Abstract
The “nasal glands” occur in many bird species and are powerful sodium ion-excretory organs. In ducks, they are located in supraorbital bony recesses. Granulomatous inflammation of these glands occurs with an incidence of approximately 1% in ducklings (Anas platyrhynchos), and is not associated with specific clinical symptoms. We investigated nine glands of eight animals with granulomas by gross pathology and histopathology, and compared results of bacteriology with 20 non-lesioned nasal glands. Adenitis was characterized by multifocal to coalescent heterophilic granulomas with central necrotic heterophils, and multinucleate giant cells, lymphocytes and plasma cells. Within the centres of the granulomas, there were clusters of Gram-negative bacteria that were identified as halotolerant Pseudomonas aeruginosa, Proteus mirabilis and Aeromonas hydrophila. Normal glands contained exclusively various halotolerant Gram-positive bacteria, mostly Streptococcus sp. and Enterococcus sp. The distribution of lesions and lack of clinical symptoms were suggestive of a localized ascending infection via the secretory ductules.
Inflammation granulomateuse des glandes à sel, associée à une lésion interne due à une bactérie Gram-négatif, chez le caneton (Anas platyrhynchos)
Les glandes nasales sont présentes chez beaucoup d'espèces d'oiseaux et sont de puissants organes d'excrétion d'ions sodium. Chez les canards, elles sont localisées dans les culs-de-sac osseux supra orbitaires. L'incidence de l'inflammation granulomateuse de ces glandes chez les canetons (Anas platyrhynchos) est d'environ 1%, et n'est pas associée a des symptômes spécifiques. Nous avons étudié 9 glandes de 8 animaux présentant un granulome observé macroscopiquement et histologiquement, puis nous avons comparé les résultats bactériologiques à ceux de 20 glandes nasales sans lésion. L'adénite a été caractérisée par des granulomes multifocaux à coalescents avec au centre des hétérophiles nécrotiques et des cellules géantes multinuclées, des lymphocytes et de cellules plasmatiques. Au centre des granulomes il y avait des groupes de bactéries Gram-négatif qui ont été identifiées comme des Pseudomonas aeruginosa, Proteus mirabilis et Aeromonas hydrophila halotolérants. Les glandes normales contenaient exclusivement des bactéries Gam-négatif, principalement des Streptococcus sp. et Enterococcus sp. La distribution des lésions et l'absence de symptôme laissent supposer une infection localisée, ascendante via les canalicules sécréteurs.
Granulomatöse Entzündung der Salzdrüsen bei Entenküken (Anas platyrhynchos) mit gramnegativen Bakterien in den Läsionen
Bei vielen Vogelarten gibt es “Nasaldrüsen”, bei denen es sich um leistungsstarke, Salzionen sezernierende Organe handelt. In Enten befinden sie sich in den supraorbitalen Knochenvertiefungen. Granulomatöse Entzündungen dieser Drüsen kommen mit einer Häufigkeit von ungefähr 1% bei Entenküken (Anas platyrhynchos) vor und sind nicht mit klinischen Symptomen verbunden. Wir untersuchten neun Drüsen von acht Tieren mit makroskopisch und histopathologisch erkennbaren Granulomen und verglichen die Ergebnisse der bakteriologischen Untersuchungen mit denen von 20 nicht veränderten Nasendrüsen. Die Adenitis war charakterisiert durch multifokale teilweise ineinander übergehende heterophile Granulome mit nekrotischen Heterophilen im Zentrum sowie vielkernigen Riesenzellen, Lymphozyten und Plasmazellen. Innerhalb der Granulomzentren befanden sich Kluster von gramnegativen Bakterien, die als halotolerante Pseudomonas aeruginosa, Proteus mirabilis und Aeromonas hydrophila identifiziert wurden. Normale Drüsen enthielten ausschließlich verschiedene grampositive Bakterien, meistens Streptococcus sp. und Enterococcus sp. Die Verteilung der Läsionen sowie das Nichtvorhandensein klinischer Symptome lassen auf eine lokalisierte, über die Sekretionsgänge aufsteigende Infektion schließen.
Inflamación granulomatosa de las glándulas de la sal en patos jóvenes (Anas platyrhynchos) asociados a bacterias gram negativas intralesionales
Las ‘glándulas nasales’ se encuentran en la mayoría de especies de aves y son potentes de excreción de iones sodio. En patos, se localizan en recesos óseos supraorbitales. La inflamación granulomatosa de estas glándulas ocurre con una incidencia de aproximadamente el 1 % en patos jóvenes (Anas platyrhynchos), y no se asocia con síntomas clínicos específicos. Se investigaron las lesiones macroscópicas y microscópicas de 9 glándulas de 8 animales con granulomas y se comparó la bacteriología con 20 glándulas nasales no lesionadas. La adenitis se caracterizó por granulomas heterofílicos con un área central de heterófilos necróticos, células gigantes multinucleadas, linfocitos y células plasmáticas, de distribución de multifocal a confluyente. En el centro de los granulomas, se observaron colonias de bacterias gram negativas que se identificaron como Pseudomonas aeruginosa, Proteus mirabilis y Aeromonas hydrophila halotolerantes. Las glándulas normales contenían exclusivamente bacterias gram positivas halotolerantes, mayoritariamente Streptococcus sp. y Enterococcus sp. La distribución de las lesiones y la inexistencia de signos clínicos son compatibles con una infección localizada vía ascendente a partir de los conductos secretores.
Introduction
For marine birds, hypertonic sea water may be the only source of drinking water for extended periods. The massive salt intake associated with drinking sea water requires efficient mechanisms of osmoregulation in these animals. Since avian kidneys have a limited capability to concentrate urine (Dantzler, Citation1989), extrarenal salt glands are necessary for eliminating excess sodium and chloride ions. Paired crescent-shaped glands located supraorbitally, which are able to efficiently excrete sodium chloride while retaining free water in the body, were first described in the herring gull (Schmidt-Nielsen, Citation1960). These “nasal glands” or “salt glands” occur in virtually all bird species (Technau, Citation1936). In marine birds, these glands are usually fully developed and functional active soon after hatching. In other bird species with infrequent exposure to excess salt, the glands remain quiescent and appear histologically in a mostly undifferentiated state until challenged (Ernst & Ellis, Citation1969). Mechanisms and regulation of salt secretion have been extensively studied in the duck (Anas platyrhynchos). When these animals are initially exposed to osmotic stress, the nasal glands proliferate and show an increase in the number of secretory cells (hyperplasia) and reorganization of existing ones to reach full differentiation within a few days (Ernst & Ellis, Citation1969; Hildebrandt, Citation2001). In domestic ducks (A. platyrhynchos) the nasal glands possess two supraorbital parts, divided into a medial lobe and a lateral lobe. Both drain into the nasal cavity adjacent to the rostral part of the ventral turbinate with two separate ducts (McLelland et al., Citation1968; Butler et al., Citation1991).
Diseases associated with functional disturbances of this organ are not known. To the best of our knowledge, only one case report describes inflammation and hyperplasia of the nasal glands in turkeys but the cause of these lesions could not be determined (Riddell & Roepke, Citation1991). In young ducklings up to 3 weeks of age, however, pathological changes in the nasal glands have been found incidentally. This condition occurs in approximately 1% of domestic ducklings independent of the breeding stock and the location of hatching; it was observed in ducklings that were bred in the US or in Europe. An aetiology has not been defined.
This prompted us to investigate spontaneous cases of this entity. We describe in the present paper a severe granulomatous inflammation, clinically silent, of the salt glands of ducklings associated with various Gram-negative bacteria.
Materials and Methods
Case history
The ducklings (A. platyrhynchos) described were part of a study investigating the intracellular signalling mechanisms controlling adaptive cell proliferation and cell differentiation in the glands (Hildebrandt, Citation2001; Müller & Hildebrandt, Citation2003). Over a period of 4 years, more than 1000 duck heads were dissected to obtain glands for biochemical and molecular biology studies. Altogether, nine affected glands originating from eight ducklings were included in the study: one animal had bilaterally affected glands and three ducklings had unilaterally affected glands. In addition, four single glands with lesions, and 10 heads with 20 normal glands of ducklings (14 to 21 days old) were submitted for the investigation (). The affected ducklings were 2 days old (one animal), 9 days old (one animal), 16 days old (one animal) and 17 to 23 days old (five animals), of both sexes. They had been kept in a conventional stable with free access to chicken starter crumbs and drinking water. Some of the ducklings (affected and unaffected) were exposed to 1% NaCl solution instead of their normal drinking water, which is the routine procedure to impose osmotic stress upon these animals and activate their salt glands in vivo. All animals were observed for clinical signs and humanely euthanized.
Table 1. Results of histopathologic and microbiologic investigation of salt glands with and without granulomatous inflammation
Pathology
The salt glands were dissected out after removal of the skin under sterile conditions. Where inflammation was present, one-half of the tissue was fixed by immersion in 4% neutral buffered formalin, processed routinely and embedded in paraffin-wax. Sections were cut at 2 μm, mounted on glass slides and stained with haematoxylin and eosin. Additional sections were stained with Ziehl–Neelsen to detect acid-fast bacteria, and Brown–Hopps was applied for Gram-staining.
Bacteriology
Samples of nasal glands were weighed, crushed and subsequently dissolved and serially diluted in isotonic NaCl saline and were cultured under aerobic conditions on Columbia agar with 5% bovine blood at 37°C for 24 to 48 h. Two of the nine affected glands were submitted to our laboratory as formalin-fixed tissue samples only. The same procedures were undertaken with samples of the soiled litter and the water of the hutch. The heart and lung of the 10 control ducklings were smeared onto the surface of the Columbia agar with 5% bovine blood and cultured aerobically at 37°C for at least 48 h. The total number of colonies was determined and single colonies were picked, and for subcultivation smeared again on Columbia agar with 5% bovine blood. Further biochemical differentiation was performed using the API-STAPH, API-STREP and API-20NE systems (bioMerieux). Salt tolerance of the different isolates was tested on Columbia agar with NaCl concentrations of up to 40% or in thioglycolate medium with variable salt contents and subsequent subculture on Columbia-agar with 5% bovine blood. The BIOLOG system (OXOID) was used for the biochemical fingerprinting of the halotolerant isolates.
Results
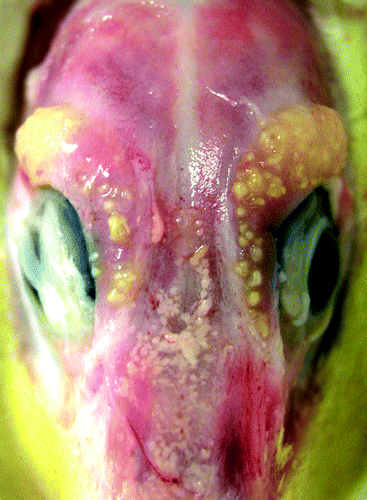
All of the examined 18 animals were bright and alert and exhibited no clinical signs, either before or after exposure to salt water. Macroscopically, the nine affected glands were diffusely and moderately enlarged with numerous, multifocal to coalescing, yellow, slightly elevated nodules of pinpoint size up to 3 mm in diameter admixed with small streaky red areas (). Lesions were detected in ducklings that were 2 to 23 days old. Compared with non-affected glands of animals of the same age and the same drinking regimen, these glands had increased weights of up to 2.5 times the normal.
Figure 1. Bilateral granulomatous adenitis of salt glands, duckling number 4 (A. platyrhynchos), 13 days old. Glands there are focally to partly coalescing within the moderately enlarged and slightly elevated yellow nodules.
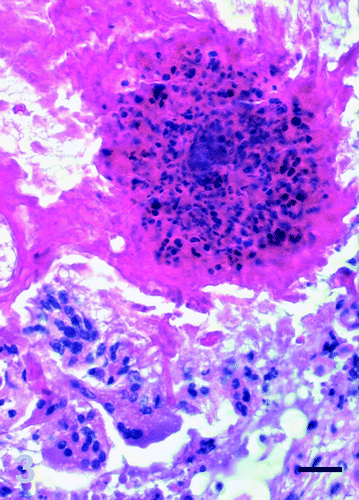
Histologically, approximately 70% of the gland was diffusely and severely disrupted by large, multifocal to coalescing, sharply demarcated areas of granulomatous inflammation that replaced the glandular structure (). The heterophilic granulomas were characterized by central remnants of heteropilic aggregates and clustered bacteria surrounded by numerous multinucleate giant cell, lymphocytes and plasma cells and few heterophils (). There was mild to moderate, diffuse, interstitial oedema with separation of the necrotic glandular lobules by a dense fibrous tissue composed of multiple, slender and spindle-shaped fibroblasts. Multifocally, there was a diffuse interstitial inflammatory infiltration with numerous lymphocytes and plasma cells and scattered heterophils. The majority of the remaining glandular ducts were dilated and obliterated with erythrocytes, hypereosinophilic cellular debris, pyknotic and karyorrhectic nuclei, and some clustered bacteria. Focally, there was evidence of ductal and acinar regeneration characterized by tightly packed, proliferating basophilic epithelial cells with prominent euchromatic nuclei and evidence of ductal squamous metaplasia.
Figure 2. Granulomatous adenitis, salt gland, duckling number 4. The normal glandular architecture is diffusely disrupted and replaced by numerous large and coalescing foci of granulomatous inflammation. Haematoxylin and eosin, bar=500 μm.
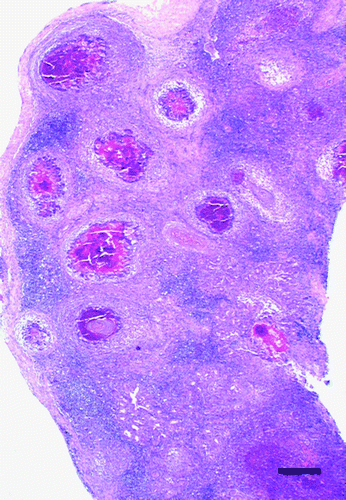
Figure 3. Granulomatous adenitis, salt gland, duckling number 4. Inflammatory foci are composed of central coagulative necrosis with accumulation of numerous degenerate heterophils, surrounded by multinucleate giant cells. Haematoxylin and eosin, bar=25 μm.
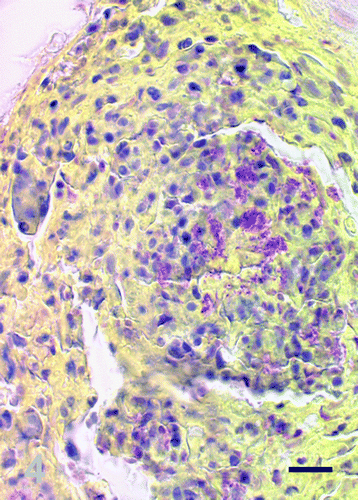
Gram-staining consistently showed clusters of Gram-negative rod-shaped bacteria within the granulomas (). Acid-fast bacteria were not detected by Ziehl–Neelsen staining.
Figure 4. Brown–Hopps Gram-staining, salt gland, duckling number 4. Scattered throughout the necrotic heterophilic debris there are numerous Gram-negative bacteria. Bar=25 μm.
Bacteriology revealed diverse results in the seven affected glands and 20 normal glands. The most consistent finding was the detection of Gram-negative bacteria exclusively in salt glands with granulomas. From four of the affected glands, different strains of Pseudomonas aeruginosa were recovered. From the remaining glands Aeromonas hydrophila could be detected in two glands of a bilaterally affected duck and Proteus mirabilis from one gland. In one animal with unilateral adenitis associated with P. aeruginosa, the same bacterium was isolated from the contralateral unaffected gland. The P. aeruginosa isolates from different animals belonged to at least two biochemically distinct strains, as shown by adipic acid assimilation in the API-System. Testing of the salt tolerance revealed that all P. aeruginosa isolates grew in media containing NaCl up to 7% (w/v).
In all unaffected glands, a mixed culture of Gram-positive bacteria was detectable. An overall bacterial count of up to 105 colony forming units (CFU) per gram of tissue was determined in these glands. To a variable extent and in various combinations, infections with Enterococcus faecium, Enterococcus faecalis, Staphylococcus lentus, Staphylococcus warneri, Staphylococcus simulans, Staphylococcus epidermidis and Micrococcus spp. as well as Corynebacterium sp. were found. Within affected glands, only a few E. faecium and E. faecalis were present.
A mixed culture of 10 to 60 CFU/g tissue Streptococcus spp., Escherichia coli, Corynebacterium spp. and Staphyloccus spp. was isolated from the lungs and hearts of all unaffected animals and four of the affected animals.
Bacteriology of the litter mainly revealed growth of 109 CFU E. coli and 108 CFU α-hemolytic Streptococcus spp. Further differentiation and incubation in thioglycolate media with a NaCl concentration up to 25% (w/v), followed by plating on blood agar, showed growth of halotolerant Micrococcus and Staphylococcus. In the drinking water of the animals, 107 CFU Enterobacteriaceae but no Gram-positive bacteria could be found.
Discussion
To our knowledge, bacterial infections of the nasal glands have not been described previously. In this report, we show an association of severe heterophilic granulomatous inflammation with fibrosis of this gland, with infection by Gram-negative bacteria. P. aeruginosa strains were the most common species found in these animals. We also identified P. mirabilis or A. hydrophila associated with granulomatous adenitis in two ducklings. Unaffected glands of birds younger than 23 days that had no or unilateral lesion in the examined stock were consistently colonized by a mixture of mainly Gram-positive bacteria (e.g. S. lentus, S. warneri, S. epidermidis, S. simulans, Corynebacterium sp. as well as E. faecium and E. faecalis).
Persistent microbes like mycobacteria, fungi, yeasts and, to a lesser degree, foreign bodies are the most common causes of granulomatous inflammation in mammals and birds (Bochsler & Slauson, Citation2002). Nevertheless, Gram-negative bacteria are known to stimulate the formation of heterophilic “acute” granulomas in avian species that might develop into “chronic” granulomas with epitheloid cells, lymphocytes, plasma cells and connective tissue elements (Montali, Citation1988). Well-known diseases include coligranulomatosis in chicken caused by E. coli (Nakamura et al., Citation1985) and Salmonella sp.-associated granulomas in the caecum and liver (Sato et al., Citation1993; Desmidt et al., Citation1997). Less frequently, P. aeruginosa and P. mirabilis have been shown to cause granulomatous infections in ostrich and turkey (Momotani et al., Citation1995; Gomis et al., Citation2002). The main virulence factors of Gram-negative bacteria are their endotoxins. Lipopolysaccharides are part of the outer membrane of Gram-negative bacteria, are released following degradation of the cell wall, and may induce remarkable immunological reactions.
In facilities constantly used for keeping the same species, a stable specific bacterial flora might cause significant problems. In this case, a housing-specific problem can be most probably excluded, since three different Gram-negative species were isolated from diseased animals. Additionally, the isolated Pseudomonas spp. differed in their biochemical characteristics. The diversity of the isolated Gram-negative strains makes it unlikely that the infection spreads by a horizontal transmission. A high Gram-negative bacterial load in the environment and crowding-related conditions were ruled out since only a very mild bacterial load of the litter with Gram-positive Staphylococcus and Micrococcus spp. and no contamination of the drinking water with the relevant Gram-negative bacteria could be detected. In this context, it is noticeable that similar incidences of granulomatous inflammation of salt glands in ducklings are recognized in ducklings obtained from different breeding facilities.
The exact pathogenetic mechanism of infection and disease remains elusive. The pattern of distribution of the granulomas, which was remarkably correlated to the normal anatomic ductular and lobular structure of the gland, makes an ascending infection from the nose through the draining ducts most probable. This hypothesis is supported by the clinical picture and results of bacteriology. No clinical signs of systemic infection with bacteraemia and septicaemia were found. In the lungs and hearts only stress-associated enteric translocation of E. coli and Streptococcus spp. were detected, but none of the granuloma-associated Gram-negative bacteria. The presence of a mixed culture of Gram-positive bacteria without inflammation in the nasal glands and other organs of ducklings is regarded as normal flora of animals in this age group.
The results of our investigations demonstrate a bacterial involvement in the pathogenesis of this multifocal necrotizing and granulomatous adenitis. Whereas a Gram-positive bacterial infection in the salt glands of younger ducklings in this stock could be detected in all examined unaffected glands, the appearance of an inflammatory reaction due to Gram-negative rods was limited to approximately 1% of the animals. However, to fulfil Koch's postulates, infection experiments are needed to verify the infectious character of, and to further characterize infectious agents responsible for, the described lesions.
Translations of the abstract in French, German and Spanish are available on the Avian Pathology website.
Acknowledgments
The authors are grateful for the excellent technical assistance provided by Gabriele Czerwinski, Christine Putzar and Regine Schröder.
Notes
Dedicated to Prof. Dr Dr h.c. mult. Eugen Weiss on the occasion of his 75th birthday.
References
- Bochsler PN Slauson DO (2002) Inflammation and repair of tissue In D.O. Slauson & B.J. Cooper (Eds.) Mechanisms of Disease 3rd edn pp. 159–160 St Louis MO Mosby
- Butler , DG , Youson , JH and Campolin , E . (1991) . Configuration of the medial and lateral segments of duck (Anas platyrhynchos) salt glands . Journal of Morphology , 207 : 201 – 210 .
- Dantzler WH (1989) Comparative Physiology of the Vertebrate Kidney Berlin, Heidelberg, New York, London, Paris, Tokyo Springer Verlag
- Desmidt , M , Ducatelle , R and Haesebrouck , F . (1997) . Pathogenesis of Salmonella enteritidis phage type four after experimental infection of young chickens . Veterinary Microbiology , 56 : 99 – 109 .
- Ernst , SA and Ellis , RA . (1969) . The development of surface specialization in the secretory epithelium of the avian salt gland in response to osmotic stress . Journal of Cellular Biology , 40 : 305 – 321 .
- Gomis , S , Amoako , AK , Ngeleka , AM , Belanger , L , Althouse , B , Kumor , L , Waters , E , Stephens , S , Riddell , C , Potter , A and Allan , B . (2002) . Histopathologic and bacteriologic evaluations of cellulitis detected in legs and caudal abdominal regions of turkeys . Avian Diseases , 46 : 192 – 197 .
- Hildebrandt , J-P . (2001) . Coping with excess salt: adaptive functions of extrarenal osmoregulatory organs in vertebrates . Zoology , 104 : 209 – 220 .
- McLelland , J , Moorhouse , PDS and Pickering , EC . (1968) . An anatomical and histochemical study of the nasal gland of Gallus gallus domesticus . Acta Anatomica , 71 : 122 – 133 .
- Momotani , E , Kiryu , M , Ohshiro , M , Murakami , M , Ashida , Y , Watanabe , S and Matsubara , Y . (1995) . Granulomatous lesions caused by Pseudomonas aeruginosa in the ostrich (Struthio camelus) . Journal of Comparative Pathology , 112 : 273 – 282 .
- Montali , RJ . (1988) . Comparative pathology of inflammation in the higher vertebrates (reptiles, birds and mammals) . Journal of Comparative Pathology , 99 : 1 – 26 .
- Müller , C and Hildebrandt , J-P . (2003) . Salt glands—the perfect way to get rid of too much sodium chloride . Biologist , 50 : 255 – 258 .
- Nakamura , K , Maeda , M , Imada , Y , Imada , T and Sato , K . (1985) . Pathology of spontaneous colibacillosis in a broiler flock . Veterinary Pathology , 22 : 592 – 597 .
- Riddell , C and Roepke , D . (1991) . Inflammation of the nasal gland in domestic turkeys . Avian Diseases , 35 : 982 – 985 .
- Sato , Y , Kumeta , A , Koyama , T , Takada , T , Aoyagi , T , Ichikawa , K , Wada , K , Furuya , T and Tanaka , K . (1993) . An outbreak of Salmonella typhimurium infection in bengalees, a variety of Lonchura striata . The Journal of Veterinary Medical Science , 55 : 1073 – 1076 .
- Schmidt-Nielsen , K . (1960) . The salt-secreting gland of marine birds . Circulation , 21 : 955 – 967 .
- Technau , GE . (1936) . Die Nasendrüse der Vögel. Zugleich ein Beitrag zur Morphologie der Nasenhöhle . Journal of Ornithology , 48 : 511 – 617 .