y no presentaron lesiones. Los resultados confirman que tanto S. gallinaceus como E. hirae pueden causar endocarditis en pollos infectados experimentalmente.
Introduction
The most common Streptococcus and Enterococcus spp. causing both septicaemia and localized infections in poultry are Streptococcus zooepidemicus, Streptococcus bovis, Enterococcus hirae, Enterococcus durans and Enterococcus faecalis (Smyth & McNamee, Citation2001). Endocarditis may develop when bacteraemic/septicaemic infections progress to a subacute or chronic stage, leading to colonization and subsequent inflammation of the heart valves. Infections might also result in myocarditis and/or pericarditis. Endocarditis in chickens often appears as nodular friable yellow vegetations, adhering to the surface of one or more heart valves, and containing fibrin, bacteria, erythrocytes and macrophages (Gross, Citation1984; Riddell, Citation1987, Citation1991). Streptococcal and enterococcal endocarditis in avian species has previously been associated with E. faecalis, Enterococcus faecium, E. durans, E. hirae, and Streptococcus equi spp. zooepidemicus (Nogaard & Mohler, Citation1902; Povar & Brownstein, Citation1947; Gross & Domermuth, Citation1962; Peckham, Citation1966; Domermuth & Gross, Citation1969; Jortner & Helmboldt, Citation1971; Jensen, Citation1979; Gross, Citation1991: Randall & Pearson, Citation1991; Greenwood et al., Citation1996; McNamee & King, Citation1996). Septicaemia and endocarditis caused by S. zooepidemicus and E. durans seem to be limited to mature birds, whereas infections with E. hirae appear to include young chickens with a majority of endocarditis affection in the right-side of the heart (Randall & Pearson, Citation1991). The habitat of E. hirae and E. durans has been reported to be in the small intestine until the chickens are 3 to 4 weeks old, whereas E. faecalis is one of the main bacteria in the gut flora of 1-day-old chicks (Devriese et al., Citation1991a).
Recently, increased mortality in a broiler parent flock caused by a new Streptococcus sp., Streptococcus gallinaceus (Collins et al., Citation2002), was found associated with septicaemia and mitral valvular endocarditis in 29% of clinical cases in mature birds of between 26 and 56 weeks of age (Chadfield et al., Citation2004). The outbreak was clonal in nature, as demonstrated by genotyping, with 85% of the birds infected with a single clone of S. gallinaceus. In addition, further outbreaks associated with septicaemia and endocarditis due to E. hirae infections have also been observed within the Danish poultry industry (Chadfield et al., Citation2005), demonstrating different genotypes but clonality of these dominant genotypes within the individual outbreaks. In all outbreaks there was mural or valvular endocarditis in birds ranging from 1 to 3 weeks of age (Chadfield et al., Citation2005). Previously, E. hirae infections have been reported to cause septicaemia and endocarditis in 4-week-old broilers (McNamee & King, Citation1996) and encephalomalacia in 1-week-old to 2-week-old broilers and commercial layers (Devriese et al., Citation1991b; Randall et al., Citation1993). Focal necrosis of the brain in chicks and septicaemia of psittacine birds have also been reported (Devriese et al., Citation1991b, Citation1992, Citation1995). E. hirae was originally associated with growth depression in young chickens (Farrow & Collins, Citation1985). Investigation of the bacterial flora of normal chickens has shown that E. hirae is seldom found in biological niches other than the small intestine and is common in chickens of between 3 and 4 weeks of age (Devriese et al., Citation1991a).
The clonal nature of the outbreaks of S. gallinaceus and E. hirae already described (Chadfield et al., Citation2004, Citation2005) indicates their pathogenic potential. However, in some instances additional opportunistic bacterial and viral infections were evident in the flocks, and may have compromised host immunity to the prevailing major infections. This study used experimental challenge of 4-week-old birds in order to establish whether the clinical isolates were able to cause endocarditis in non-compromised hosts.
Materials and Methods
Experimental animals
Three-week-old Ross 308 broiler chickens were purchased from commercial breeders with no history of disease problems and with very high biosecurity standards. For both experiments, birds were acclimatized for 1 week before being assigned randomly to three treatment groups; two groups infected with bacteria and one group sham-infected with phosphate-buffered saline (PBS). To investigate the possibility of horizontal transmission, the birds were kept in the same room without separation throughout both experiments. Chickens were marked to distinguish between the three different groups, and all birds received water and feed ad libitum.
Bacteria
S. gallinaceus CCUG 42692T (C13156) was originally isolated from an outbreak in a broiler parent flock from the liver of a 52-week-old bird, while E. hirae DSM 20160 (C17410) was isolated from an outbreak in a broiler flock from the liver of a 6-day-old bird. Both birds had shown endocarditis. Bacterial cultures were stored at −80°C in a solution of 30% (v/v) glycerol (KVL Pharmacy, Denmark) and 70% heart infusion broth (DIFCO, USA) until use.
Preparation of inocula
Both strains were incubated at 37°C overnight on blood agar containing 5% bovine blood (Blood Agar Base, CM 55; Oxoid, Basingstoke, UK) to ensure pure cultures, followed by inoculation of three to five colonies in 10 ml brain heart infusion (BHI) broth (Oxoid) and incubation at 37°C with shaking. Finally, 10 μl bacterial suspension was inoculated into 10 ml fresh BHI broth and re-incubated under aerobic conditions with shaking for 9 h. After incubation, broth cultures were washed three times and resuspended in PBS and adjusted to an optical density (OD) at 620 nm of 0.1. The inocula viable count was subsequently confirmed by serial 10-fold dilutions on BHI agar and approximated to 108 colony forming units (CFU). The size of the inoculum chosen was approximately 108 CFU/0.5 ml. The same inocula for both strains were used in this study. The OD values for S. gallinaceus and E. hirae were used in a linear regression to predict the viable count: log [CFU/ml]=1.08×log(OD)+9.16.
Experimental design
In the first experiment, 50 4-week-old birds were inoculated intravenously through the brachial vein as follows: 0.5 ml S. gallinaceus (2.5×108 CFU) in PBS in 20 birds, 0.5ml E. hirae (3.5×108 CFU) in PBS in 20 birds, and 0.5 ml PBS alone in 10 birds (which constituted the control group). The birds were observed daily for 28 days, and every 7 days a group of three to six birds was killed humanely and examined post mortem as described later. Any birds that died were similarly examined, as were any that developed obvious signs of disease, which were killed humanely.
In the second experiment, 58 4-week-old birds were inoculated in the jugular vein with either 0.5 ml S. gallinaceus (1.4×108 CFU) in PBS in 24 birds, 0.5 ml E. hirae (1.9×108 CFU) in PBS in 22 birds, or 0.5 ml PBS alone in 12 birds (control group). The birds were observed daily for 23 days and three to five birds were killed humanely at days 7, 14, 21 and 23 post-infection and examined post mortem. Otherwise, birds were killed humanely and subjected to postmortem examinations when obvious clinical signs developed.
Postmortem examination and bacteriology
All birds were subjected to a lege artis postmortem examination, either immediately after euthanasia or after overnight storage at 4°C. Swabs were taken aseptically from the liver, spleen, heart (mitral valve), bone marrow (right femur) and right hock joint for bacteriological culture. The swabs were cultured on blood agar containing 5% bovine blood and incubated at 37°C overnight. From blood agar plates showing pure growth, a single colony was subcultured for pulsed-field gel electrophoresis to identify the bacterial isolates recovered by comparison with the challenge strains. The protocol used was as described by Ojeniyi et al. (Citation1991), using SmaI (R0141S; New England Biolabs, USA) for restriction enzyme endonuclease digestion.
Histopathology
Heart tissues, including mitral valves were fixed in 10% buffered formalin solution for 24 h. After fixation, the tissues were trimmed, dehydrated and embedded in paraffin wax prior to preparation of sections 3 to 4 μm thick, which were mounted on adhesive slides (Super Frost/Plus; Menzel-Gläser, Germany) and stained with haematoxylin–eosin stain. Sections were examined by light microscopy and photographed with a Zeiss AxioCam digital camera.
Data analysis
Pairwise comparison of the proportion of culture-positive animals overall was carried out for relevant parameters; that is, both strains and both inoculation sites versus the controls, jugular versus brachial veins for both strains, and infection with S. gallinaceus versus E. hirae at the same inoculation sites. The proportions were compared using the Fisher exact test for the comparison of proportions for independent groups. Statistical significance was taken as P<0.05.
The per cent of positive cultures post mortem from the five anatomical sites at the four time intervals after inoculation was estimated, and the estimates plotted graphically. No statistical analyses were performed due to the limited number of observations at each time point. The birds that were killed prematurely due to signs of clinical disease were excluded from these estimations.
Results
Bacterial growth
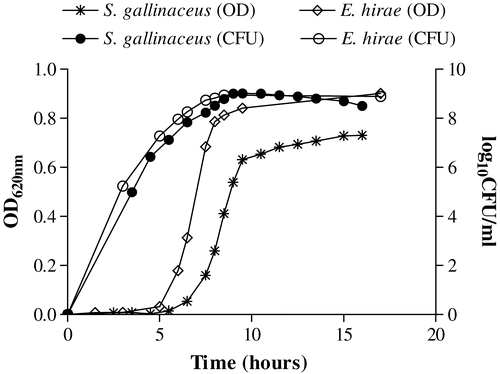
Growth of S. gallinaceus CCUG 42692T (C13156) in BHI broth was generally slower than that of the E. hirae strain DSM 20160 (C17410), with bacterial viability (CFU/ml) declining after approximately 10 h () even though the OD at 620 nm continued to increase. Bacteria were harvested in the exponential phase after 9 h incubation to ensure a reliable correlation between OD and viability of the inocula.
Figure 1. Growth curves of S. gallinaceus (C13156) and E. hirae (C17410) expressed as optical density (OD 620 nm) and corresponding viable counts (log10 CFU/ml). Inocula used for infection were adjusted to an OD 620 nm of 0.1 in PBS (approximately 108 CFU/ml) taken from exponential growth of culture (approximately 9 h).
Samples collected at necropsy yielded pure cultures of streptococcal/enterococcal-like colonies from the liver, spleen, bone marrow, joints and heart. After 24 h aerobic growth, the colonies on blood agar varied from pin-point to 0.5 mm (S. gallinaceus) and from 0.5 mm to 1.0 mm in diameter (E. hirae), and were grey-transparent to white. Colonies were circular with an entire margin, convex and surrounded by a more or less pronounced zone of α-haemolysis. All isolates appeared to be genetically identical to the inoculated parent strains as demonstrated by pulsed-field gel electrophoresis (data not shown), confirming that the lesions were indeed induced by the clinical strains.
Challenge studies
All control birds used for both experiments were negative by culture for the challenge strains used in the study and no clinical signs or gross lesions were demonstrated in these birds, emphasizing that disease did not develop through contact transmission.
S. gallinaceus infections
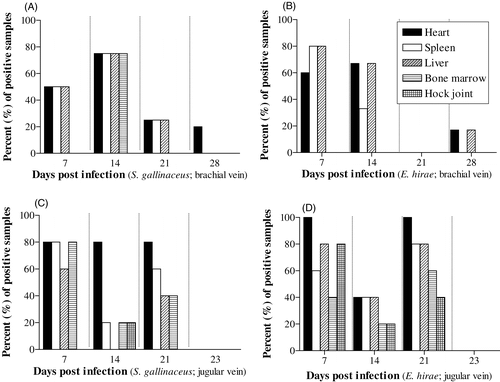
Challenge of 4-week-old birds with S. gallinaceus via the brachial vein resulted in three birds (numbers 18–20) having to be humanely killed within 12 days of infection due to morbidity/obvious clinical signs of disease (). All three birds had evidence of septicaemia, including hepatomegaly and splenomegaly. Two of the birds that were killed within 1 week of inoculation had endocarditis with positive cultures from the mitral valves, liver and spleen, and in one of them also from the bone marrow (). The third bird had no evidence of gross valvular endocarditis or culture but was judged to be septicaemic on the basis of pericarditis hepatomegaly, splenomegaly and renomegaly. Together with the remaining birds, which were killed at weekly intervals for 4 weeks after inoculation, positive cultures from heart valves were obtained in 45% (9/20) of birds and lesions in heart tissue were seen in 35% (6/20) (). With the exception of three birds, all birds had positive heart cultures and variable lesions within the two first weeks post-challenge; thereafter, the culture and pathology were negative with two exceptions. There was no growth from hock joints from any of the birds.
Table 1. Recovery of S. gallinaceus post mortem from birds experimentally infected via the brachial vein
Inoculation via the jugular vein with an approximate equivalent inoculum of S. gallinaceus resulted in a higher incidence of positive culture from the heart valves, with 75% (18/24) and 79% (19/24) birds showing heart lesions (). Hepatomegaly and splenomegaly were evident, as were multiple, irregular, well demarcated necro-haemorrhagic zones throughout the liver and spleen (). Gross lesions of endocarditis included focal necrotizing myocarditis and/or yellow–white or greyish verruciform vegetative endocarditis associated with the mitral valve (). Six birds were killed due to obvious clinical signs within 13 days of inoculation and all were culturally positive from heart valves for S. gallinaceus (). In contrast to inoculation via the brachial vein route, birds were culture-positive up to 3 weeks post-infection and generally had a higher incidence and severity of lesions associated with septicaemia ().
Figure 2. Gross postmortem lesions following S. gallinaceus jugular intravenous infection. 2a: hepatomegaly. 2b: splenomegaly with multiple, randomly distributed, irregular, well-demarcated areas of necrosis surrounded by a haemorrhagic zone (arrow). 2c: yellow–white vegetative valvular endocarditis (arrow).
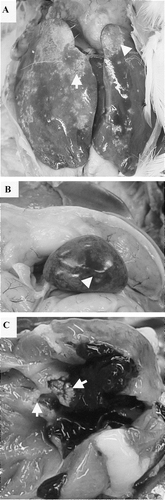
Table 2. Recovery of S. gallinaceus post mortem from birds experimentally infected via the jugular vein
E. hirae infections
Infection via the brachial vein demonstrated that 35% (7/20) of birds cultured E. hirae-positive from heart valves, of which 20% (4/20) had pathological lesions of valvular endocarditis as an opaque thickening of the mitral valves (). Only two birds were killed by day 5 post-infection and only one had endocarditis. During the first 2 weeks post-infection, bacteria were re-isolated from the liver, spleen and heart but not from the bone marrow or hock joints. Appearances interpreted as septicaemia accompanied by serositis were evident in week 1 and week 3 post-infection, although the challenge strain was not re-isolated. In birds killed in week 4, bacteria were isolated from a single bird only but lesions were not observed ().
Table 3. Recovery of E. hirae and lesions seen post mortem in birds experimentally infected via the brachial vein
Inoculation via the jugular vein, as with S. gallinaceus, produced a higher incidence of endocarditis, with 73% (16/22) of birds positive in culture from heart valves and 55% (12/22) showing thickening and/or vegetative growth on the mitral valves (). Postmortem examination demonstrated lesions interpreted as septicaemic, including general hyperaemia, subserosal discoloration of fat, hepatomegaly and splenomegaly, and necroses varied from pin-point to larger irregular lesions demarcated by a haemorrhagic zone (). Fibrinous perihepatitis and pericarditis were also occasionally observed. Birds were culture-positive for 3 weeks post-infection and, although negative by day 23, lesions including splenomegaly and valvular endocarditis were evident. A total of 45% (10/22) of the birds were culture-positive in hock joints, although the majority of these birds had low bacterial counts (CFU) ().
Figure 3. Gross postmortem lesions following E. hirae jugular intravenous infection. 3a: hepatomegaly. 3b: splenomegaly with multiple, randomly distributed, irregular, well-demarcated areas of necrosis surrounded by a haemorrhagic zone (arrow). 3c: yellow–white lesion of vegetative valvular endocarditis (arrow denotes thickening of the mitral valve due to vegetative growth).
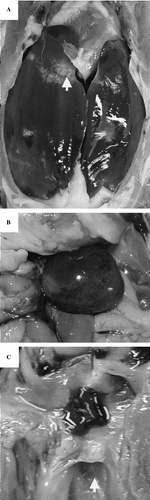
Table 4. Recovery of E. hirae and lesions seen post mortem in birds experimentally infected via the jugular vein
shows the per cent of samples positive for the respective challenge strains, after inoculation by two different routes, at four different post-infection times at different anatomical sites. The per cent of samples culture-positive for S. gallinaceus and E. hirae from the heart in birds at 3 weeks post-infection via the brachial vein was low (0% to 25%) () compared with infection via the jugular vein (80% to 100%) ). With both strains and inoculation routes, the per cent of culture positives after 3 weeks post-infection at all anatomical sites was minimal (≤20%). Overall, culture from the heart with both strains was more likely to be positive than from any other anatomical site, and the culture from hock joints was positive only with E. hirae (jugular vein).
Histopathology
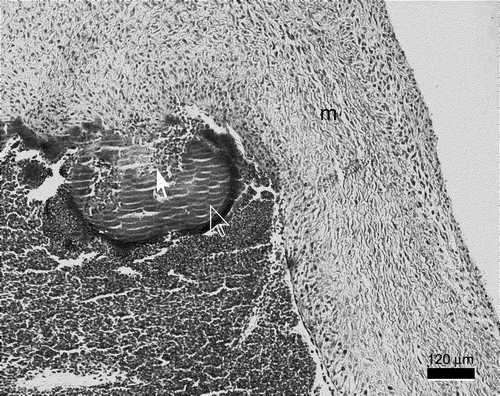
There were vegetative lesions of both mural and valvular endocardium. Vegetations had a central focus of bacteria, fibrin and cellular debris attached to the endocardium. A layer of inflammatory cells, dominated by heterophils and a few macrophages, surrounded the central part of the vegetations. The outermost parts of the vegetations on the atrioventricular valves consisted of erythrocytes, thrombocytes and fibrin ().
Figure 5. Mitral valve endocarditis induced by S. gallinaceus 3 weeks post-infection. The bird had hepatomegaly and splenomegaly with necrosis, renomegaly and both valvular and mural endocarditis. There is formation of a vegetative lesion at the mitral valve (m) consisting of bacteria, fibrin and basophils (full arrow), surrounded by a corona consisting mainly of heterophils and erythrocytes in the periphery (open arrow).
Data analysis
Comparison of control and infected birds for recovery of each of the challenge strains by each inoculation route from different anatomical sites in the birds demonstrated a significant difference with respect to isolation from the heart with both S. gallinaceus and E. hirae and inoculation via the brachial (P=0.03 to 0.01) and jugular veins (P<0.001) (). However, with S. gallinaceus there was no significant difference compared with the control for recovery from the liver, spleen, bone marrow (via brachial vein) and hock joint (via brachial and jugular veins). With E. hirae there was no significant difference in culture from bone marrow and hock joints following inoculation via the brachial vein compared with the controls.
Table 5. Comparison by Fisher Exact test of recovery of S. gallinaceus and E. hirae from different anatomical sites after experimental infection of 4-week-old birds the brachial vein and jugular vein
With regard to differences between the two challenge strains, only isolation of E. hirae from hock joint (via the jugular vein) was statistically significant (P<0.007) (). Differences between routes of inoculation of challenge strains were seen only with E. hirae, where inoculation via the jugular vein resulted in significantly more bacterial isolation from the heart (P=0.029), bone marrow (P=0.002) and hock joints (P<0.001) compared with inoculation via the brachial veins. There were no significant differences between male and female birds with regard to infection (data not shown).
Discussion
In this study endocarditis was induced in intravenously inoculated birds that were given approximately 108 CFU challenge strains of either S. gallinaceus or E. hirae. Few previous studies on experimental infective endocarditis have been conducted in avian species, although a comparable study by Gross & Domermuth (Citation1962) reported reproduction of endocarditis in 67% of adult chickens inoculated intravenously with Streptococcus faecalis (1010 CFU). In the study reported here, S. gallinaceus inoculation by the same route resulted in 75% of the birds being culture positive in the heart, and 84% of these birds had lesions of valvular endocarditis. Similar results were shown with infection of E. hirae at 73% and 55%, respectively. Although only a few birds were killed due to obvious clinical disease in this study, the onset of disease at 5 to 13 days with S. gallinaceus and 5 to 16 days for E. hirae was comparable with the study of Gross & Domermuth (Citation1962), who demonstrated peak mortality in birds at 5 to 16 days post-inoculation. The apparent difference in mortality levels could be explained by the use of a 100-fold higher infecting dose, but may also be directly related to strain virulence. It is also worth noting that infections associated with Enterococcus spp. often produce increased early mortality without specific clinical signs (Chadfield et al., Citation2005). Due to the fact that mortality is often only slightly increased, birds are not necessarily submitted for laboratory investigations and the infections remain unnoticed.
Mitomo (Citation1972) also demonstrated vegetative endocarditis in chickens intravenously inoculated with 5×107 CFU S. faecalis (now E. faecalis) and demonstrated that valvular lesions developed progressively over 24 h, leading to full bacterial vegetations on mitral valves by 7 days post-infection. This agrees with the present study, where pathology indicated vegetative proliferation on mitral valves in birds from as early as 5 to 7 days post-infection with both S. gallinaceus and E. hirae. Interestingly, Mitomo (Citation1972) demonstrated that some birds were negative for culture from vegetative growth on the heart valves at day 5, but had positive blood cultures. In some instances in our study, vegetative lesions in the heart were negative for culture at day 7; however, contemporary blood samples were not taken to ascertain the state of blood infection. Negative culture was also apparent at day 23 in vegetations in birds infected with the challenge strains ( and ). Given that all other anatomical sites were also negative, this could suggest clearance of bacteria. Even if the infection were self-limiting, the pathological damage incurred, particularly in the heart, would leave the birds vulnerable to further infection by opportunistic pathogens.
Lesions associated with naturally occurring bacterial endocarditis in chickens have been reported to occur commonly in the left ventricle, involving both the atrioventricular (mitral) and aortic valves (Dahme et al., Citation1965; Peckham, Citation1966; Riddell, Citation1987; Droual et al., Citation1992). However, enterococcal endocarditis affecting the right atrioventricular valve has been reported in young broilers (Randall & Pearson, Citation1991; Chadfield et al., Citation2005). In our study, endocardial lesions were seen only on the left mitral valves. In addition, lesions were also evident in the mural endocardium, but only with infection of S. gallinaceus and only in two birds that also had valvular lesions. Whether the route of inoculation influences the site of infection in the heart is uncertain, but the lesions were consistent with those in the natural infection outbreaks (Chadfield et al., Citation2004, Citation2005).
Previous studies have concluded that reproduction of endocarditis required alteration of normal valves, followed by intravenous injection of bacteria (Sande, Citation1976). Streptococci have been the most common isolates from endocarditis lesions in chickens (Wages, Citation1997), and studies in man have shown that streptococci localize on deformed or previously damaged endothelium (Levison, Citation1976). Attempts to reproduce infection with clinical isolates of E. hirae and inoculation of 1-day-old chicks failed to demonstrate signs (El-Sukhon & Abdul-Aziz, Citation1993) even after immunosuppression with betamethasone (Abdul-Aziz & El-Sukhon, Citation1994). It was subsequently proposed that predisposing factors might be involved in establishing natural infections. This is in contrast to the results of this study in which, to our knowledge, no predisposing factors were evident in the birds prior to infection. Age-related changes in the heart valves often seen in mature birds (Mitomo, Citation1972) might provide suitable conditions for initiation of vegetative endocarditis, but in this study only 4-week-old birds were used and therefore this scenario is unlikely. Furthermore, only single inoculations were administered to the birds, as opposed to repeated inoculations, which would avoid excessive bacterial overload in the blood stream and related increase in viscosity and/or toxin production that may damage the endothelium (Woodfield & Sisson, Citation1989; Robinson & Maxie, Citation1993).
It was apparent from the results in this study that, except for localization of E. hirae in the joints, major differences were not observed between S. gallinaceus and E. hirae infection of 4-week-old birds. These experiments should also be repeated in 1-day-old and mature birds to either confirm similarities in production of disease or differentiate disease states specific to each bacterial species. The lack of observed differences in the present study may indicate common virulence factors. Further work will also address the presence of previously defined virulence traits associated with streptococci and enterococci.
The field strain of E. hirae used in this study was isolated from a broiler flock during a longitudinal study over a period of 4 weeks, and constituted the predominant bacterial pathogen associated with mortality (Chadfield et al., Citation2005). After 3 weeks, mortality associated with E. hirae apparently disappeared from the flock. Although the outbreak demonstrated the pathogenic potential of E. hirae with regard to encephalomalacia and induction of infective endocarditis, overall mortality rates were relatively low. Encephalomalacia observed under field conditions was not reproduced experimentally. The reasons for these differences remain to be investigated but might be age related. In the third week of the outbreak, both Staphylococcus aureus and Escherichia coli were isolated from birds post mortem and were responsible for 17% and 8% of mortality, respectively, at 20 days. At the same time, E. hirae was isolated from 42% of the birds. However, E. hirae was previously isolated in pure culture on day 6 without any evidence of concurrent infection in the flock. Similarly, the S. gallinaceus strain was isolated from a parent broiler flock and was also associated with other major diseases identified within the flock, including myelocytomatosis and E. coli and S. aureus infections (Chadfield et al., Citation2004). However, given the clonal nature of both outbreak strains it seems that both were capable of inducing endocarditis independently of predisposing factors. The findings in this study support the contention that both S. gallinaceus and E. hirae are pathogenic in chickens, resulting in lesions interpreted as septicaemic, and demonstrate for the first time experimental reproduction of valvular endocarditis with these bacterial species.
Translations of the abstract in French, German and Spanish are available on the Avian Pathology website.
Acknowledgments
The authors wish to thank Gitte Petersen for excellent technical assistance and Frederik Andersen for expert care of experimental birds. This work was funded by the Danish Research Council (SJVF Sagsnr. 53-00-0295).
References
- Abdul-Aziz , TA and El-Sukhon , SN . (1994) . Pathogenicity of Enterococcus hirae for chicken embryos and betamethasone-treated chicks . Research in Veterinary Science , 56 : 397 – 398 .
- Chadfield , MS , Christensen , JP , Christensen , H and Bisgaard , M . (2004) . Characterization of streptococci and enterococci associated with septicaemia in broiler parents in which a high prevalence of endocarditis was observed . Avian Pathology , 33 : 610 – 617 .
- Chadfield , MS , Christensen , JP , Juhl-Hansen , J , Christensen , H and Bisgaard , M . (2005) . Characterisation of Enterococcus hirae outbreaks in broiler flocks demonstrating increased mortality due to septicaemia and endocarditis and/or altered production parameters . Avian Diseases , 49 : 16 – 23 .
- Collins , MD , Hutson , RA , Falsen , E , Inganäs , E and Bisgaard , M . (2002) . Streptococcus gallinaceus sp. nov., from chickens . International Journal of Systematic Evolutionary Microbiology , 52 : 1161 – 1164 .
- Dahme , E , Dorn , P and El Etreby , MF . (1965) . The problem of endocarditis in fowl . Berliner und Munchener Tierarztliche Wochenschrift , 78 : 222 – 227 .
- Devriese , LA , Hommez , J , Wijfels , R and Haesebrouck , F . (1991a) . Composition of the enterococcal and streptococcal intestinal flora of poultry . Journal of Applied Bacteriology , 71 : 46 – 50 .
- Devriese , LA , Ducatelle , R , Uyttebroek , E and Haesebrouck , F . (1991b) . Enterococcus hirae infection and focal necrosis of the brain of chicks . Veterinary Record , 129 : 316
- Devriese , LA , Cruz Colque , JI , Haesebrouck , F , Desmidt , M , Uyttebroek , E and Ducatelle , R . (1992) . Enterococcus hirae in septicaemia of psittacine birds . Veterinary Record , 130 : 558 – 559 .
- Devriese , LA , Chiers , K , De Herdt , P , Vanrompay , D , Desmidt , M and Ducatelle , R . (1995) . Enterococcus hirae infections in psittacine birds: epidemiological, pathological and bacteriological observations . Avian Pathology , 24 : 523 – 531 .
- Domermuth , CH and Gross , WB . (1969) . A medium for isolation and tentative identification of fecal streptococci, and their role as avian pathogens . Avian Diseases , 13 : 394 – 399 .
- Droual , R , Walker , RL , Shivaprasad , HL , Jeffery , JS , Meteyer , CU , Chin , RP and Shapiro , DP . (1992) . An atypical strain of Pasteurella gallinarum: pathogenic, phenotypic, and genotypic characteristics . Avian Diseases , 36 : 693 – 699 .
- El-Sukhon , SN and Abdul-Aziz , TA . (1993) . Experimental infection of chicks with Enterococcus hirae . Veterinary Record , 133 : 144
- Farrow , JAE and Collins , MD . (1985) . Enterococcus hirae, a new species that includes amino acid assay strain NCDO 1258 and strains causing growth depression in young chickens . International Journal of Systematic Bacteriology , 35 : 73 – 75 .
- Greenwood , AG , Marshall , J and Tinsley , EGF . (1996) . Vegetative endocarditis in a Waldrapp ibis . Avian Pathology , 25 : 387 – 391 .
- Gross WB (1984) Bacterial endocarditis In M.S. Hofstad, H.J Barnes, B.W Calnek, W.M. Reid & H.W Yoder (Eds.) Diseases of Poultry 8th edn pp. 268–269 Ames Iowa State University Press
- Gross , WB . (1991) . Use of corticosterone and ampicillin for treatment of Streptococcus faecalis infection in chickens . American Journal of Veterinary Research , 52 : 1288 – 1291 .
- Gross , WB and Domermuth , CH . (1962) . Bacterial endocarditis of poultry . American Journal of Veterinary Research , 23 : 320 – 329 .
- Jensen , WI . (1979) . An outbreak of streptococcosis in eared grebes (Podiceps nigricollis) . Avian Diseases , 23 : 543 – 546 .
- Jortner , BS and Helmboldt , CF . (1971) . Streptococcal bacterial endocarditis in chickens . Veterinary Pathology , 8 : 54 – 62 .
- Levison ME (1976) Pathogenesis of infective endocarditis In D. Kaye (Ed.) Infective Endocarditis pp. 29–41 Baltimore MD University Park Press
- McNamee , PT and King , DC . (1996) . Endocarditis in broiler breeder rearers due to Enterococcus hirae . Veterinary Record , 138 : 240
- Mitomo , Y . (1972) . Electron microscopic study of the early changes in heart valve of chickens infected with Streptococcus faecalis . Bulletin of Tokyo Medical and Dental University , 19 : 287 – 300 .
- Nogaard , VA and Mohler , JR . (1902) . Apoplectiform septicemia in chickens . United States Department of Agriculture BAI Bull , 36 : 21 – 22 .
- Ojeniyi , B , Høiby , N and Rosdahl , VT . (1991) . Genome fingerprinting as a typing method used on polyagglutinable Pseudomonas aeruginosa isolates from cystic fibrosis patients . Acta Pathologica, Microbiologica et Immunologica Scandinavica , 99 : 492 – 498 .
- Peckham , MC . (1966) . An outbreak of streptococcosis (apoplectiform septicemia) in white rock chickens . Avian Diseases , 10 : 413 – 421 .
- Povar , ML and Brownstein , B . (1947) . Valvular endocarditis in the fowl . Cornell Veterinarian , 37 : 49 – 54 .
- Randall , CJ and Pearson , DB . (1991) . Enterococcal endocarditis causing heart failure in broilers . Veterinary Record , 129 : 535
- Randall , CJ , Wood , AM and Mackenzie , G . (1993) . Encephalomalacia in 1st-week chicks . Veterinary Record , 132 : 419
- Riddell C (1987) Cardiovascular system Avian Histopathology pp. 31–36 Kennett Square PA American Association of Avian Pathologists
- Riddell C (1991) Endocarditis In B.W Calnek, H.J Barnes, C.W Beard, W.M Reid & H.W Yoder (Eds.) Diseases of Poultry 9th edn p. 844 Ames Iowa State University Press
- Robinson WF Maxie MG (1993) Endocarditis In K.V.G Jubb, P.C Kennedy & N. Palmer (Eds.) Pathology of Domestic Animals 4th edn pp. 24–27 San Diego Academic Press
- Sande MA (1976) Experimental endocarditis In D. Kaye (Ed.) Infective Endocarditis pp. 11–28 Baltimore MD University Park Press
- Smyth JA McNamee PT (2001) Staphylococci, Streptococci and Enterococci In F. Jordan, M. Pattison, D. Alexander & T. Faragher (Eds.) Poultry Diseases 5th edn pp. 163–169 London W.B. Saunders
- Wages DP (1997) Streptococcosis In B.W. Calnek, H.J. Barnes, C.W. Veard, L.R. McDougald & Y.M. Saif (Eds.) Diseases of Poultry 10th edn pp. 297–302 London Mosby-Wolfe
- Woodfield JA Sisson D (1989) Infective endocarditis In S.J. Ettinger (Ed.) Textbook of Veterinary Internal Medicine pp. 1151–1162 Philadelphia PA W.B. Saunders Co