Abstract
Early cases of colibacillosis with omphalitis, yolk sac infection and increased mortality were observed in five broiler chicken flocks (A1, A2, A3, A4 and B1) from two broiler breeder flocks A and B, respectively. Avian pathogenic Escherichia Coli (APEC) serotype O78, Fim/Tsh/Iuc pathotype, were isolated from flocks A, A1, A2, A3 and A4, and APEC serotype O139, pathotype Fim/Iuc, from flocks B and B1. APEC O78 strains isolated from broiler chicks A1, A2, A3 and A4, originating from breeder flock A, had the same antibiotic resistance pattern as APEC O139 strains isolated from broiler chicks B1 and breeder B.
The random amplified polymorphic DNA technique performed on APEC strains revealed two distinct clusters of genetic similarity: cluster I consisted of some APEC O78 and cluster II of APEC O139. These results indicated that a transmission of APEC strains from adults A and B to their respective progeny could occur.
Transmission d' Escherichia coli pathogène aviaire (APEC) à partir de reproducteurs de type chair à leur descendance dans une production intégrée.
Les cas précoces de colibacillose avec omphalite, infection du sac vitellin, et augmentation de mortalité, ont été observés dans cinq troupeaux de poulets de chair A1, A2, A3, A4 et B1 issus de deux troupeaux de reproducteurs respectivement A et B. Des Escherichia coli pathogènes aviaires (APEC) sérotype O78, pathotype Fim/Tsh/Iuc ont été isolés des troupeaux A1, A2, A3, A4 et des APEC O139, pathotype Fim/Iuc ont été isolés des troupeaux B et B1. Les souches d'APEC O78 isolées à partir des poulets de chair A1, A2, A3 et A4 issus du troupeau de reproducteurs A, avaient le même profil de résistance aux antibiotiques que les souches de l'APEC O139 isolés des poulets de chair B1 et des reproducteurs B.
Les souches d'APEC ont fait l'objet d'une analyse par RAPD qui a révélé que ces souches se répartissaient en deux groupes distincts de similarité génétique : le groupe I comprenant quelques APEC O78, et le groupe II comprenant les APEC O139. Ces résultats ont montré que la transmission des souches d'APEC des adultes A et B à leur descendance respective peut se produire.
Übertragung von Escherchia coli (APEC) von Broilerelterntieren auf ihre Nachkommen innerhalb einer integrierten Geflügelproduktionskette
In fünf Broilerherden (A1, A2, A3, A4 und B1) von zwei Broilerelterntierherden (A und B) wurden frühe Fälle von Colibazillose verbunden mit Omphalitis, Dottersackinfektion und erhöhter Mortalität beobachtet. Aus den Herden A, A1, A2, A3 und A4 wurden pathogene aviäre Escherichia coli (APEC)-Serotyp O78,-Pathotyp Fim/Tsh/iuc und aus den Herden B und B1 APEC-Serotyp O139, -PathotypFim/Iuc isoliert. Die O78-APEC-Stämme aus den Broilerherden A1, A2, A3 und A4, die von der Broilerelterntierherde A abstammten, wiesen das gleiche Antibiotikaresistenzmuster auf wie die O139-APEC-Stämme aus den Broilern aus Herde B1 und ihrer Elterntierherde B. Die mit den APEC-Stämmen durchgeführte RAPD-Technik ließ zwei verschiedene genetische Cluster erkennen: Cluster I beinhaltete einige der APEC O78- Stämme und Cluster II die APEC O139. Diese Ergebnisse weisen darauf hin, dass eine Übertragung von APEC-Stämmen von den Adulten der Herden A und B auf ihre Nachkommen vorkommen kann.
Transmisión de Escherichia coli patógena aviar (APEC) de reproductores a su progenie en una cadena de producción avícola integrada
Se observaron casos tempranos de colibacilosis con onfalitis, infección del saco vitelino y mortalidad incrementada en cinco lotes de pollos de engorde A1, A2, A3, A4 y B1 provenientes de dos lotes de reproductores, A y B respectivamente. Se aisló una Escherichia Coli patógena aviar (APEC) serotipo O78, patotipo Fim/Tsh/Iuc de los lotes A, A1, A2, A3, A4 y APEC del serotipo O139 y patotipo Fim/Iuc de los lotes B y B1. Las cepas APEC O78 aisladas de los pollitos A1, A2, A3 y A4, originarios de los lotes de reproductores A, presentaron el mismo patrón de resistencia a los antibióticos que las cepas APEC O139 aisladas de los pollitos de engorde B1 y de los reproductores B.
La técnica de RAPD realizada sobre las cepas APEC reveló dos grupos distintos de similaridad genética: el grupo I consistió en algunas APEC O78 y el grupo II en APEC O139. Estos resultados indican que la transmisión de cepas APEC de adultos A y B a su progenie respectiva puede ocurrir.
Introduction
Colibacillosis is considered one of the leading causes of economic loss in the poultry industry worldwide (Zanella et al., Citation2000). Serotypes O1, O2 and O78 are present in 15% to 61% of colibacillosis clinical cases (Dho-Moulin & Fairbrother, Citation1999). Escherichia coli is present in the normal intestinal flora of birds. Only some strains with specific virulence attributes, designated as avian pathogenic E. coli (APEC), are able to cause disease such as acute colisepticaemia, fibrinopurulent polyserositis, aerosacculitis, pericarditis, salpingitis, synovitis, omphalitis, yolk sac infection, swollen head syndrome, coligranuloma, and cellulitis (Vidotto et al., Citation1990; Dozois et al., Citation1994; Gomis et al., Citation1997; Pourbakhsh et al., Citation1997; Dho-Moulin & Fairbrother, Citation1999). Among the virulence factors involved in the pathogenesis of colibacillosis, F1 (type 1) fimbriae have been shown to adhere to chicken respiratory epithelial cells of the pharynx and trachea (Dozois et al., Citation1994; Dho-Moulin & Fairbrother, Citation1999), Temperature-sensitive haemagglutinin (Tsh) plays a role in the colonization of air sacs (Dozois et al., Citation2000), the aerobactin iron-sequestering system allows E. coli growth in a low concentration of free iron in physiological liquids such as blood (Dho-Moulin & Fairbrother, Citation1999) and P fimbriae are important in the later stages of infection for the adhesion to internal organs (Dho-Moulin & Fairbrother, Citation1999), giving resistance to phagocytosis (Pourbakhsh et al., Citation1997).
In a previous study, E. coli strains in broiler chicks with clinical colibacillosis appeared to correlate with those isolated from their breeders (Giovanardi et al., Citation2003). The objective of this present study was to investigate whether APEC strains, isolated in clinical cases of colibacillosis during the first week of age, could be transmitted from breeders to broilers. Knowledge of the APEC infection process, through an integrated production chain, could be useful to take the appropriate measures to prevent the early cases of colibacillosis.
Materials and Methods
Study site
The study was conducted mainly in Veneto, a region located in the northeast of Italy.
Sampling procedure
Five broiler chick flocks were selected among the early cases of colibacillosis with increased mortality ranging from ages 1 to 7 days. Four of them, flocks A1 (7 days old), A2 (1 day old), A3 (5 days old) and A4 (1 day old), originated from broiler breeder flock A, and flock B1 (6 days old) originated from breeder flock B. The breeder flocks did not show relevant clinical signs: egg production and mortality were maintained in the normal range. Random samples were also taken from selected broiler flocks, derived from different breeders flocks (C, D, and E).
Culture and biochemical characterization
Visceral organs such as the brain, liver, spleen, lung, air sac, yolk sac, and pericardial swabs were cultured into 3% sheep blood agar (OXOID, Basingstoke, UK) and incubated aerobically at 37°C for 18 to 24 h. Suspect E. coli colonies were subsequently inoculated on eosin–methylene blue agar (OXOID) and incubated at the same time and temperature as described previously. The identification of E. coli was based on the results of diagnostic tests, which included Gram stain, catalase and oxidase (Quinn et al., Citation1994). Metabolic profiles were analysed for each isolate using the API system (Bio Mèrieux, Marcy L'Etoile, Lyon, France), designed for the identification of Enterobacteriaceae.
Serological characterization
Serological characterization of E. coli was performed using the agglutination test as described by Finazzi et al. (Citation2000).
Antimicrobial sensitivity
A sensitivity test for seven antimicrobial agents frequently used in local poultry flocks was carried out on E. coli isolated by the standard disk procedure as recommended by NCCLS (Citation1999). A 4-h broth culture was prepared for all E. coli isolated and swabbed onto the surface of Muller-Hinton agar (OXOID).
Amoxicillin, tetracycline, gentamycin, apramycin, trimethoprim-sulfamethoxazole (OXOID) and enrofloxacin (Bayer, Leverkusen, Germany) standard paper disks were laid on the medium. The plates were incubated for 24 h at 37°C and inhibition zones were measured by SIRSCAN 2000 (i2a, Montpellier, France), an automated image analyser.
Polymerase chain reaction
E. coli strains were inoculated into EC broth (Difco, Detroit, Michigan, USA), incubated aerobically at 37°C for 12 h and then submitted to polymerase chain reaction (PCR).
DNA was extracted with DNAzol (Invitrogen Life Technologies, Carlsbad, California, USA) as described by the supplier. Amplification was carried out in 45 µl reaction mixture containing 5 µl bacterial DNA, 100 µM oligonucleotide primers () for detection of different virulence associate genes: iucD, tsh, papC, fimC (JanBen et al., Citation2001), 1 x PCR Buffer A (Promega Corporation, San Luis Obispo, California, USA), 1.5 mM MgCl2, 10 mM dNTPs and 1 U Taq (Promega). The DNA was amplified using a denaturation step a 94°C for 1 min, an annealing step at 55°C for 2 min and then increased to 72°C for 1 min, for a total of 30 cycles, and a final step at 72°C for 10 min. The PCR products were separated by electrophoresis on 2% agarose gel stained with ethidium bromide and photographed using an ultraviolet transilluminator and a digital image capture system (Gel Doc 2000; BIO-RAD, Hercules, California, USA).
Table 1. PCR and RAPD sequence primers
Random amplified polymorphic DNA
Bacterial DNA was extracted as previously described and quantified by spectrophotometer. Twenty nanograms of bacterial DNA were used as a template in the random amplified polymorphic DNA (RAPD) kit containing room-temperature stable dried Ready-to-Go beads (Amersham Biosciences, Little Chalfont, UK). The kit was used as described by the supplier with the decamer primer 1290 (Maurer et al., Citation1998) and primer 6 (Chansiripornchai et al., Citation2001) (). Amplification products were resolved by electrophoresis on 2% agarose gel and detected by ethidium bromide staining; in the same gel, a molecular marker 100 base pair ladder (Invitrogen) was run. The image was captured using Gel Doc 2000 (BIO-RAD) and recorded as TIFF files.
The fingerprinting was compared with Gel Compare II (version 2.0; Applied Maths, Belgium) and the measure of the similarity was based upon densiometric curves using the Pearson correlation (product moment correlation coefficient). A dendrogram was generated by an unweighted pair group method with arithmetic average.
E. coli inocula for in vivo experimental studies
For in vivo experimental studies, E. coli O78-Fim/Tsh/Iuc pathotype isolated from the chick's yolk sac in flock A1 (E1) and E. coli O139-Fim/Iuc pathotype isolated from the chick's yolk sac in flock B1 (E2) were selected.
E. coli (E3) isolated from chickens without any recognized virulence factors was used as a negative control.
E1, E2 and E3 were grown overnight in 5 ml brain heart infusion broth (BHI OXOID) incubated at 37°C. The infective dose was then quantified by correlating the optical density of broth cultures at 625 nm with colony forming units (cfu) determined by the plate count method.
Chicken embryo lethality test
For this test, embryonated eggs from a commercial hatchery were used as suggested by Nolan et al. (Citation1992). Recently, Gibbs & Wooley (Citation2003) showed that this test exhibits similar results and high correlation with the intravenous chicken challenge model.
Overnight cultures of the E. coli strains in BHI broth (OXOID) were adjusted to a concentration of 1 x 104 cfu/ml and 0.1 ml inoculum was injected into the allantoic cavity of each embryo. Nine embryos and one control per E. coli strain were used. Eggs were incubated at 37°C and were candled daily for 7 days to identify dead embryos.
Chicken pathogenicity test and mean lethal dose
Unvaccinated 1-day-old male commercial broiler chickens were used as suggested by Vidotto et al. (Citation1990) and housed on floored battery cages. Water and a standard commercial feed without antibiotics were provided ad libitum. Rearing temperatures were similar to those used commercially.
For the chicken pathogenicity test, eight birds per isolate were subcutaneously inoculated with 0.5 ml BHI broth (OXOID) culture containing 1 x 108 cfu/ml. The birds were maintained for 7 days post-inoculation and monitored daily for mortality.
For the mean lethal dose (LD50) test, a series of 10-fold dilution of overnight BHI broth (104 to 108 cfu/0.5 ml) were subcutaneously injected into groups of six 1-day-old chickens. The chickens were observed for 7 days and mortality was recorded. The LD50 was calculated by the method of Reed and Muench. Lethality classes (LC) as previously described by Dozois et al. (Citation2000) were defined as follows: LC1, LD50<108 cfu; LC2, LD50 ≥ 108 cfu; LC3, not lethal at LD50 ≥ 108 cfu.
Results
Postmortem examination
Visceral organs such as the liver, lung, spleen, brain, pericardium, air sac and yolk sac swabs were collected from 51 broiler chicks in flocks A1, A2, A3, A4 and B1 and from six breeders in flocks A and B, respectively. In the chicks, omphalitis and yolk sac infection were the most prevalent lesions (73%). The chick's abdomen was distended with blood vessels often hyperaemic and the yolk sac increased and inflamed. Pericarditis, aerosacculitis and perihepatitis were also present in 27% of the dead birds. Rare cases of thoracic aerosacculitis were observed in both broiler breeder flocks A and B. Birds from both flocks did not have signs of salpingitis.
Culture, biochemical and serological characterization
All 59 E. coli isolates were oxidase-negative, catalase-positive and had a dark green, black metallic sheen on eosin–methylene blue agar. The API commercial differentiation system identified all the strains isolated as E. coli. The E. coli isolated that did not belong to serotypes O78 and O139 were discarded.
E. coli O78 and O139 were mainly isolated from the yolk sac (51.5% and 22.2% respectively) and the brain (24.2% and 22.2%) of chicks and from the breeder's liver and spleen (33.3% and 33.33%).
As presented in , 33 E. coli serotype O78 were isolated from broiler chicks; five from the yolk sac and three from the brain in flock A1; four from the yolk sac and three from the brain in flock A2; one from the yolk sac and one from the brain in flock A3; and seven from the yolk sac, one the from brain, five from the pericardium and three from the liver in flock A4. Six E. coli O78 were also isolated from the brain, lung, air sac, spleen and the liver of three breeders in flock A.
Table 2. Origin and number (%) of E. coli O78 and O139 isolation in breeders and chicks
Nine E. coli O139 were isolated from broiler chicks B1, of which two were from the yolk sac, two from the brain, two from the spleen, two from the liver and one from the air sac. Nine E. coli O139 were isolated from the spleen, liver and the air sac in three breeders in flock B.
E. coli isolates from the others broiler flocks C, D and E were not serotype O78 or O139 (data not shown).
Antibiotic sensitivity
The antibiotic sensitivity test for 39 E. coli O78 strains showed a high level of resistance of all the isolates to four antimicrobial agents (amoxicillin, enrofloxacin tetracycline and trimethoprim-sulfamethoxazole), whereas 18 E. coli O139 showed resistance to three antimicrobial agents (amoxicillin, enrofloxacin and tetracycline) ().
Table 3. Antibiotic resistance of E. coli isolates O78 and O139
Polymerase chain reaction
The PCR for the presence of virulence factors was performed for 57 E. coli O78 and O139 isolates.
Type 1 fimbriae and the aerobactin system were present in both E. coli serotypes O78 and O139 (100%) while Tsh was only in E. coli O78 (68.4%). All strains were negative for P fimbriae (). Analysing the PCR data we assumed that all E. coli O78 isolated were of Fim/Tsh/Iuc pathotype while all E. coli O139 were of Fim/Iuc pathotype.
Table 4. Distribution of fimC, iucD, tsh, papC DNA sequences along the E. coli isolates O78 and O139
Random amplified polymorphic DNA
In this study, the RAPD technique was used to analyse the cloned relationship among some of the E. coli isolated. The information from the phylogenetic analysis was used to determine whether there were any genetic differences between the E. coli of the same serotype, pathotype and pattern of antibiotic resistance.
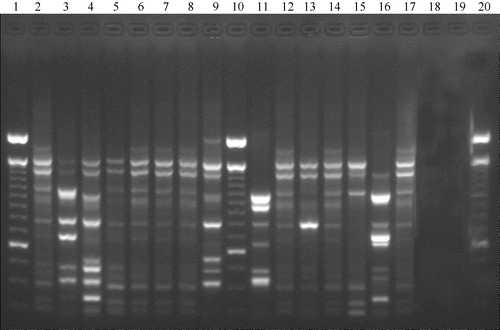
The RAPD analysis of the E. coli isolates with primer 1290 revealed 10 distinct patterns. E. coli correlated with similarities in their RAPD DNA patterns were clustered (). The most interesting clusters for the epidemiological study were cluster I, which consisted of 11 E. coli O78 isolates in breeder A and chicks A1, A2, A3 and A4, and cluster II, which consisted of three E. coli O139 isolated in breeder B and its progeny B1. To test the reproducibility of the RAPD technique, the samples were analysed in two independent reactions and there was no loss or shift in the position of banding pattern. When the reactions were run without primers or template, amplification was not obtained. A typical RAPD gel of some APEC isolates from flocks A, A1 and A4 is shown in . The E. coli serologically untypeable had a different pattern from the E. coli O78 in the same flocks.
Figure 1. Unweighted pair group method with arithmetic average dendrogram constructed from RAPD using the Pearson coefficient; data indicating genetic similarity among the 11 E. coli O78 isolates in cluster I and the three E. coli O139 in cluster II.
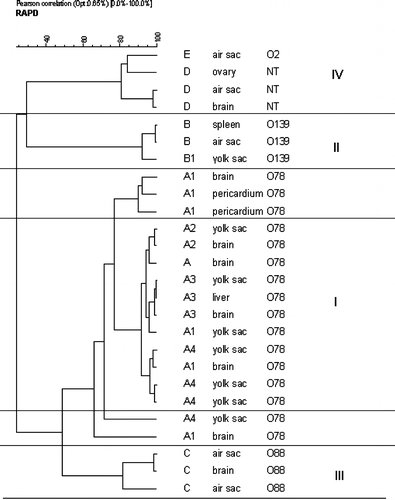
Figure 2. RAPD profiles of 15 avian E. coli strains belonging to broiler chicks in flocks A1, A4 and to breeders in flock A. Lanes 1, 10 and 20, molecular marker 100 base pair DNA ladder; lane 2, E. coli O78 (flock A); lanes 3 and 4, E. coli untypeable (flock A); lanes 5 to 8, E. coli O78 (flock A4); lanes 9 and 11, E. coli untypeable (flock A4); lanes 12 to 15, E. coli O78 (flock A1); lane 16, E. coli untypeable (flock A1); lane17, E. coli O78 (flock A1).
To improve the RAPD ability to discriminate, a different primer (primer 6) (Chansiripornchai et al., Citation2001) was used. Strains of the same cluster I and cluster II were not differentiated with this additional primer (data not shown).
Five E. coli O78 isolated from broiler chicks A1 and A4 did not belong to cluster I, so we inferred they had less genetic similarity compared with the strains of the same serotype in that cluster. E. coli strains of clusters I and II were genetically different from the ones isolated from broilers C, D and E.
Chicken pathogenicity and embryo lethality tests
In the chicken pathogenicity test, E. coli strains E1 and E2 have shown an extremely high mortality rate (100%), while no mortality was observed for the negative control strain E3 (). The chicks died 1 day after injection showed hepatitis and the remaining chicks developed omphalitis and pericarditis after 2 days.
Table 5. Results of the chicken pathogenicity and embryo lethality tests
E1 and E2 used for the experiments were isolated from the dead chicks in the yolk sac, brain, pericardium, liver and spleen.
After the calculation of the LD50, E. coli strains E1 and E2 were classified in the high lethality class (LC1) while E3 was classified in the non-lethal class (LC3).
In the embryo lethality, the higher mortality (66%) was observed in embryos infected with strain E1.
Discussion
As Barnes & Gross (Citation1997) suggested, yolk sac infection can cause the spread of E. coli into the bloodstream, which may lead to septicaemia; in this case, the pathogenesis is different from the more studied respiratory-origin colibacillosis.
Da Silveira et al. (Citation2002 Citation2003) suggested that omphalitis E. coli isolates are non-pathogenic strains since they have not been isolated in 1-day-old chicks with this lesion, and that these strains may act as opportunistic agents in causing disease, crossing the egg barriers during the egg-laying process. These E. coli strains could also potentially become pathogenic; the presence of large and small plasmids in these strains may explain the rapid and efficient transfer of virulence factors after hatching. Moreover, the genetic background (clones) could influence the ability of E. coli to acquire, maintain or express pathogenic traits.
Montgomery et al. (Citation1999) demonstrated that the contamination of a chick with a relatively non-embryo lethal E. coli could derive from an infected hen in utero and/or by ovo-depositing a fresh egg in a contaminated environment. In both cases, a possible horizontal transmission of this E. coli may occur via inhalation from the infected progeny to other chicks in contact after hatching.
At the beginning of this study, we selected five chick broiler flocks (A1, A2, A3, A4, and B1) with problems of omphalitis, yolk sac infection and mortality during the first week of life. The isolation of APEC strains O78 and O139 from these early cases led us to wonder whether these E. coli strains could be transmitted by the broiler breeder flocks, A and B from where the chicks originally came. We investigated these APEC strains for the presence of virulence-associated genes by PCR. The pathotype Fim/Tsh/Iuc was detected in all the E. coli O78 isolated from breeder A and its progeny, and the pathotype Fim/Iuc was detected in E. coli O139. Recently, Stordeur et al. (Citation2004) showed that 76% of E. coli septicemic strains were negative for the presence of the pap gene cluster. All the strains analysed in this study were negative for papC gene. In another study, different pathotypes of E. coli O78 were isolated from other breeder flocks in our integrated company so this serotype is not correlated to a specific pathotype (data not shown).
In addition to serotyping, pathotyping and the antibiotic resistance pattern of the isolates from breeder A and B and their progeny, the genetic similarity among these strains was investigated.
Different molecular and genetic techniques have been used for typing E. coli strains for epidemiological investigations such as RAPD, pulsed field gel electrophoresis typing (Osek, Citation2000) and restriction fragment length polymorphism typing (Maurer et al., Citation1998). The RAPD technique is simple, cheap, fast, requires only nanogram amounts of DNA and easily handles large numbers of samples (Ramasoota et al., Citation2000; Chansiripornchai et al., Citation2001). Vogel et al. (Citation2000) found that RAPD analysis for typing E. coli isolates has the highest discriminatory capacity compared with serotyping and ribotyping. RAPD has also been used successfully to discriminate E. coli strains isolated from humans (Pacheco et al., Citation1997; Hopkins & Hilton, Citation2001), cattle, swine (Ramasoota et al., Citation2000; Aslam et al., Citation2004) and avian species (Maurer et al., Citation1998; Chansiripornchai et al., Citation2001); Ramasoota et al. (2001) differentiated different clones inside the same E. coli serotype using RAPD.
The results obtained with RAPD are sometimes difficult to reproduce; however, by using pre-formulated RAPD analysis beads that contain all PCR reagents, we were able to achieve stable banding patterns as previously described (Vogel et al., Citation2000; Ramasoota et al., Citation2000); Grundmann et al. (Citation1997) also achieved RAPD inter-laboratory reproducibility.
By combining the RAPD information using different primers with serotyping, pathotyping and antibiotic sensitivity pattern, we were able, with more detail, to group in one cluster the APEC strains isolated from flocks A, A1, A2, A3 and A4, and in another cluster the APEC strains isolated from B and B1. Our results indicated that a transmission of APEC strains from adults A and B to their respective progeny could occur. The isolation of other E. coli O78 strains that do not belong to cluster I could indicate the presence of different clones in flocks A1 and A4.
The results obtained from of this study suggest that an APEC transmission from breeders to broiler chickens could be possible as previously described by Glavits et al. (Citation1984) for Salmonella typhimurium and Staphylococcus aureus. The penetration of these pathogenic bacteria, present on the outer surface of the shell membrane, into the egg during incubation is not always fatal for embryos, so they may hatch and become carriers of the pathogenic agent.
In the study it was relevant that the majority of APEC strains in chicks were isolated from yolk sacs (45.2%) and field cases of omphalitis and yolk sac infections were already observed in 1-day-old chicks from where a systemic infection spread. These findings, and the fact that we found a genetic similarity between APEC clones inside a single production chain from breeders to broilers, support the hypothesis that, possibly, field cases of omphalitis or colibacillosis originated from an APEC embryo infection before hatching.
The timing of infection reflects the chicken mortality pattern: with a bacterial infection acquired after hatching, the mortality begins after more than 20 h; with an embryo infection it begins at hatching, as seen in this study (Horrox, Citation2000).
The lack of APEC isolation from yolk sac, mentioned previously (Da Silveira et al. Citation2002 Citation2003), could explain the fact that, for this infection, E. coli strains do not necessarily need the virulence factors necessary to colonize the respiratory tract resulting in septicaemia. By that, yolk sac colonization could randomly occur both with APEC and with non-virulent strains of E. coli; later APEC, even if present even in very small amounts in the yolk sac, could selectively overgrow, causing septicaemia.
Translations of the abstract in French, German and Spanish are available on the Avian Patholgy website.
Acknowledgments
The authors are grateful to Dr L. Alborali and Dr S. Tagliabue, Istituto Zooprofilattico della Lombardia ed Emilia Romagna, for help with serotyping of E. coli, and to Dr H. Wieler, Institute of Microbiology, Free University of Berlin for providing the reference E. coli strains.
References
- Aslam , M. , Greer , G.G. , Nattress , F.M. , Gill , C.O. and McMullen , L.M. 2004 . Genetic diversity of Escherichia coli recovered from the oral cavity of beef cattle and their relatedness to fecal E. coli . Lett Appl Microbiol , 39 : 523 – 527 .
- Barnes , J.H. and Gross , W.B. 1997 . “ Colibacillosis ” . In Diseases of Poultry , 10th edn , Edited by: Calnek , B.W. 131 – 140 . Ames : Iowa State University Press .
- Chansiripornchai , N. , Ramasoota , P. , Sasipreeyajan , J. and Svenson , S.B. 2001 . Differentiation of avian pathogenic Escherichia coli (APEC) strains by random amplified polymorphic DNA (RAPD) analysis . Veterinary Microbiology , 80 : 75 – 83 .
- Da Silveira , M. , Ferriera , W.D. , Brocchi , M. , De Hollanda , L.M. , De Castro , A.F.P. , Yamada , A.T. and Lancellotti , M. 2002 . Biological characteristics and pathogenicity of avian Escherichia coli strains . Veterinary Microbiology , 85 : 47 – 53 .
- Da Silveira , W.D. , Lancellotti , M. , Ferriera , A. , Solferini , V.N. , De Castro , A.F.P. , Stehling , E.G. and Brocchi , M. 2003 . Determination of the clonal structure of avian Escherichia coli strains by isoenzyme and ribotyping . Journal of Veterinary Medicine Series B , 50 : 63 – 69 .
- Dho-Moulin , M. and Fairbrother , J.M. 1999 . Avian pathogenic Escherichia coli (APEC) . Veterinary Research , 30 : 299 – 316 .
- Dozois , M.C. , Chanteloup , N. , Dho-Moulin , M. , Brèe , A. , Desautels , C. and Fairbrother , J.M. 1994 . Bacterial colonization and in vivo expression of F1 (type 1) fimbrial antigens in chickens experimentally infected with pathogenetic Escherichia coli . Avian Diseases , 38 : 231 – 239 .
- Dozois , M.C. , Dho-Moulin , M. , Brèe , A. , Fairbrother , J.M. , Desautels , C. & Curtiss III , R. (2000) . Relationship between the Tsh autotransponder and pathogenicity of avian Escherichia coli and localization and analysis of the tsh genetic region . Infection and Immunity , 68 , 4145 – 4154 .
- Finazzi , G. , Cardeti , G. , Pacciarini , M.L. , Losio , M. and Tagliabue , S. 2000 . Characterization of strains of Escherichia coli isolated from rabbits with enteritis in Lombardia and Emilia Romagna during the triennium 1997–1999 . Journal of The World Rabbits Science Association , 8 ( Suppl. 1 ) : 241 – 247 .
- Gibbs , P. and Wooley , R.E. 2003 . Comparison of the intravenous chicken challenge method with the embryo lethality assay for studies in avian colibacillosis . Avian Diseases , 47 : 672 – 680 .
- Giovanardi , D. , Campagnari , E. , Sperati , R.L. , Ortali , G. , Furlattini , V. & Pesente , P. (2003) . Bacteriological and biomolecular diagnosis of poultry colibacillosis . Proceedings of the Italian Society of Avian Pathology , 1 , 77 – 78 .
- Glavits , R. , Ratz , F. , Fehervari , T. and Povazsan , J. 1984 . Pathological studies in chicken embryos and day-old chicks experimentally infected with Salmonella typhimurium and Staphylococcus aureus . Acta Veterinaria Hungarica , 32 : 39 – 49 .
- Gomis , S.M. , Watts , T. , Riddell , C. , Potter , A.A. and Allan , B.J. 1997 . Experimental reproduction of Escherichia coli cellulitis and septicemia in broiler chickens . Avian Diseases , 41 : 234 – 240 .
- Grundmann , H.J. , Towner , K.J. , Dijkshoorn , P. , Gerner-Smidt , P. , Maher , M. , Seifert , H. and Vaneechoutite , M. 1997 . Multicenter study using standardized protocols and reagents for evalutation of reproducibility of PCR-Based fingerprinting of Acinetobacter spp . Journal of Clinical Microbiology , 35 : 3071 – 3077 .
- Hopkins , K.L. and Hilton , A.C. 2001 . Use of multiple primers in RAPD analysis of clonal organism provides limited improvement in discrimination . Biotechniques , 30 : 1266 – 1267 .
- Horrox , N. 2000 . Controlling yolk sac infection . International Hatchery Practice , 14 : 27 – 28 .
- JanBen , T. , Schwarz , C. , Preikschat , P. , Voss , M. , Hans-C , P. and Wiewer , L. 2001 . Virulence-associated genes in avian pathogenic Escherichia coli (APEC) isolated from internal organs of poultry having colibacillosis . International Journal of Medical Microbiology , 291 : 371 – 378 .
- Maurer , J.J. , Lee , M.D. , Lobsinger , C. , Brown , T. , Maier , M. and Thayer , S.G. 1998 . Molecular typing of avian Escherichia coli isolates by random amplification of polymorphic DNA . Avian Diseases , 42 : 431 – 451 .
- Montgomery , R.D. , Boyle , C.R. , Lenarduzzi , T.A. and Jones , L.S. 1999 . Consequences to chicks hatched from Escherichia coli inoculated embryos . Avian Diseases , 43 : 553 – 563 .
- NCCLS (1999) . Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals Approved Standard M 31-A , Vol. 19 , No. 11 . Waryne, Pennsylvania : NCCLS .
- Nolan , L.K. , Wooley , R.E. , Brown , J. , Spears , K.R. , Dickerson , H.W. and Dekich , M. 1992 . Comparison of a complement resistance test, a chicken embryo lethality test, and a chicken lethality test for determining virulence of avian Escherichia coli . Avian Diseases , 36 : 395 – 397 .
- Osek , J. 2000 . Clonal analysis of Escherichia coli strains isolated from pigs with post-wearing diarrhea by pulsed-field gel electroforesis . FEMS Microbiology Letters , 186 : 327 – 331 .
- Pacheco , A.B.F. , Guth , B.E.C. , Soares , K.C.C. , Nishimura , L. , De Almeida , D.F. and Ferreira , L.C.S. 1997 . Random amplification of polymorphic DNA reveals serotype-specific clonal clusters among enterotoxigenic Escherichia coli strains isolated from humans . Journal of Clinical Microbiology , 35 : 1521 – 1525 .
- Pourbakhsh , S.A. , Boulianne , M. , Martineau-Doizè , B. , Dozois , C.M. , Desautels , C. and Fairbrother , J.M. 1997 . Dynamics of Escherichia coli infection in experimentally inoculated chickens . Avian Diseases , 41 : 221 – 233 .
- Ramasoota , P. , Krovacek , K. , Chansiripornchai , N. , Pedersen Mörner , A. and Svenson , S.B. 2000 . Identification of Escherichia coli recovered from milk of sows with coliform mastitis by random amplified polymorphic DNA (RAPD) using standardized reagents . Acta Veterinaria Scandinavica , 41 : 249 – 259 .
- Quinn , P.J. , Carter , M.E. , Markey , B.K. and Carter , G.R. 1994 . Clinical Veterinary Microbiology , London : Mosby-Year Book Europe Limited, Wolfe Publishing .
- Stordeur , P. , Brèe , A. , Mainil , J. and Moulin-Schouler , M. 2004 . Pathogenicity of pap-negative avian Escherichia coli isolated from septocemic lesions . Microbes and Infections , 6 : 637 – 646 .
- Vidotto , M.C. , Muller , E.E. , De Freitas , J.C. , Alfieri , A.A. , Guimaraes , I.G. and Santos , D.S. 1990 . Virulence factors of Avian Escherichia coli . Avian Diseases , 34 : 531 – 538 .
- Vogel , L. , van Oorschot , E. , Mass , H.M. , Minderhoud , B. and Dijkshoorn , L. 2000 . Epidemiologic typing of Escherichia coli using RAPD analysis, ribotyping and serotyping . Clin Microbiol Infection , 6 : 82 – 87 .
- Zanella , A. , Alborali , G.L. , Bardotti , M. , Candotti , P. , Guadagnini , P.F. , Anna Martino , P. and Stonfer , M. 2000 . Severe Escherichia coli septicemia and polyserositis in hens at the start of lay . Avian Pathology , 29 : 311 – 317 .