Abstract
One-day-old specific pathogen free White Leghorn chicks were vaccinated with live avian pneumovirus (APV) vaccine, live Newcastle disease virus (NDV) vaccine or both. At intervals up to 28 days after vaccination, distribution of the virus in the tissues was studied, together with humoral and mucosal antibody responses in lachrymal fluid and tracheal washes. APV vaccine was detected for almost twice as long in the dual vaccinates as in the single vaccinates. Higher numbers of isolations of ND virus vaccine were obtained from the dual rather than the single vaccinates at 7 days post-vaccination but the reverse occurred at 14 days. APV serum antibodies were significantly lower in the dual rather than the single vaccinates. However, there were similar levels of local APV-specific IgA in the lachrymal fluids of both single and dual APV vaccinates. NDV serum antibody titres in the dual vaccinates were significantly higher than in the singly NDV-vaccinated chickens. It appears that simultaneous vaccination of chicks with live APV and NDV vaccines causes temporary suppression of APV vaccine proliferation and reduces humoral antibody responses to it, although the antibody response to NDV is enhanced.
Interaction entre les vaccins à virus vivants de la pneumovirose aviaire et de la maladie de Newcastle chez des poulets exempts de microorganismes pathogènes spécifiés
Des poussins White Leghorn, exempts de microorganismes pathogènes spécifiés, âgés d'un jour, ont reçu un vaccin à virus vivant de la pneumovirose aviaire (APV) ou un vaccin à virus vivant de la maladie de Newcastle (ND) ou les deux. A intervalles réguliers, jusqu'à 28 jours après vaccination, la distribution du virus dans les tissus a été étudiée ainsi que les réponses en anticorps au niveau humoral et au niveau muqueux dans le liquide lacrymal et dans les lavages trachéaux. Le vaccin APV a été détecté presque deux fois plus longtemps chez les animaux ayant reçu les deux vaccins que chez ceux n'ayant reçu qu'un vaccin. Sept jours après vaccination, un nombre supérieur d'isolements de NDV vaccinal a été obtenu à partir des animaux ayant reçu les deux vaccins comparés à ceux ayant reçu un vaccin, mais à 14 jours l'inverse a été observé. Les anticorps sériques APV ont été significativement inférieurs chez les animaux ayant reçu les deux vaccins. Cependant, des niveaux similaires d'IgA spécifiques d'AVP ont été enregistrés dans le liquide lacrymal des animaux ayant reçu un ou deux vaccins. Les titres des anticorps sériques NDV chez les animaux ayant reçu les deux vaccins ont été significativement supérieurs comparés à ceux n'ayant reçu qu'un vaccin. Il apparaît que la vaccination simultanée des poussins avec les vaccins vivants APV et NDV entraîne une suppression temporaire de la prolifération du vaccin APV et en réduit la réponse immunitaire humorale, bien que la réponse en anticorps NDV soit accrue.
Interaktion zwischen Lebendvirusvakzinen mit aviärem Pneumovirus und dem Virus der Newcastle-Krankheit in spezifiziert pathogenfreien Hühnern
Spezifiziert pathogenfreie weiße Leghorn-Eintagsküken wurden entweder mit einer Lebendvirusvakzine mit aviärem Pneumovirus (AVP) oder mit dem Virus der Newcastle-Krankheit (NKV) oder mit beiden Vakzinen geimpft. In Intervalen bis zum 28. Tag post vaccinationem wurde die Virusverteilung im Körper sowie die humorale und lokale Immunantwort in der Tränen- und Trachealspülflüssigkeit untersucht. Das APV-Vakzinevirus wurde nach der Doppelvakzination beinahe zweimal so lang nachgewiesen wie in der Einfachvakzination. Am 7. Tag post vaccinationem lag die Anzahl der Isolierungen von NK-Vakzinevirus bei den Doppelvakzinierten über der der Einfachvakzinierten, was sich am 14. Tag umkehrte. Die Serumantikörper gegen APV waren in der doppelt vakzinierten Gruppe signifikant niedriger als in der einfach vakzinierten. Die lokalen APV-spezifischen IgA in den Tränenflüssigkeiten waren jedoch in den einfach und doppelt Vakzinierten auf ähnlicher Höhe. Die Serumantikörpertiter gegen NKV waren bei den doppelvakzinierten signifikant höher als bei den einfach vakzinierten Küken. Es scheint, dass die simultane Vakzination von Hühnerküken mit APV- und NKV-Lebendvirusvakzinen eine temporäre Suppression der Proliferation von APV-Vakzinevirus verursacht und die humorale Immunantwort gegen es reduziert, obwohl die Antikörperantwort auf NKV verstärkt ist.
Interacción entre las vacunas vivas de pneumovirus aviar y de enfermedad de Newcastle en pollos libres de patógenos específicos
Pollos White Leghorn libres de patógenos específicos de un día de edad fueron vacunados con vacuna viva de pneumovirus aviar (APV), con una vacuna viva de enfermedad de Newcastle (ND) o con ambas vacunas. Se estudiaron la distribución del virus en tejidos, junto con la respuesta de anticuerpos humorales y de mucosa en fluido lacrimal y en lavados traqueales a diferentes intervalos, hasta 28 días tras la vacunación. La vacuna de APV se detectó casi el doble, tanto en la vacunación doble como en la única. Se obtuvieron altos números de aislamientos de vacuna de NDV de la vacunación dual a los 7 días post-vacunación comparados con la vacunación única, pero el contrario ocurrió a los 14 días. Los anticuerpos séricos frente a APV fueron significativamente menores en la vacunación dual en comparación con la única. Aún así, se encontraron niveles similares de IgA específicas de APV en fluidos lacrimales de los vacunados con una sóla vacuna de APV o con la vacunación dual. Los títulos de anticuerpos séricos frente a NDV en los vacunados con las dos vacunas fueron significativamente mayores en los pollos vacunados con una sola vacuna de NDV. Parece que la vacunación simultánea de pollos con vacunas vivas de APV y NDV causa una supresión temporal de la proliferación de la vacuna de APV y reduce la respuesta de anticuerpos humorales, aunque mejora la respuesta frente a NDV.
Introduction
Avian pneumoviruses (APVs) cause turkey rhinotracheitis in turkeys and have been associated with swollen head syndrome in chickens (Cook, Citation2000; Cook & Cavanagh, Citation2002). Newcastle disease virus (NDV) caused by avian paramyxovirus serotype 1 (Aldous & Alexander, Citation2001) occurs throughout the world in poultry and is frequently associated with high mortality and morbidity (Alexander & Jones, Citation2001; Alexander, Citation2003).
Live vaccines are used in chickens for protection against NDV (Meulemans, Citation1988; Gallili & Ben-Nathan, Citation1998; Alexander & Jones, Citation2001; Cserep, Citation2001) and APV (Cook et al., Citation1989; Williams et al., Citation1991; Alexander & Jones, Citation2001). In some instances, other live vaccines are given concurrently with NDV vaccines (Gallili & Ben-Nathan, Citation1998). Whenever a new vaccine is introduced into a regular vaccination programme the question arises as to its compatibility with other vaccines already in the protocol. In previous studies, it has been demonstrated that live IBV vaccines interfere with the efficacy of NDV vaccines in chickens (Hanson et al., Citation1956; Thornton & Muskett, Citation1975), and Cook et al. (Citation2001) demonstrated that it also interferes with live APV vaccine persistence and the ability to induce humoral antibodies in chickens.
This paper describes a study of the interaction between live APV and NDV vaccine viruses when they were administered simultaneously to chicks. The vaccine viruses’ distribution, local and humoral immune responses were recorded.
Materials and Methods
Chicks
White Leghorn 1-day-old specific pathogen free chicks were randomly allocated into four groups and were placed in separate isolation rooms. Food and water were provided ad libitum.
Vaccines
NDV (strain VG/GA NDV, Avinew®) and AVP (subtype B APV, Nemovac®) vaccines were used— Avinew® and Nemovac® are registered trademarks of Merial S.A.S. (Lyon, France). The vaccines were reconstituted as recommended by the manufacturer and one vial of NDV vaccine was thoroughly mixed with 100 ml sterile water (SW). APV was prepared in a similar manner. For dual vaccination, one vial of APV vaccine and one vial of NDV vaccine were reconstituted and mixed in 100 ml SW. The dosages of vaccines are presented in .
Table 1. Experimental design to study the effects of vaccination with APV or NDV alone or together
Detection of vaccine viruses
Oropharyngeal (OP) and infra-orbital (IOS) swabs and selected homogenized tissues from the chicks were subjected to detection of the viruses by virus isolation and molecular methods.
Virus isolation
For APV vaccine, isolation was attempted in Vero cells (Williams et al., Citation1991) and tracheal organ cultures (TOC) (Cook et al., Citation1976). Samples were passaged three times and those with ciliostasis at the third passage were tested by a polymerase chain reaction (PCR) to confirm the presence of APV. For NDV vaccine, 9-day-old to 10-day-old embryonated specific pathogen free chicken eggs were used and the presence of the virus was demonstrated by haemagglutinating activity (Alexander, Citation1998).
Polymerase chain reaction
Samples of OP, IOS and homogenized tissues from each group at each sampling interval were pooled and subjected to detection of the viruses by reverse transcriptase (RT-PCR). For APV and NDV, RT-PCRs were carried out as described by Cavanagh et al. (Citation1999) and Aldous & Alexander (Citation2001), respectively.
Detection of vaccinal antibodies
Serum antibodies
NDV antibodies were detected by a haemagglutination inhibition test (Allan & Gough, Citation1974). For APV virus antibodies, sera were tested by an in-house enzyme-linked immunosorbent assay (ELISA) as described by Worthington et al. (Citation2003), but the coating antigen was subtype B APV.
Virus-specific IgA and IgG in lachrymal fluid and tracheal washes
NDV-specific and APV-specific IgA and IgG in the lachrymal fluid and tracheal washes were assayed using an indirect ELISA (Dhinakar Raj & Jones, Citation1996) except that the coating antigen used was either APV or NDV. Corrected optical density values were calculated by deducting the optical density values of non-antigen-coated wells from those of the test wells (Fournier-Caruana et al., Citation2003).
Experimental design
One-day-old specific pathogen free chicks were allocated into four groups (). The control (C) group was sham-inoculated with SW. Each chick received 50 µl SW ocularly and another 50 µl orally. Through the same route of application, the same volume of inoculum of APV or NDV was given to chicks in groups N and A, respectively. For the dually vaccinated (AN) group, SW containing both APV and NDV were administered in the same way. Dosages received by each bird were as recommended by the manufacturers, and these are presented in . The chicks were monitored daily for clinical signs.
Oropharyngeal swabs
Dry swabs from the oropharynx were collected 2, 5, 10, 16 and 24 days post-vaccination (d.p.v.) from all birds in each group for detection of NDV and APV by RT-PCR. A separate set of oropharyngeal swabs moistened in TOC medium (Eagle's serum-free modified Eagle's medium with glutamine, streptomycin [50 µg/ml] and penicillin [50 IU/ml]) were used for attempted re-isolation of the vaccine viruses.
Tissues
At 7, 14, 21 and 28 d.p.v. four chicks from each group were killed humanely, and pieces of the turbinate, trachea, lung, proventriculus, caecal tonsil, pancreas and liver were collected aseptically and used for attempted isolation of the vaccine viruses. The same samples were used for RT-PCR. At postmortem examination, IOS swabs were also collected for virus isolation and RT-PCR.
Sera
On days 7, 14 and 21 blood was taken randomly from not less than five chicks per group for detection of antibodies against the vaccines. On day 28, blood samples were collected from the remaining 4 birds.
Lachrymal fluid and tracheal washes
At 7, 14, 21 and 28 d.p.v. lachrymal fluid and tracheal washes were collected from three or four birds per group, and were assayed for virus-specific IgA and IgG.
Histopathology
On days 7, 14, 21 and 28 pieces of the trachea, lung, proventriculus, pancreas and liver were collected in 10% formaldehyde for conventional histopathology, stained by haematoxylin and eosin.
Statistics
The mean antibody titres were compared using a Student's t-test.
Results
Clinical signs and postmortem lesions
No clinical signs or postmortem lesions were observed in any group, but chicks in the AN group appeared somewhat dull and depressed for the first 5 d.p.v.
Detection of vaccine viruses (virus isolation or RT-PCR)
For NDV vaccine, virus isolation (VI) and RT-PCR were found to be of identical sensitivity, so only VI results are shown. For APV, both methods are shown since results were different. For isolation of APV, despite three passages of samples in Vero cells, no evidence of viral growth was observed, even though the vaccine was developed in this culture system. Thus, isolation was attempted using TOCs and these are the results shown. Control swabs and tissues remained negative throughout.
OP swabs
presents the results of attempted detection of the vaccine viruses from OP swabs. APV was detected by RT-PCR on day 10 in the single APV group and on days 16 and 24 in the dual-vaccination group. Re-isolation of APV was successful only on day 10 in the single APV vaccinates and day 16 following the dual vaccination. For NDV, OP swabs from both NDV-vaccinated groups were positive at 2 and 5 d.p.v. ().
Table 2. Detection of APV in swabs and tissues by RT-PCR and passage in tracheal organ cultures
Table 3. Attempted isolation of NDV from swabs and tissues by passage in embryonated chicken eggs
Tissue samples and IOS
In group N, APV antigen was detected by RT-PCR on days 7 and 14 in the IOS and turbinates, but the only re-isolation of virus was on day 7 in the IOS (). It was not detected in the trachea. In the dually vaccinated group on day 7, APV was detected only by RT-PCT and only in the turbinate; and by both methods in the IOS and turbinate on day 14. It was detected in the trachea by RT-PCR on day 21 and was isolated from the trachea on days 14 and 21. Lungs remained negative throughout.
NDV vaccine virus was isolated at 7 d.p.v. from tissues of the dual vaccinates more frequently than single vaccinates (). The difference was particularly marked in respiratory tract samples and liver. At 14 d.p.v. no virus was recovered from the respiratory tracts of either group but, while it was rarely found in the proventriculus, caecal tonsil and liver, it was most common in single vaccinates.
Serology
NDV antibodies
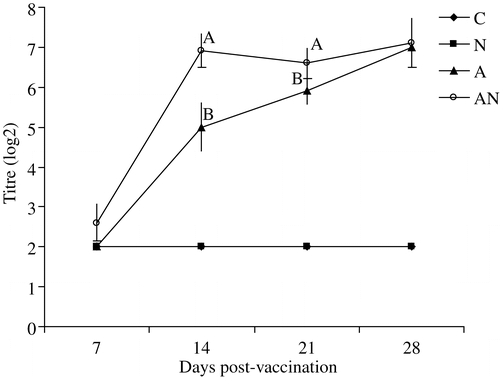
shows the result of testing sera by haemagglutination inhibition. On days 14 and 21, antibody titres in the dually vaccinated group were significantly higher than in the singly vaccinated chickens.
APV antibodies
shows the results of testing the sera by ELISA using a type B strain as antigen. At 21 and 28 d.p.v. significantly higher antibody titres were detected in the group vaccinated with APV alone (group N) compared with the dually vaccinated group.
Figure 2. APV ELISA antibodies in the unvaccinated (C), APV (N), NDV (A) or dual-vaccinated (AN) groups measured using APV type B-coated ELISA plates. The birds were vaccinated with same strain as the ELISA antigen. Different superscripts between groups indicate that the values differ significantly (P<0.05).
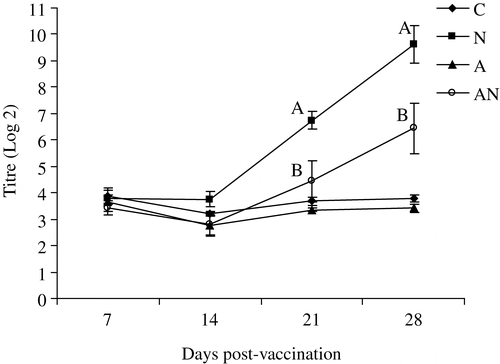
NDV-specific IgA and IgG in lachrymal fluid and tracheal washes
a and 3b show the levels of IgA and IgG, respectively, in pooled samples of LF. The corrected optical densities of NDV-vaccinated chickens were markedly higher than those of the unvaccinated and APV-vaccinated (N) chickens. In group A, there was a marked increase in the IgA level at 14 d.p.v. compared with the dually vaccinated group, but results were similar at 21 and 28 d.p.v. The levels of IgG were similar in both groups (A and AN) up to 21 d.p.v., but at 28 d.p.v. the level declined sharply in the dually vaccinated group.
Figure 3. 3a: NDV-specific IgA in lachrymal fluid of unvaccinated (C), APV (N), NDV (A) or dual-vaccinated (AN) chickens. 3b: NDV-specific IgG in lachrymal fluid of unvaccinated (C), APV (N), NDV (A) or dual-vaccinated (AN) chickens.
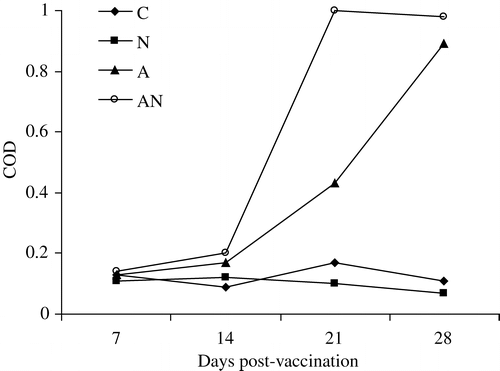
In tracheal washes at 21 d.p.v. the levels of IgG in the dual vaccinates were markedly higher than in the NDV-vaccinated chickens (). For IgA, only trace amounts were detected in either group (data not shown).
APV-specific IgA and IgG in lachrymal fluid and tracheal washes
Identical levels of IgA were found in the lachrymal fluid of both APV-vaccinated groups (a). For IgG, a gradual and greater increase in the corrected optical density was recorded in the APV-vaccinated chickens compared with the dually-vaccinated chickens (b).
Figure 5. 5a: APV-specific IgA in lachrymal fluid of unvaccinated (C), APV (N), NDV (A) or dual-vaccinated (AN) chickens. 5b: APV-specific IgG in lachrymal fluid of unvaccinated (C), APV (N), NDV (A) or dual-vaccinated (AN) chickens.
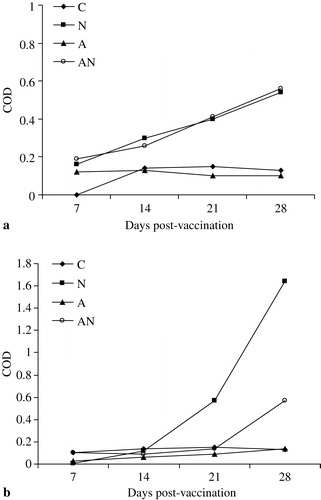
Only trace amounts of APV-specific IgA and IgG were detected in tracheal washes of either group (data not shown).
Discussion
The higher and earlier detection of NDV in tissues of dually vaccinated chickens suggests that, in the presence of APV, NDV became more invasive. Despite this, NDV isolations were reduced by 14 d.p.v. and were less frequent than in the singly vaccinated NDV group.
For APV, there were more frequent detections of virus in the singly vaccinated group up to and including 14 d.p.v., but thereafter the virus was detected more often in the dual vaccinates. It appears that the dual vaccination resulted in a delayed detection of APV. Cook et al. (Citation2001) and Khehra (Citation1998), using vaccine or virulent viruses, found suppression of APV replication in the presence of IBV, and both authors considered that vigorous multiplication of IBV with consequent destruction of respiratory tract epithelium was likely to have resulted in reduced replication of APV, as both viruses competed for the cells of the respiratory tract epithelium. In the current study, similar suppression of APV was found in the early stages after dual vaccination. However, our results indicate that the suppression was only temporary, as APV was detected up to 24 d.p.v. in the dually vaccinated group when NDV was not. To detect APV this late after experimental infection or vaccination is very unusual. For example, Cook et al. (Citation2001) vaccinated commercial broiler chickens against IBV at 1 day old and 7 days later against APV. They did not detect APV beyond 11 d.p.v.
Isolation of the APV vaccine was found to be more successful in TOC than in Vero cells, its medium of adaptation. Williams et al. (Citation1991) reported identical findings. The reason for this is not clear. However, Vero cells are used for isolation and propagation of subtype C APV (Gough et al., Citation1998).
In the current study, the enhanced levels of NDV antibodies in dual vaccinates is likely to be due to increased NDV invasiveness and ability to multiply because of the dual vaccination. In contrast to the enhancement found here, Khehra (Citation1998) reported similar levels of IBV antibodies in chickens infected with a virulent strain of IBV (M41) alone or simultaneously with APV, even though longer persistence of IBV was detected in the dually infected group. Similarly, Cook et al. (Citation2001) infected chickens with vaccine strains of APV and IBV, and found no significant antibody titre differences between singly and dually vaccinated chickens. More research is required to explore the reasons for the enhanced antibody response to NDV in the presence of subtype B APV.
In contrast to the enhanced humoral immune response to NDV after dual vaccination, the APV-specific antibody was depressed. Khehra (Citation1998) and Cook et al. (Citation2001) reported similar findings after simultaneous administration of APV and IBV vaccines to chickens. The depressed response is probably due to the inability of APV to compete with the NDV vaccine as outlined earlier. Interestingly, despite a delayed response to APV in the early stages after the dual vaccination, antibody levels still showed a rising pattern with an increase in age of the chickens. This correlates with the belated detection of APV by both RT-PCR and VI. Thus it appears that the depressed APV proliferation and subsequent humoral antibody production was a temporary effect. Where the APV persisted during early stages after vaccination is unknown.
In the local antibody response to NDV vaccine in lachrymal fluid and tracheal washes, two different types of response were found. In lachrymal fluid, IgA and IgG levels were higher in the single NDV-vaccinated group compared with the dual vaccinates. However, the reverse was found in the case of tracheal washes, where IgA and IgG levels in dual vaccinates were higher than in the single NDV-vaccinated chickens, reflecting the humoral responses. The latter finding probably correlates with the increased isolation of NDV in the trachea and lungs of dual vaccinates.
Although dual vaccination clearly caused a significant depression in the humoral response to APV, levels of IgA in lachrymal fluid were identical in both APV-vaccinated groups. The significance of this in relation to protection remains to be determined. However, Cook et al. (Citation2001) reported that despite lowered replication of APV after dual-vaccination with IBV, chickens vaccinated with APV alone or together with IBV were protected against subsequent virulent APV challenge. From our results, it appears that such protection might occur due to similar levels of local immune responses, rather than different humoral antibody levels in the singly or dually vaccinated chickens. Our findings further support the contention that humoral antibody levels may not correlate well with protection against APV. Previous studies have also emphasized the importance of local or cellular immune responses in resistance to and clearance of APV infection (Cook et al., Citation1989; Jones et al., Citation1992).
Translations of the abstract in French, German and Spanish are available on the Avian Patholgy website.
References
- Aldous , E.W. and Alexander , D.J. 2001 . Detection and differentiation of Newcastle disease virus (avian paramyxoviruses type 1) . Avian Pathology , 30 : 117 – 128 .
- Alexander , D.J. 1998 . “ Newcastle disease virus and other avian paramyxoviruses ” . In A Laboratory Manual for the Isolation and Identification of Avian Pathogens , 4th edn , Edited by: Swayne , D.E. , Glisson , J.R. , Jackwood , M.W. , Pearson , J.E. and Reed , W.M. 156 – 163 . Pennsylvania, PA : American Association of Avian Pathologists .
- Alexander , D.J. 2003 . “ Newcastle disease ” . In Diseases of Poultry , 11th edn , Edited by: Saif , Y.M. , Barnes , H.J. , Glisson , J.R. , Fadly , A.M. , McDougald , L.R. and Swayne , D.E. 64 – 87 . Ames : Iowa State University Press .
- Alexander , D.J. and Jones , R.C. 2001 . “ Paramyxoviridae ” . In Poultry Diseases , 5th edn , Edited by: Jordan , F.T.W. , Pattison , M. , Alexander , D.J. and Faragher , T. 257 – 272 . London : W.B. Saunders, Harcourt Publishers .
- Allan , W.H. and Gough , R.E. 1974 . A standard haemagglutination-inhibition test for Newcastle disease. (1) A comparison of macro and micro methods . Veterinary Record , 95 : 120 – 123 .
- Cavanagh , D. , Mawditt , K. , Britton , P. and Naylor , C.J. 1999 . Longitudinal field studies of infectious bronchitis virus and avian pneumovirus in broilers using type-specific polymerase chain reactions . Avian Pathology , 28 : 593 – 605 .
- Cook , J.K.A. 2000 . Avian pneumovirus infections of turkey and chickens . Veterinary Journal , 160 : 118 – 125 .
- Cook , J.K.A. and Cavanagh , D. 2002 . Detection and differentiation of avian pneumoviruses (metapneumoviruses) . Avian Pathology , 31 : 117 – 132 .
- Cook , J.K.A. , Darbyshire , J.H. and Huggins , M.B. 1976 . The use of chicken tracheal organ cultures for the isolation and assay of avian infectious bronchitis virus . Archives of Virology , 50 : 109 – 118 .
- Cook , J.K.A. , Ellis , M.M. , Dolby , C.A. , Holmes , H.C. , Finney , P.M. and Huggins , M.B. 1989 . A live attenuated turkey rhinotracheitis virus vaccine. 1. Stability of the attenuated strain . Avian Pathology , 18 : 511 – 522 .
- Cook , J.K.A. , Huggins , M.B. , Orbell , S.J. , Mawditt , K. and Cavanagh , D. 2001 . Infectious bronchitis virus vaccine interferes with the replication of avian pneumovirus vaccine in domestic fowl . Avian Pathology , 30 : 233 – 242 .
- Cserep , T. 2001 . “ Vaccines and vacination ” . In Poultry Diseases , 5th edn , Edited by: Jordan , F.T.W. , Pattison , M. , Alexander , D.J. and Faragher , T. 55 – 70 . London : W.B. Saunders, Harcourt Publishers .
- Dhinakar Raj , G. and Jones , R.C. 1996 . Local antibody production in the oviduct and gut of hens infected with a variant strain of infectious bronchitis virus . Veterinary Immunology and Immunopathology , 53 : 147 – 161 .
- Fournier-Caruana , J. , Poirier , B. , Haond , G. , Jallet , C. , Fuchs , F. , Tordo , N. and Perrin , P. 2003 . Inactivated rabies vaccine control and release: use of an ELISA method . Biologicals , 31 : 9 – 16 .
- Gallili , G.E. and Ben-Nathan , D. 1998 . Newcastle disease vaccines . Biotechnology Advances , 16 : 343 – 366 .
- Gough , R.E. , Alexander , D.J. and Wyeth , P.J. 1998 . “ Avian rhinotracheitis (pneumovirus) ” . In A Laboratory Manual for the Isolation and Identification of Avian Pathogens , 4th edn , Edited by: Swayne , D.E. , Glisson , J.R. , Jackwood , M.W. , Pearson , J.E. and Reed , W.M. 164 – 168 . Pennsylvania, PA : American Association of Avian Pathologists .
- Hanson , L.E. , White , F.H. and Alberts , J.O. 1956 . Interference between Newcastle disease and infectious bronchitis viruses . American Journal of Veterinary Research , 17 : 294 – 298 .
- Jones , R.C. , Naylor , C.J. , Al-Afaleq , A. , Worthington , K.J. and Jones , R. 1992 . Effect of cyclophosphamide immunosuppression on the immunity of turkey to viral rhinotracheitis . Research in Veterinary Science , 53 : 38 – 41 .
- Khehra , R.S . (1998) . Avian Pneumovirus Infection in Chickens and Turkeys: Studies on Some Aspects of Immunity and Pathogenesis . Ph.D. Thesis , University of Liverpool .
- Meulemans , G. 1988 . “ Control by vaccination ” . In Newcastle Disease , Edited by: Alexander , D.J. 318 – 329 . London : Kluwer Academic Publishers .
- Thornton , D.H. and Muskett , J.C. 1975 . Effect of infectious bronchitis vaccination on the performance of live Newcastle disease vaccine . Veterinary Record , 96 : 467 – 468 .
- Williams , R.A. , Savage , C.E. , Worthington , K.J. and Jones , R.C. 1991 . Further studies on the development of a live attenuated vaccine against turkey rhinotracheitis . Avian Pathology , 20 : 585 – 596 .
- Worthington , K.J. , Sargent , B.A. , Davelaar , F.G. and Jones , R.C. 2003 . Immunity to avian pneumovirus infection in turkeys following in ovo vaccination with an attenuated vaccine . Vaccine , 21 : 1355 – 1362 .