Abstract
Avian reoviruses that have been shown to be genetically distinct from chicken origin reoviruses were isolated from commercial turkey flocks in the Southeastern US and Texas that were experiencing enteritis. The pathogenesis of these turkey origin reoviruses (TRVs) was evaluated in commercial and specific pathogen free (SPF) turkey poults and SPF chickens. Mortality, clinical disease, gross lesions, microscopic lesions and body weights were observed. TRVs replicated poorly and did not cause disease in chickens. Clinical disease induced by the TRV isolates, characterized by diarrhoea and depression, was mild in both SPF and commercial origin poults. Several TRV isolates caused moderate to severe bursal atrophy in poults. Additionally, each of the TRV isolates caused significant body weight decreases in SPF and/or commercial poults as compared with sham inoculates. Molecular characterization of the isolates revealed that the TRVs and chicken origin reoviruses had identical electropherotype profiles.
Pathogénie des réovirus d'origine dinde chez la dinde et le poulet
Les réovirus aviaires, qui se sont révélés être différents génétiquement des réovirus d'origine poulet (CRVs), ont été isolés de troupeaux commerciaux de dindes qui présentaient de l'entérite, au Sud Est des USA et au Texas. La pathogénie de ces réovirus d'origine dinde (TRVs) a été évaluée chez des dindonneaux SPF et des poulets SPF. Les critères étudiés ont été la mortalité, les symptômes, les lésions macroscopiques et microscopiques ainsi que les poids. Chez le poulet, les TRVs se répliquent faiblement et n'entraînent pas de maladie. Chez les dindonneaux SPF et commerciaux, la maladie clinique induite par les souches de TRV est caractérisée par de la diarrhée et de une dépression peu sévère. Plusieurs souches de TRV ont entraîné une atrophie modérée à sévère de la bourse chez les dindonneaux. De plus chaque souche de TRV a entraîné des pertes de poids, significatives, chez les dindonneaux SPF et/ou commerciaux comparées aux témoins. La caractérisation moléculaire de ces isolats a révélé que les TRVs et les CRVs avaient des profils électrophorétiques identiques.
Pathogenese von Infektionen mit Reovirusstämmen aus Puten in Puten und Hühnern
Aus kommerziellen Putenherden im Südosten und in Texas in den USA mit Durchfallerkrankungen wurden aviäre Reoviren isoliert, die sich genetisch von den Hühnerreoviren (CRVs) unterschieden. Die Pathogenese dieser Putenreoviren (TRVs) wurde in kommerziellen sowie SPF-Putenküken und in SPF-Hühnerküken untersucht. Es wurden Mortalität, klinische Symptome, pathologisch-anatomische und –histologische Befunde und die Körpergewichte ermittelt. In den Hühnerküken vermehrten sich die TRVs nur in geringgradig und riefen keine Krankheitssymptome hervor. Sowohl in den SPF- als auch in den Putenküken kommerzieller Herkunft waren die durch die TRV-Isolate induzierten klinischen Symptome wie Diarrhoe und Störung des Allgemeinbefindens nur schwach ausgeprägt. Einige TRV-Isolate verursachten mittel- bis hochgradige Bursaatrophie in den Putenküken. Außerdem führten alle TRV-Isolate in den SPF-und/oder kommerziellen Putenküken im Vergleich zu scheininfizierten Putenküken zu signifikanten Körpergewichtsreduktionen. Die molekulare Charakterisierung der Isolate zeigte, dass die TRVs und die CRVs identische Elektropherotypenprofile hatten.
Patogénesis de reovirus orginarios de pavos en pavos y pollos
Se aislaron unos reovirus aviares, que se ha demostrado que son genéticamente diferentes a los reovirus que se originan en los pollos (CRVs), de un lote de pavos comerciales del Sudeste de los U.S. y de Texas que presentaban enteritis. La patogénesis de estos reovirus originarios de pavos (TRVs) fue evaluada en pavitos comerciales y SPF, y en pollos SPF. Se evaluaron la mortalidad, la enfermedad clínica, las lesiones macroscópicas y microscópicas y los pesos. Los TRVs se replicaron poco y no causaron enfermedad en pollos. La enfermedad clínica inducida por aislados de TRV, caracterizada por diarrea y depresión, fue leve tanto en pavitos comerciales como en SPF. Varios aislados de TRV causaron atrofia de la bolsa, de moderada a severa, en pavitos. Además, cada uno de los aislados de TRV causó disminución significativa del peso corporal en pavitos SPF y comerciales en comparación con los controles. La caracterización molecular de estos aislados reveló que los TRVs y CRVs presentaban perfiles de electroferotipo idénticos.
Introduction
Clinical disease caused by avian reoviruses has been well described and has a variety of presentations, including viral arthritis, respiratory disease, immunosuppression, malabsorption with stunting and runting, and subclinical infection (reviewed in Rosenberger, Citation2003). While most reports focus on disease in chickens, clinical disease in turkeys has been reported to be similar to what is observed in chickens, and includes viral arthritis (Page et al., Citation1982) and poult enteritis (Dees et al., Citation1972; Simmons et al., Citation1972; Gershowitz & Wooley, Citation1973; Reynolds et al., Citation1987; Lozano et al., Citation1989; Heggen-Peay et al., Citation2002). Historically, it has been assumed that all avian reoviruses from chickens and turkeys are closely related. Recently, however, a group of avian reoviruses that are genetically distinct from chicken origin reoviruses (CRVs) (Kapczynski et al., Citation2002; Sellers et al., Citation2004) have been collected from commercial turkey flocks during episodes of poult enteritis.
The recently described turkey origin reoviruses (TRVs) have 64% nucleotide identity with CRVs in the S3 gene and 77% amino acid identity with the σ2 protein it encodes (Kapczynski et al., Citation2002), whereas among CRVs or among TRVs there is 95% to 100% amino acid identity. No other TRV gene segment sequences have been determined yet; however, based on differential detection by reverse transcription-polymerase chain reaction (RT-PCR)-based methods, it appears that the S1 genome segment that encodes the σ3 protein, which is the cell attachment protein and a neutralizing epitope, is also not conserved between CRVs and TRVs (Spackman et al., Citation2005; unpublished data).
In this study an initial evaluation of the pathogenesis of selected TRV isolates has been performed with commercial origin turkey poults, specific pathogen free (SPF) turkey poults and SPF chickens. Additionally, the genomes of selected TRV isolates were compared with the S1133 CRV strain genome by electropherotyping.
Materials and Methods
Viruses
TRV isolates were originally obtained from commercial turkey flocks experiencing enteritis. Two chicken-origin isolates—S1133 (Van der Heide et al., Citation1974), a low virulent strain, and 1733 (Rosenberger et al., Citation1989), a highly virulent strain—were used as reference isolates. The TRV isolate strains evaluated include NC/85, TX/99, TX/98 (Sellers et al., Citation2004) and NC/98 (Kapczynski et al., Citation2002). Additional turkey origin reoviruses, NC/SEP-R44/03 and NC/SEP-R108/03, were isolated on first passage in Vero cells from intestinal samples of commercial turkeys experiencing enteritis. Each TRV was propagated for no more than two to four passages in Vero cells to exclude turkey astrovirus and turkey coronavirus. The absence of these agents was confirmed by real-time RT-PCR as described previously (Spackman et al., Citation2005).
Preparation of samples for virus isolation
Tissues were processed for virus isolation as follows: approximately 1 g intestinal tissue was homogenized with a fast-prep glass bead homogenizer (Savant Instruments, Holbrook, New York, USA) in 0.5 ml sterile 50% Dulbecco's modified Eagle's medium (DMEM)/50% F12 medium (Mediatech, Herndon, Virginia, USA). Samples were clarified by centrifugation for 15 min at 15 000×g. Supernatants were decanted, 0.8 µM filtered and treated with 10 000 IU/ml penicillin, 2000 µg/ml streptomycin and 20 µg/ml amphotericin B (Mediatech) for 30 to 45 min at room temperature prior to inoculation into cell culture.
Cloacal swabs were prepared by centrifuging 500 µl swab material for 15 min at 14 000×g. The supernatant was removed and incubated with 10 000 IU/ml penicillin, 2000 µg/ml streptomycin and 20 µg/ml amphotericin B (Mediatech) for 30 to 45 min at room temperature prior to inoculation into cell culture.
Virus isolation in Vero cells
A continuous cell line, Vero cells, were used for virus isolation because previous work with these strains had shown that Vero cells were equivalent to chicken embryo liver cells for the isolation of these particular isolates (unpublished data). Vero cells were grown in 96-well cell culture plates in 50% DMEM/50% F12 (Mediatech), 8% foetal bovine serum (Mediatech) and 100 IU/ml penicillin, 100 µg/ml streptomycin, 0.25 µg/ml amphotericin B (Mediatech), until a monolayer density of between 70% and 80% confluence was achieved. The medium was removed and 100 µl processed sample was inoculated. Each sample was plated in duplicate. Plates were incubated for 30 to 60 min to allow the inoculum to adsorb, then maintenance medium (the same formulation as growth medium but with only 2% foetal bovine serum) was added and the plates were incubated for 5 to 6 days at 37°C, 5% CO2 and observed daily for cytopathic effects (CPE). Samples from the experiment with SPF turkeys were passed three times; however, since no additional isolations occurred after one passage, samples from subsequent experiments with commercials turkeys and chickens were passed only once and infection status was confirmed by real-time RT-PCR. Wells exhibiting CPE were considered positive. Selected wells exhibiting CPE were confirmed to be virus-positive by real-time RT-PCR, and wells not exhibiting CPE were confirmed to be negative by real-time RT-PCR (Spackman et al., Citation2005).
Virus neutralization assay
Serum was collected from five poults prior to inoculation and from all remaining birds at 28 days post inoculation (d.p.i.). Sera from poults collected at inoculation were tested by virus neutralization assay to determine the levels of maternal antibody to the inoculated viruses. Briefly, two-fold serial dilutions of heat-inactivated serum were made in 50% DMEM/50% F12 media (Mediatech) in a microtitre plate. An equal volume of the appropriate virus at a titre of approximately 103 median tissue culture infectious doses (TCID50) was added to each well and incubated overnight at 4°C, then 100 µl material from each well was transferred to the corresponding well of another microtitre plate with Vero cells, prepared as described earlier, at a density of between 70% and 80% confluence. Virus neutralization plates were incubated at 37°C, 5% CO2 and observed daily for CPE. Virus neutralization titres were evaluated when a simultaneous back-titration showed CPE to a dilution of 10−3. The titre was recorded as the reciprocal of the highest dilution that did not display CPE.
Pathogenesis in commercial poults
Poults were obtained at 3 days of age (referring to the first 24 h after hatch as 1 day of age) from a commercial breeder source, individually tagged and divided into groups of 15. Each group was inoculated (at 3 days of age) with 103.5 (TCID50) of the appropriate isolate in 0.2 ml () by the oral route with an oral gavage needle, and housed in Horsfal isolators with ad libitum access to feed and water. One group received 0.2 ml sterile 50% DMEM/50% F12 medium and served as sham inoculated controls. At 2, 4, 7, 11 and 14 d.p.i., all poults were weighed, cloacal swabs were collected from five birds in each group, and two birds from each group were killed and examined post mortem. The caeca, duodenum, gastrocnemius tendon, liver, spleen, bone marrow, thymus and bursa were collected and fixed in 10% neutral buffered formalin, paraffin embedded and stained with haemotoxylin and eosin by standard methods for microscopic evaluation.
Table 1. Genetic groupings of turkey reovirus isolates used in this study
Virus isolation was attempted in Vero cells, using cloacal swabs and pooled intestinal tissue (caeca, jejunum and ileum). Real-time RT-PCR (Spackman et al., Citation2005) was also used to detect virus in cloacal swabs, intestinal and bursal tissue. Pre-inoculation and post-inoculation sera were tested for antibody with a commercial avian reovirus antibody enzyme-linked immunosorbent assay (ELISA) (IDEXX, Inc., Westbrook, Maine, USA) in accordance with the manufacturer's instructions.
Pathogenesis in SPF poults
SPF Small White Beltsville turkeys were obtained at 2 days of age from Southeast Poultry Research Laboratory flocks. Among other agents, these flocks are monitored for turkey astrovirus, turkey coronavirus, haemorrhagic enteritis virus and avian reovirus. Birds were divided into groups of 20, individually tagged, weighed and housed in Horsfal isolators with ad libitum access to feed and water.
Each group was inoculated with 0.2 ml appropriate virus at 2 days of age () (approximately 104.0 TCID50/bird) by the intratracheal and oral routes (0.1 ml each route). Sham inoculated control birds were inoculated with 0.2 ml sterile 50% DMEM/50% F12 medium (Mediatech).
At 2, 5, 7 and 9 d.p.i. all poults were weighed and two birds from each group were killed. The bursa, thymus, spleen, liver, caeca, jejunum, ileum, liver and heart were collected and processed for microscopic evaluation as previously described. Virus isolation and real-time RT-PCR (Spackman et al., Citation2005) was used to detect virus in intestinal tissue. Birds dying during the experiment were examined and gross lesions recorded.
Pre-inoculation and post-inoculation sera were tested for antibody by ELISA as already described.
Replication in chickens
Six TRV isolates were evaluated for their ability to replicate in and cause disease in chickens (). The 1733 isolate was used as a reference CRV isolate.
SPF white-rock chickens were obtained from Southeast Poultry Research Laboratory flocks at 2 days of age, individually tagged and divided into nine groups of 10 birds each. Among other agents these flocks are monitored for the following agents; avian reovirus, adenovirus, infectious bursal disease virus and chicken anaemia virus. Chickens were housed in Horsfal isolators with ad libitum access to feed and water. Each group was inoculated at 2 days of age with 0.2 ml virus (approximately 104.5 TCID50/ml bird) by both the intratracheal and oral routes. Control birds were inoculated with 0.2 ml sterile 50% DMEM/50% F12 media (Mediatech). The caeca, ileum, jejunum, liver, spleen, bursa, thymus, heart and gastrocnemius tendons were collected from two birds in each group at 2, 4 and 7 d.p.i. for microscopic evaluation as described for the poult experiments. Cloacal swabs were collected at the same time. Cloacal swabs and intestinal tissue (pooled caeca, ileum and jejunum) were processed for virus isolation in Vero cells and by real-time RT-PCR.
Birds dying during the experiment were examined and gross lesions recorded. Serum was collected from birds remaining at 28 d.p.i. for antibody detection by ELISA as described earlier.
Electropherotype analysis of avian reovirus genomic RNA
Each isolate of avian reovirus was propagated in Vero cells until a minimum of 80% CPE had been achieved. All cell material was removed, pelleted by centrifugation and stored at −80°C. Total RNA was extracted from infected cell pellets using TRIzol LS reagent (Invitrogen Inc., Carlsbad, California, USA) according to the manufacturer's recommendations. Total RNA was reconstituted in nuclease-free water and ssRNA was precipitated in a final concentration of 2 M LiCl overnight at 4°C. The ssRNA was pelleted by centrifugation and viral dsRNA was subsequently precipitated from the supernatant in a final concentration of 4 M LiCl at −20°C for 1 h. Purified viral dsRNA was reconstituted in nuclease-free water and stored at −20°C. Approximately 1 µg dsRNA from each isolate was separated into L, M, and S genome segments on a 2% agarose gel, stained with ethidium bromide, and visualized with an ultraviolet transilluminator.
Statistical analysis
The statistical significance (P<0.05) of body weight differences between the virus inoculated groups and sham inoculated poults was determined by one-way repeated-measures analysis of variance (Dunnett's method) (SigmaStat 3.0; Systat Software, Richmond, California, USA).
Results
Pathogenesis in commercial poults
Clinical signs included mild diarrhoea and depression in the NC/SEP-R44/03 inoculated group at 7 d.p.i. and in the NC/85 group at 11 d.p.i. Two birds inoculated with NC/85 were severely depressed and had ruffled feathers from days 4 to 7 post-inoculation when they died. One poult had severe ascites; no gross lesions were observed in the other poult. Mortality was low; in addition to the two birds previously described, two sham inoculates were dead at 7 d.p.i. and two TX/98 inoculates were dead at 2 d.p.i. Clinical signs were not observed in groups inoculated with S1133, 1733, NC/98, TX/99 or NC/SEP-R108/03. No gross lesions were observed in any treatment groups.
Poults were weighed at 2, 4, 7 and 11 d.p.i. At 2 d.p.i. the NC/85 group had higher body weights than the rest of the groups. At 4 d.p.i. the S1133, NC/SEP-R44/03, TX/98 and TX/99 inoculated groups had statistically significant (P<0.05) decreases in group mean body weights compared with the sham inoculates, and at 7 d.p.i. the TX/98 group had significantly lower body weights. At 11 d.p.i. mean body weights in all treatment groups were statistically the same.
No microscopic lesions were observed in tissues from sham inoculated poults or poults inoculated with reovirus isolates S1133 and 1733. Microscopic lesions in the bursa of Fabricius were observed in poults inoculated with the TRV isolates. Moderate to severe follicular lymphocyte depletion was observed in poults inoculated with NC/SEP-R44/03 at 2, 4, 7, and 11 d.p.i., and mild heterophilic infiltration was present at 2 and 4 d.p.i. At 2, 4, and 7 d.p.i. both cortical and medullary regions of the bursa had severe lymphoid depletion with increased fibroplasia between the follicles. At 11 and 14 d.p.i. lymphoid depletion of the bursa was mild to moderate, follicle size was not uniform and intrafollicular cysts were present. Poults inoculated with this strain also had lymphocytic infiltrates in the liver and pancreas at 7 d.p.i. Mild increase of crypt depth in the intestine was present at 7 d.p.i.
Poults inoculated with reovirus isolates NC/98, TX/98, TX/99, and NC/85 had mild to severe lymphocyte depletion of the bursa at 4 and 7 d.p.i. Lymphocyte infiltrates were present at 7 d.p.i. in the livers of all poults inoculated with NC/98, TX/98, TX/99, and NC/85. Lymphocytic infiltrates were also present in the pancreas of poults inoculated with NC85 and TX/98 at 11 and 14 d.p.i. Intestinal lesions were minimal and consisted of mild crypt hyperplasia, with lymphocyte, heterophil and eosinophil infiltration of the lamina propria and submucosa of the small intestine at 11 and 14 d.p.i.
Virus was not detected in intestinal tissue or cloacal swabs by virus isolation or real-time RT-PCR from any treatment group at any sample time. Virus was detected in the bursa by real-time RT-PCR at 2 and 4 d.p.i. in NC/SEP-R44/03 and TX/99 inoculated poults. Antibody was detected in serum from poults in each virus inoculated group. Sham inoculates and poults bled prior to inoculation were negative for reovirus antibody.
Pathogenesis in SPF poults
Clinical signs in TRV inoculated SPF poults were generally mild or absent, although diarrhoea was noted in the NC/98 and TX/98 treatment groups on day 7 post-inoculation. No gross lesions were observed.
Mortality was low, except at day 2 post-inoculation when between one and seven poults died in all treatment groups, including the sham inoculates. This mortality was probably non-specific. At 5 d.p.i. there were four dead birds in the NC/98 group, three poults in the NC/SEP-R44/03 group and one poult in the 1733 group. At 7 d.p.i. one 1733 inoculated poult died.
Statistically significant (P<0.05) decreases in treatment group mean body weights were observed in all virus inoculated groups at 5 d.p.i. except for NC/85. Body weights in only the NC/SEP-R44/03 and 1733 inoculated groups were significantly lower at 7 d.p.i. At 9 d.p.i. all virus inoculated group body weight means were significantly lower than in the sham inoculates.
No microscopic lesions were observed in the tissues of sham inoculated poults. Poults inoculated with reovirus isolate 1733 had lymphocytic infiltrates in the liver at 5 and 9 d.p.i. but no other microscopic lesions.
Poults inoculated with reovirus isolate NC/SEP-R44/03 had severe bursal atrophy at 2, 5, 7, and 9 d.p.i. At 2 d.p.i. there was severe lymphocytolysis characterized by pyknotic and karryorhectic nuclei in the bursa. At 5, 7, and 9 d.p.i. there was diffuse lymphoid depletion in bursal follicles with foamy macrophages within the medullary region. Fibroplasia between bursal follicles was also increased and multiple large intrafollicular cysts were present (). Mild crypt hyperplasia with heterophil infiltration was observed in the duodenum and jejunum at 2 and 5 d.p.i. Lymphocytic infiltrates were present in the liver at 5, 7, and 9 d.p.i., in the pancreas at 7 d.p.i., and in the heart at 9 d.p.i.
Figure 1. Photomicrographs (20× magnification) of the bursa of Fabricius from SPF poults 7 d.p.i. with (1a) sham inoculum, (1b) NC/SEP-R44/03, (1c) TX/98, and (1d) NC/98.
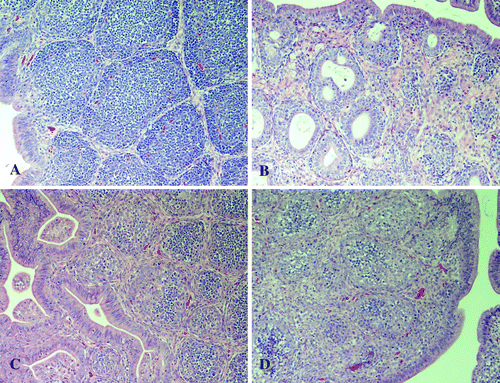
Poults inoculated with the NC/98, TX/98, and NC/85 TRV isolates had moderate to severe bursal lymphoid depletion at 5, 7, and 9 d.p.i. (). Lymphocytic infiltrates were observed in the liver at 5, 7, and 9 d.p.i. and in the pancreas at 9 d.p.i.
Virus was detected by real-time RT-PCR in intestinal tissues at 2 d.p.i. in TX/98, NC/SEP-R44/03 and NC/98 inoculated groups, at 5 d.p.i. in NC/98 inoculated poults and at 7 d.p.i. in 1733 inoculated poults. Virus isolation attempts were negative for all samples. Antibody was detected in serum from all reovirus inoculated groups. Sham inoculates and poults bled prior to inoculation were antibody negative.
Virus replication in chickens
The 1733 isolate was used as a challenge control and produced severe depression in all inoculated birds. All 1733 inoculated chickens were dead by 11 d.p.i. There were no clinical signs or gross lesions observed in chickens inoculated with any of the six TRV isolates. One chicken inoculated with a TRV (NC/SEP-R108/03) was dead at 7 d.p.i.
With the exception of intestinal tissue from two chickens inoculated with 1733 collected 7 d.p.i., virus was not detected in either intestinal tissue or cloacal swabs by virus isolation or real-time RT-PCR at any time.
No microscopic lesions were present in the tissues of sham inoculated chickens or chickens inoculated with reovirus isolates NC/SEP-R44/03, NC/98, TX/99, and NC/SEP-R108/03. However, chickens inoculated with reovirus isolates NC/85 and TX/98 had lymphocytic infiltrates in the liver and pancreas at 2, 4 and 7 d.p.i. Isolate 1733 produced lesions in chickens consistent with those previously reported for CRV in chickens (Rosenberger, Citation2003).
Antibody was not detected by ELISA in serum collected from any chickens inoculated with any of the TRV isolates. Serum from 1733 inoculated chickens was antibody positive.
Electropherotype analysis of avian reovirus genomic RNA
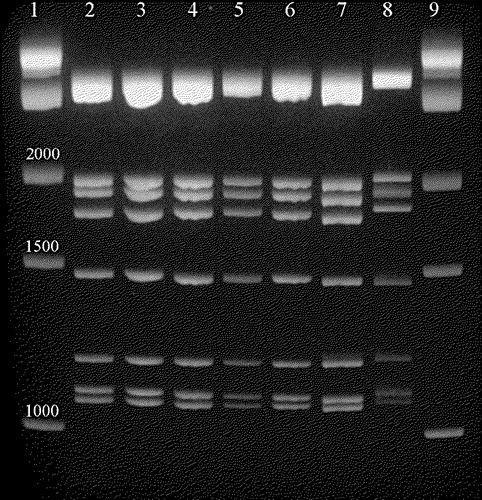
The electropherotypes of the genomes of six TRV isolates were compared with S1133, a representative CRV. The electrophoretic patterns were the same for all isolates examined ().
Figure 2. Electropherotype comparing selected turkey-origin and chicken-origin reovirus dsRNA genomes. Lanes 1 and 9, 1 kb DNA ladder (1 kb, 1.5 kb and 2 kb bands are indicated); lane 2, S1133 genome; lane 3, 1733 genome; lane 4, TX/98 genome; lane 5, NC/85 genome; lane 6, TX/99 genome; lane 7, NC/98 genome; lane 8, NC/SEP-R44/03 genome.
Discussion
Avian reoviruses are orthoreoviruses and contain 10 genome segments (Schnitzer, Citation1985; Varela & Benavente, Citation1994). Of these genome segments, sequence data have only been reported for the S3 segment of TRVs (Kapczynski et al., Citation2002; Sellers et al., Citation2004). The S3 segment encodes the σ2 protein. Although the function of the σ2 protein is currently not clear, it has dsRNA binding regions and is thought to be a capsid protein with group-specific epitopes (Kapczynski et al., Citation2002). Much more sequence data are available for CRVs, where all four S gene segments have been sequenced from numerous isolates (Yin et al., Citation1997; Liu & Huang, Citation2001; Kant et al., Citation2003).
Although sequence data for the S1 gene segment of TRV have not been reported, numerous RT-PCR primers based on the CRV S1 gene sequence do not amplify the S1 segment of some TRVs (Spackman et al., Citation2005; unpublished data), indicating that the S1 gene sequence of TRV is divergent from CRVs in at least some of the isolates. The fact that some TRV S1 genes can be detected by the CRV S1-specific primers suggests that CRVs and TRVs may not exist as truly distinct virus families, and/or may be able to reassort, which is an important mechanism for segmented virus evolution and has been reported to occur among mammalian reoviruses (reviewed in Joklik & Roner, Citation1995). As an initial evaluation of the role these genes may have in host range restriction and disease induction, we included isolates with each of the possible S1 and S3 gene combinations described ().
Reovirus infections in turkeys, often in flocks experiencing enteritis, have been reported previously (Simmons et al., Citation1972; Reynolds et al., Citation1987; Lozano et al., Citation1989; Heggen-Peay et al., Citation2002). However, the role of reovirus in disease induction has not been clearly established as the virus can be found in specimens from healthy flocks and it is not uncommon to detect other enteric viruses in the same specimens (Dees et al., Citation1972).
The results of this study indicate that TRVs are probably not a primary cause of poult enteritis or other disease, as clinical signs in poults were minimal, gross lesions were absent and microscopic lesions in the intestines were mild and non-specific in both commercial origin and SPF poults. Furthermore, the ability of these viruses to replicate in intestinal tissue is poor, as evidenced by only rare detection of virus in intestinal tissue after experimental inoculation, which differs from some previous reports where reovirus replicated well in the intestine (Simmons et al., Citation1972; Nersessian et al., Citation1985) and may be a strain-specific characteristic.
Although the role of TRVs as primary pathogens in poult enteritis is unlikely, TRVs may contribute to poult enteritis, and perhaps other diseases, as a predisposing agent. Based on the severe bursal atrophy caused by the TRV isolates, it is probable that poults infected at a young age would experience transient and possibly permanent immunosuppression. Induction of immunosuppression at an early age could increase susceptibility to infection and disease caused by other infectious agents. Future work will evaluate the effect of TRVs on the immune system.
Another notable result of TRV infection was decreased body-weight gain. This effect is consistent with the ability of avian reoviruses to cause stunting, runting and malabsorption, (reviewed by Rosenberger, Citation2003), and in commercial turkeys most of the economic loss from poult enteritis comes from decreases in production where the birds do not grow to the weights expected by their genetic potential. Whether the decreases in body-weight gain observed here are related to this aspect of poult enteritis or stunting in commercial turkeys is not clear at this time. Another possibility is that other infectious agents exacerbate the weight-gain depression caused by TRVs during episodes of poult enteritis. The long-term effect of TRVs on weight gain and the mechanism involved also needs further evaluation.
None of the TRV isolates caused disease in chickens and the ability of the TRV isolates to replicate in chickens was poor or absent. Chickens failed to develop detectable antibody and virus was not detected in intestinal tissues at any time, indicating that the virus did not replicate or replicated to a level below our ability to detect them in this tissue. The only indication that replication may have occurred is based on minimal, non-specific microscopic lesions in the liver and pancreas in NC/85 and TX/98 inoculated chickens. Interestingly, both of these isolates have S1 gene segments similar to CRV isolates.
The S1 genome segment encodes the σC protein, the capsid protein that is the major antigenic determinant and the cell attachment protein, and may contribute to host restriction (Martinez-Costas et al., Citation1997; Bodelon et al., Citation2001). If this is the case, the host restriction must be one way as CRV isolates can replicate in turkeys; however, disease severity and presentation is not equivalent to what is produced in chickens, as evidenced by only mild disease in turkeys inoculated with the 1733 isolate. Similar results have been shown previously with viral arthritis inducing strains (al Afaleq & Jones, Citation1989). Additionally, all TRV and CRV isolates replicate in Vero cells, a mammalian cell line, illustrating that numerous undefined factors are involved.
Since avian reovirus isolates with the TRV type genes have been identified from as early as 1985 (i.e. the NC/85 isolate) (Sellers et al., Citation2004), these viruses are not a newly introduced type of virus and have probably been co-circulating and possibly re-assorting with CRVs. Additionally, because these TRVs and CRVs have identical electropherotypes, similar morphology by electron microscopy and antibody to TRVs can be detected by the commercial ELISA used in this report, it is probable that they have been previously been identified as CRVs. Previous work with TRVs has not included genetic characterization, which may be a more accurate measure of relatedness among avian reoviruses than pathogenesis tests, serological tests and electropherotyping. Until further molecular characterization of avian reoviruses in general is completed, it is not clear whether these TRVs represent a variant group of CRVs or are truly distinct, but it is clear that avian reoviruses constitute a diverse group of viruses biologically, pathogenically and molecularly.
Translations of the abstract in French, German and Spanish are available on the Avian Patholgy website.
Acknowledgments
The authors would like to acknowledge Scott Lee and Dawn Williams-Coplin for technical assistance with this paper. They would also like to thank the turkey health professionals who have provided expertise and samples. This work was supported by USDA CRIS project #6612-32000-036.
Mention of trade names or commercial products in this manuscript is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture.
References
- al Afaleq , A.I. and Jones , R.C. 1989 . Pathogenicity of three turkey and three chickens reoviruses for poults and chicks with particular reference to arthitis/tenosynovitis . Avian Pathology , 18 : 433 – 440 .
- Bodelon , G. , Labrada , L. , Martinez-Costas , J. and Benavente , J. 2001 . The avian reovirus genome segment S1 is a functionally tricistronic gene that expresses one structural and two nonstructural proteins in infected cells . Virology , 290 : 181 – 191 .
- Dees , T.A. , Wooley , R.E. and Gratzek , J.B. 1972 . Infectious enteritis of turkeys: pathogenicity of bacteria-free filtrates and a viral agent isolated from turkeys with infectious enteritis . American Journal of Veterinary Research , 33 : 165 – 170 .
- Gershowitz , A. and Wooley , R.E. 1973 . Characterization of two reoviruses isolated from turkeys with infectious enteritis . Avian Diseases , 17 : 406 – 414 .
- Heggen-Peay , C.L. , Cheema , M.A. , Ali , R.A. , Schat , K.A. and Qureshi , M.A. 2002 . Interactions of poult enteritis and mortality syndrome-associated reovirus with various cell types in vitro . Poult Science , 81 : 1661 – 1667 .
- Joklik , W.K. and Roner , M.R. 1995 . What reassorts when reovirus genome segments reassort? . Journal of Biological Chemistry , 270 : 4181 – 4184 .
- Kant , A. , Balk , F. , Born , L. , van Roozelaar , D. , Heijmans , J. , Gielkens , A. and ter Huurne , A. 2003 . Classification of Dutch and German avian reoviruses by sequencing the sigma C protein . Veterinary Research , 34 : 203 – 212 .
- Kapczynski , D.R. , Sellers , H.S. , Simmons , V. and Schultz-Cherry , S. 2002 . Sequence analysis of the S3 gene from a turkey reovirus . Virus Genes , 25 : 95 – 100 .
- Liu , H.J. and Huang , P.H. 2001 . Sequence and phylogenetic analysis of the sigmaA-encoding gene of avian reovirus . Journal of Virological Methods , 98 : 99 – 107 .
- Lozano , L.F. , Bickford , A.A. , Castro , A.E. , Swartzman-Andert , J. , Chin , R. , Meteyer , C. , Cooper , G. , Reynolds , B. and Manalac , R.L. 1989 . Association of Reoviridae particles in an enteric syndrome of poults observed in turkey flocks during 1988 . Journal of Veterinary Diagnostic Investigation , 1 : 254 – 259 .
- Martinez-Costas , J. , Grande , A. , Varela , R. , Garcia-Martinez , C. and Benavente , J. 1997 . Protein architecture of avian reovirus S1133 and identification of the cell attachment protein . Journal of Virology , 71 : 59 – 64 .
- Nersessian , B.N. , Goodwin , M.A. , Page , R.K. and Kleven , S.H. 1985 . Studies on orthoreoviruses isolated from young turkeys. II. Virus distribution in organs and serological response of poults inoculated orally . Avian Diseases , 29 : 963 – 969 .
- Page , R.K. , Fletcher , O.J. Jr and Villegas , P. 1982 . Infectious tenosynovitis in young turkeys . Avian Diseases , 26 : 924 – 927 .
- Reynolds , D.L. , Saif , Y.M. and Theil , K.W. 1987 . Enteric viral infections of turkey poults: incidence of infection . Avian Diseases , 31 : 272 – 276 .
- Rosenberger , J.K. 2003 . “ Reovirus infections ” . In Diseases of Poultry , 11th edn , Edited by: Saif , Y.M. , Barnes , H.J. , Glisson , J.R. , Fadly , A.M. , McDougal , L.R. and Swayne , D.E. 283 – 298 . Ames : Iowa State University Press .
- Rosenberger , J.K. , Sterner , F.J. , Botts , S. , Lee , K.P. and Margolin , A. 1989 . In vitro and in vivo characterization of avian reoviruses. I. Pathogenicity and antigenic relatedness of several avian reovirus isolates . Avian Diseases , 33 : 535 – 544 .
- Schnitzer , T.J. 1985 . Protein coding assignment of the S genes of the avian reovirus S1133 . Virology , 141 : 167 – 170 .
- Sellers , H.S. , Linnemann , E.G. , Pereira , L. and Kapczynski , D.R. 2004 . Phylogenetic analysis of the sigma 2 protein gene of turkey reoviruses . Avian Diseases , 48 : 651 – 657 .
- Simmons , D.G. , Colwell , W.M. , Muse , K.E. and Brewer , C.E. 1972 . Isolation and characterization of an enteric reovirus causing high mortality in turkey poults . Avian Diseases , 16 : 1094 – 1102 .
- Spackman , E. , Kapczynski , D.R. and Sellers , H.S. 2005 . Multiplex real-time reverse transcription-polymerase chain reaction for the detection of three viruses associated with poult enteritis complex: turkey astrovirus turkey coronavirus, and turkey reovirus . Avian Diseases , 49 : 86 – 91 .
- Van der Heide , L. , Geissler , J. and Bryant , E.S. 1974 . Infectious tenosynovitis: serologic and histopathologic response after experimental infection with a Connecticut isolate . Avian Diseases , 18 : 289 – 296 .
- Varela , R. and Benavente , J. 1994 . Protein coding assignment of avian reovirus strain S1133 . Journal of Virology , 68 : 6775 – 6777 .
- Yin , H.S. , Shieh , H.K. and Lee , L.H. 1997 . Characterization of the double-stranded RNA genome segment S3 of avian reovirus . Journal of Virological Methods , 67 : 93 – 101 .