Abstract
The susceptibility of mule and muscovy ducks to “blackhead” disease caused by Histomonas meleagridis was studied, using an experimental intracloacal inoculation. Turkeys were used as controls. Morbidity, mortality and body weight gain were recorded regularly during the experiments. A direct examination of the caecal content was made to determine the absence or presence of the parasite. Gross and microscopic lesions were observed on days 7, 14, 21, 28 and 35 post infection to evaluate any clinical histomoniosis in ducks and to appraise the histomonad's carriage. A scoring system was developed both for gross and histological lesions of the caecum and liver. Infected mule and muscovy ducks (n=83) never developed any clinical signs of histomoniasis. Weight gains of infected mule and muscovy ducks were similar to those of uninfected ducks. In 67% of the ducks (56/83), it was possible to demonstrate the parasite in the caecal content throughout the experiment. Typical macroscopic caecal lesions were observed in five of the ducks between days 7 and 21 post infection, with a caecal necropsy main lesion score (MLS = 1.6) less severe than that in turkeys (MLS = 2.9). Only caecal histological lesions occurred in six of the cases. Therefore, ducks do not seem to be a susceptible host for “blackhead” but may act as carrier animals for H. meleagridis. The virulence was apparently not changed, since 67% of turkeys (10/15) infected with the caecal content of positive ducks displayed classical signs of blackhead disease. Even if H. meleagridis alone does not represent a substantial danger in the duck production, its infectivity should to be taken into account in the transmission to more susceptible species.
Infectiosité d'Histomonas meleagridis chez les canards
La sensibilité des canards mulards et de Barbarie à la maladie de la "tête noire" causée par Histomonas meleagridis a été étudiée à l'aide d'une inoculation intra cloacale. Des dindes ont servi de contrôles. La morbidité, la mortalité et les gains du corps ont été enregistrés régulièrement pendant les expérimentations. Un examen direct (DE) du contenu cæcal a été réalisé pour déterminer l'absence ou la présence du parasite. Les lésions macroscopiques et microscopiques ont été observées 7, 14, 21, 28 et 35 après l'infection (PI) pour évaluer toute histomonose clinique chez les canards et pour évaluer le portage d'Histomonas. Un système de notation a été développé pour les lésions macroscopiques et histologiques du caecum et du foie. Les canards mulards et de Barbarie infectés (n = 83) n'ont jamais développé de symptômes d'histomonose. Les gains de poids des canards mulards et de Barbarie ont été similaires à ceux non infectés. Chez 67% des canards (56/83) il a été possible de démontrer la présence du parasite dans le contenu cæcal tout au long de l'expérimentation. Des lésions macroscopiques cæcales typiques ont été observées chez 5 canards entre 7 et 21 jours PI, avec un indice lésionnel moyen cæcal MLS = 1.6 qui est moins grave que chez les dindes MLS = 2.9. Des lésions histologiques cæcales sont apparues dans seulement 6 des cas. Ainsi, les canards ne semblent pas être des hôtes sensibles à la "tête noire" mais peuvent jouer le rôle d'animaux porteurs d' H. meleagridis.
La virulence n'a apparemment pas changée, puisque 67% (10/15) des dindes infectées par le contenu cæcal des canards positifs ont montré de symptômes classiques de tête noire. Même si H. meleagridis seul ne représente pas un danger substantiel pour la production de canards, son infectiosité doit être prise en compte dans la transmission à des espèces plus sensibles.
Infektiosität von Histomonas meleagridis für Enten
Die Empfänglichkeit von Mule- und Moschus (Cairina moschata)-Enten für die durch Histomonas meleagridis verursachte Schwarzkopfkrankheit wurde mittels experimenteller intrakloakaler Inokulation untersucht. Als Kontrolltiere wurden Puten verwendet. Während des Versuchs wurden Morbidität, Mortalität und Körpergewichtszunahmen in regelmäßigen Abständen erfasst. Die direkte Untersuchung des Zäkalinhalts wurde zum Nachweis des Parasiten durchgeführt. Zur Evaluierung von Anzeichen einer klinischen Histomonadose und zur Beurteilung des Histomonadeninfektionsgrades wurden 7, 14, 21, 28 und 35 Tage post infectionem (PI) pathologisch-anatomische und –histologische Alterationen bei den Enten ermittelt. Sowohl für die pathologisch-anatomischen als auch für die histologischen Läsionen in Zäkum und Leber wurde ein Bewertungssystem entwickelt. Keine der infizierten Mule- und Moschusenten (n = 83) entwickelten klinische Symptome einer Histomonadose. Die Gewichtszunahmen der infizierten Mule- und Moschusenten entsprachen denen der nicht infizierten Enten. Bei 67 % der Enten (56/83) konnte der Parasit während der gesamten Versuchsdauer im Zäkalinhalt nachgewiesen werden. Bei 5 Enten wurden typische makroskopische Zäkalläsionen zwischen dem 7. und 21. Tag PI beobachtet, wobei der zäkale Nekropsie-Bewertungsgrad 1,6 betrug, was deutlich geringer als bei den Puten (2,9) war. Nur histologische Zäkalveränderungen traten in sechs Fällen auf. Aufgrund dieser Ergebnisse scheinen Enten keine empfänglichen Wirtstiere für die Schwarzkopfkrankheit zu sein, sie können jedoch als Trägertiere für H. meleagridis fungieren. Die Virulenz des Erregers hatte sich offensichtlich nicht verändert, da 67 % der Puten (10/15), die mit dem Zäkalinhalt der positiven Enten infiziert worden waren, die klassischen Symptome einer Schwarzkopfkrankheit entwickelten. Selbst wenn H. meleagridis alleine keine substantielle Gefahr für die Entenproduktion darstellt, sollte seine Infektiosität für die Übertragung zu empfänglicheren Spezies beachtet werden.
Infectividad de Histomonas meleagridis en patos
Se estudió la susceptibilidad de patos Mula y Mudo a la enfermedad de la “Cabeza negra” causada por Histomonas meleagridis, mediante infección intracloacal. Se usaron pavos como controles. Durante los ensayos se registraron regularmente la morbilidad, mortalidad y el peso corporal. Se realizó un examen directo (DE) del contenido cecal para determinar la ausencia o presencia del parásito. Para evaluar cualquier signo de histomoniasis clínica en patos y determinar los portadores de histomonas se valoraron las lesiones macroscópicas y microscópicas a los 7, 14, 21, 28 y 35 días post infección (PI). Se desarrolló un sistema de puntuación para las lesiones macroscópicas e histológicas de ciego e hígado. Los patos Mula y Mudo infectados (n = 83) no desarrollaron nunca ningún signo clínico de histomoniasis. Los incrementos de peso de los patos Mula y Mudo infectados fueron similares a los observados en los patos no infectados. Durante el experimento se demostró la presencia del parásito en el contenido cecal en el 67% de los patos (56/83). Se observaron lesiones características en el ciego en 5 de los patos entre los días 7 y 21 PI, siendo la puntuación media de las lesiones en la necropsia (MLS = 1.6) menos grave que en los pavos (MLS = 2.9). Solamente se observaron lesiones histológicas en el ciego en 6 de los casos. Por lo tanto, los patos no parecen ser huéspedes susceptibles de la “enfermedad de la cabeza negra”, aunque podrían actuar como animales portadores de H. meleagridis. Al parecer la virulencia no sufrió cambios, dado que el 67% de los pavos (10/15) infectados con el contenido cecal de los patos positivos desarrollaron signos clínicos de la enfermedad de la cabeza negra. Pese a que H. meleagridis por si solo no represente un peligro sustancial para el sector de la producción de patos, su infectividad debería tenerse en cuenta en vistas a la transmisión a especies de mayor susceptibilidad.
Introduction
Histomonas meleagridis, a trichomonad protozoan, is the aetiological agent of “blackhead” disease, commonly reported in turkeys. The role of the caecal worm Heterakis gallinarum and its eggs as a reservoir and carrier of the parasite in poultry yard soil has been demonstrated (Gibbs, Citation1962; Lee, Citation1969; Ruff et al., Citation1970) and explains the long period of infectivity of the protozoan in an uninhabited range. The rapid spread of histomoniasis through flocks under field conditions might be due to direct lateral transmission by oral ingestion (Hu & McDougald, Citation2003) or by the cloacal drop method (Hu et al., Citation2004) of viable parasites in fresh droppings of infected birds. H. meleagridis parasitizes birds of the Galliform order, poultry and game birds, with a big difference between host species in their displaying of clinical signs (Lund & Chute, Citation1972a; McDougald, Citation2003). Nothing is really known about “blackhead” in other large poultry production systems, particularly in the duck production, which is the third largest fowl production in the world. Lund et al. (Citation1974) asserted that ducks are unsatisfactory hosts for H. meleagridis, yet in France cases of diarrhoea and typhlo-hepatitis of unknown origin have been reported in domestic Anseriforms, and particularly on duck farms.
Usually, in order to identify potential hosts for a pathogen, it is important to point out the distinction that can be made between the susceptibility of a host species with respect to the disease caused by the pathogen and its ability to survive in a host species in a carrier stage (Toma et al., Citation1999). Only susceptible host species are able to display classical clinical signs of the disease, including death. In the case of histomoniasis, turkeys, pheasants and partridges commonly appear to be the most susceptible hosts.
This study has two objectives: to determine whether ducks could develop clinical histomoniasis or could serve as asymptomatic carriers for H. meleagridis and transmit the infection to turkeys.
Materials and Methods
We experimentally infected muscovy and mule ducklings, the main domestic duck species in Europe, in order to study clinical signs, gross pathology and histopathology. To compare the severity of lesions, a scoring system was developed both for the gross and histological lesions of the caecum and liver on a scale from 0 to 4.
Animals
One-day-old muscovy ducks (Cairina moschata), mule ducks (F1 Cairina moschata and Anas platyrhynchos) and Bettina turkeys (Meleagris gallopavo) were obtained from Grimaud frères (Roussay, France). The ducks were battery raised until they were 2 to 3 weeks old, the turkeys until they were 4 to 5 weeks old, and then they were assigned to an experimental group. The birds were floor housed in separate boxes lined with clean wood shavings as bedding. Feed and water were provided ad libitum.
Infections
The birds were infected through the cloaca with our HmBR-a strain of H. meleagridis (Callait et al., Citation2002). We obtained this strain from H. gallinarum ova isolated from the droppings of chickens naturally infected with H. meleagridis. After in-vitro growth in anaerobia at 39°C on Stepkowski media (Stepkowski & Kilmont, Citation1979), these infected ova were orally given to chickens and H. meleagridis was isolated in their caecal content. The strain was maintained in our laboratory through serial passages in chickens and turkeys. The experimental animals were infected with the caecal content of turkeys infected 5 days earlier. Each inoculum was made with this strain and each dose contained, on average, 3×106 parasites, estimated by counting in Malassez cells.
Evaluation of clinical histomoniasis in ducks
Experiments were performed twice in both duck species. The birds were divided into five groups of eight animals each: infected and uninfected muscovy ducks, infected and uninfected mule ducks, and infected control turkeys. Inoculation was performed at the same time for both the infected ducks and the infected control turkeys. After inoculation, the birds were examined every day for 35 days post infection (p.i.), after which they were euthanized by cervical dislocation and necropsied. The following criteria were selected to appraise the severity of histomoniasis in ducks: during the experiment, morbidity, mortality and body weight gain; at the end of the experiment, the absence or presence of the parasites in the caecal content, gross lesions of the caecum and liver, and microscopic lesions of the caecum and liver.
Morbidity included those birds that developed clinical signs of histomoniasis as described in turkeys (McDougald, Citation2003); that is, sulfur yellow droppings, diarrhoea, drowsiness, depression, drooped wings, hunched posture, and inappetence. Mortality referred to birds that died displaying typical symptoms and lesions of histomoniasis (BonDurant & Wakenell, Citation1994); that is, caseous inflammation in the caecum, with production of the yellow exudates mentioned earlier, and target-like circular depressions on the liver. To follow body weight gains, ducks were weighed twice a week.
The presence or absence of H. meleagridis was investigated using both direct examination (DE) in aliquots of caecal contents, immediately following death, and histological observation. In the caeca, the parasite is described as being of spherical or oval “flagellated lumen form”, measuring about 6 to 20 µm in diameter. The cell contains numerous food vesicles but the flagellum is generally difficult to observe with phase contrast microscopy. In negative samples, the absence of parasites was confirmed using the culture technique as described by Callait et al. (Citation2002).
Gross lesions were converted into a necropsy lesion score (LS). In the caecum, they were classified as: (0) none; (1) moderate thickening of the caecal wall + normal caecal content; (2) moderate thickening of the caecal wall + caseous core partially filling the lumen + slightly haemorrhagic mucosa; (3) severe thickening of the caecal wall + caseous core partially filling the lumen + haemorrhagic mucosa; and (4) severe thickening of the caecal wall + caseous core totally filling the lumen + epithelial necrosis of the mucosa. We graded liver lesions based on the number of target-like yellowish-green circular depressed areas: (0) none; (1) one to five small foci; (2) more than five small foci; (3) numerous small and large foci; and (4) numerous large foci + extended necrosis.
Histological examinations were performed on caecal and liver samples. The absence or presence of the parasite in the caecal wall was made: histomonads are detectable as pale, ovoid bodies (12 to 21 µm) within lacunae in the lamina propria and muscularis layers; in the liver, pockets or nests of intercellular “aflagellar tissues forms” of H. meleagridis can be found. Histological lesions were converted into a histological LS. The histological classification of caecal lesions is: (0) none; (1) small inflammatory infiltration; (2) large nodular mononuclear cells infiltration + large mucosal erosions; (3) extended epithelial necrosis; and (4) necrotic and fibrinous enteritis. Liver microscopic lesions are scored as follows: (0) none; (1) small mononuclear infiltrates near portal vessels; (2) some foci of necrosis; (3) numerous foci of necrosis; and (4) extensive necrosis of parenchyma.
Evaluation of histomonad carriage by ducks
One experiment was carried out in mule ducks and two experiments in muscovy ducks. Each group of infected ducks contained four or five animals. The presence of H. meleagridis was tested in infected ducks at 7, 14, 21 and 28 days p.i. The absence or presence of the parasite in the caecal content and the caecum and liver necropsy LS and histological LS were performed at each date as previously described.
The virulence of H. meleagridis isolated at 7, 14, 21 and 28 days p.i. from positive ducks was also immediately verified while inoculating healthy turkeys with the caecal content from these ducks. In all, 15 turkeys were infected in this way. They were then euthanized 8 days after inoculation. The pathogenicity of the isolate after one passage in the duck was assessed by clinical (mortality and morbidity) and pathological examination (gross and microscopic lesions).
Statistical analysis
The gross and microscopic LS recorded in infected ducks were compared with those found in uninfected ducks and infected turkeys with Students t test based on the necropsy LS and histological LS for each organ of the subjects. Mean lesion scores (MLS) for the caecum or liver were calculated in the different groups both for necropsy and for histology.
Results
Clinical histomoniasis
Except for one mule duck, which accidentally died, none of the experimentally infected ducks became ill or died displaying symptoms consistent with histomoniasis. Weight gains in the control and infected ducks over 35 days were similar irrespective of species: no significant difference between infected and uninfected birds was shown by Students t test (P < 0.05).
The data necropsy collected on day 35 p.i. for the different groups are presented in . The DE of caecal contents revealed the presence of H. meleagridis in 25 to 75% (mean 47%) of the groups, irrespective of duck species. No gross lesions were observed in the livers or caeca of infected ducks. The uninfected ducks did not have a positive DE or any gross lesion.
Table 1. Evaluation of clinical histomoniosis at 35 days p.i. of two duck species using experimental intracloacal innoculation with the HmBR-A strain of H. meleagridis and compared with those observed commonly in turkeys
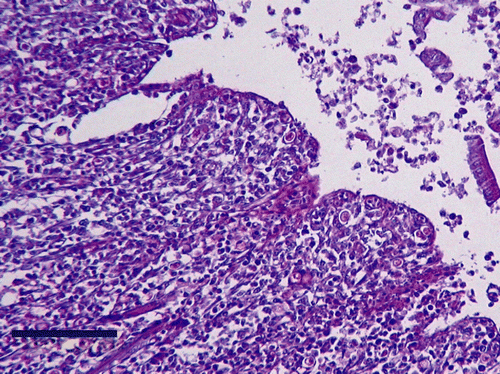
Histological examinations of infected duck organs revealed lesions in 42% of the caeca and in 19% of the livers, with a LS of 1 or 2. Twenty-eight per cent of the uninfected ducks had lesions in the caecal wall, but the histological MLS for all the infected ducks (0.74) were significantly greater (P < 0.05) than uninfected ducks (0.34). In three infected ducks, numerous histomonads were detected within the mucosa () associated with large nodular mononuclear cell infiltrations. In the liver, some mononuclear infiltrates with heterophils appeared near the portal vessels in 19% of the infected ducks and in 6% of the uninfected ducks. But no statistically significant difference (P > 0.1) was found in the histological MLS between the infected (0.22) and uninfected (0.06) ducks, or between all muscovy (0.12) and mule ducks (0.16).
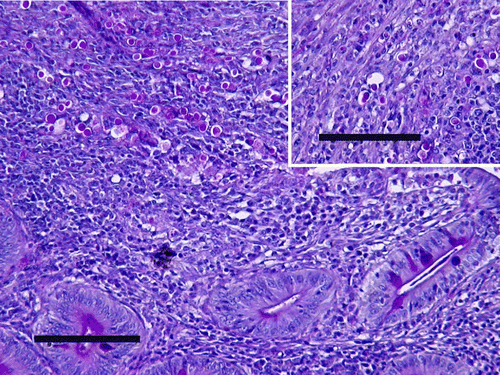
Figure 1. Histologic section of duck caeca, examined 35 days p.i. with H. meleagridis. Note histomonads within the mucosa. Periodic acid Schiff reagent, bar = 100 µm.
All the infected turkeys developed classical histomoniasis, with “sulfur droppings”, depression, hunched posture and anorexia. Fifty-eight per cent died between days 8 and 11 p.i., and the others were euthanized (). The presence of H. meleagridis was discovered in all cases by direct examination of the caecal contents and by histological examination of the caecal mucosa. All of them had caecal gross lesions, with caecal necropsy MLS = 3.09. Seventy-five per cent had gross lesions in the liver, with a liver necropsy MLS = 1.92. In histological samples, severe lesions of enteritis with large epithelial necrosis were frequently observed in infected turkeys. Large histomonad invasions were extended into all the caecal wall structures, associated with large mononuclear and heterophil infiltrations (). The caecal histological MLS of infected turkeys (2.0) was significantly (P < 0.01) greater than those of infected ducks (0.74). The same difference (P < 0.01) was noticed between the histological liver MLS of infected turkeys (2.22) and infected ducks (0.44).
Carrier status
Data regarding the chronological evolution of infected duck groups are presented in . None of the infected ducks died, and none exhibited clinical signs of histomoniasis. However, the parasite was found, by DE, in 20 to 100% of the caecal contents at 7, 14, 21 and 28 days p.i. and, by histology, once in the caecal mucosa at 7 days p.i.
Table 2. Evaluation of histomonad carriage, at 7, 14, 21 and 28 days p.i. by two duck species using experimental intracloacal innoculation with the HmBR-A strain of H. meleagridis
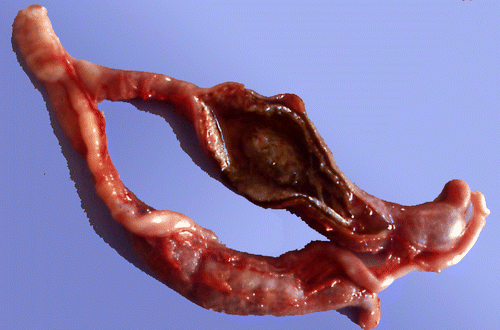
In positive ducks, liver lesions were never observed during necropsy but caecal lesions occurred in five muscovy ducks (three ducks at 7 days p.i., one duck at 14 days p.i. and one duck at 21 days p.i.). The gross lesions were observed in the caecum in two ducks and both caeca in three cases. The caecal necropsy MLS was 1.6 for these five ducks. Lesions were characterized by a thickening of the caecal wall (score 1) in all cases, with partial caseous caecal cores (score 2) in three cases (). DE of the caecal contents and gross lesions of uninfected control ducks were always negative.
Figure 3. Duck caeca, examined at 8 days p.i. with H. meleagridis. Note caseous core (black arrow) with haemorrhagic mucosa (white arrow).
Histological analysis of duck organs showed lesions in 67% of the caeca and in 17% of the livers. Severe caecal histological lesions were seen in six cases with epithelial necrosis (score 3) or necrotic and fibrinous enteritis (score 4). More severe histological lesions were observed in ducks that also demonstrated gross lesions. The liver LS remained at level 1 or 2, even if severe caecal lesions were present. Finally, no significant differences (P > 0.10) were found in the caecal and liver histological MLS according to the examination dates, irrespective of duck species.
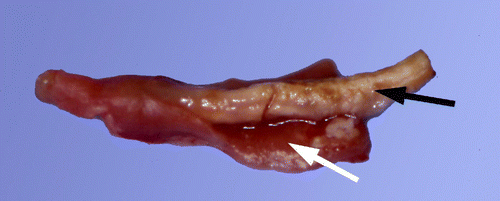
Eighty-seven per cent of the turkeys infected with the caecal content of positive ducks had a positive direct examination at 8 days p.i.; two remained healthy (no parasites in the caecum, no lesions). Sixty-seven per cent developed characteristic caecal gross lesions (), with moderate to severe thickening of the caecal wall and normal, little or clearly caseous caecal content (caecal necropsy MLS = 2.9). One turkey showed a large number of small necrotic spots on the liver (liver LS = 2). Histological lesions were present in 80% of caeca, with MLS = 2.07, and in 53% of liver, with MLS = 0.87.
Discussion
An increase in the prevalence of blackhead has been shown in poultry farms since the ban on Dimetridazole in 1995 (Appendix IV of Council Regulation EEC 1798/95). Moreover, some farming practices increase in the risk of contamination when several poultry species share the same building in standard poultry production, or the same range in traditional farms such as “Label Rouge” or organic. However, the infectivity of H. meleagridis in ducks, which so far has been considered resistant, remains a question.
The present study shows that infected mule and muscovy ducks never displayed clinical signs of histomoniasis and that their body weight gains where similar to those of uninfected ducks and to those of ducks in standard production flocks. Moreover, no gross lesion was observed in ducks at the end of the experiments (at 35 days p.i.), irrespective of their species, and despite that the parasite remained in their caecal content. Microscopic characteristic lesions were only observed in ducks between days 7 and 28 p.i., always significantly less severe than those observed in the turkeys. By histological examination, the parasitic presence in the duck tissue was infrequent and only identified in the caecal mucosa and never in the liver tissue, whereas it was always found in the turkey caecal wall structures and often in the livers. So, using experimental intracloacal inoculation without the use of heterakid eggs, mule and muscovy ducks did not seem to be susceptible to blackhead disease, but they were able to carry and to multiply histomonads in their caeca.
Nevertheless, Lund et al. (Citation1974) considered that the parasite, inoculated orally by heterakid eggs or alone per rectum, is not able to develop in the lumen of duck caeca. This difference underlines the importance of the means of inoculation for this parasite in experimental reproduction of the disease, as recently observed by Hu et al. (Citation2004). On the other hand, comparing the response of galliform birds with histomonad infection with H. meleagridis inoculated orally by heterakid eggs, Lund & Chute (Citation1972a) observed three sets for species tested at less than 8 weeks old: (i) turkey and Chukar partridge, with a high infection rate (>50%), moderate to severe weight losses and caecal lesions; (ii) New Hampshire chicken and guinea fowl, with intermediate infection rate (50%), severe weight losses and moderate to severe caecal lesions; and (iii) ring-necked pheasant with a low rate of infection (12.5%), severe weight losses and mild to moderate caecal lesions. With our results, we show that mule and muscovy ducklings cannot enter in the previous groups, with high infection rate (67%), no weight losses and mild to moderate caecal lesions. This apparent contradiction between carriage and clinical signs of blackhead may partly explain the lack of correlation between moderate to severe clinical signs sometimes observed on farms and poor lesions identified in experimental infections. This phenomenon has already been described in chickens (Homer & Butcher, Citation1991). Probably, other exacerbating circumstances may exist under field conditions, such as highly virulent histomonad strains, the presence of other pathogens (coccidia, helminths, bacteria or virus), physiological particularity or feeding of host species, husbandry practices, and so on. Particularly for ducks, other pathogens may be associated with H. meleagridis in sporadic thyphlo-hepatitis cases reported over the past few years on farms. Indeed, other flagellates, such as Tetratrichomonas gallinarum for example, have also been associated with digestive problems and typhilitis (Leibovitz, Citation1973). Experimental studies suggest that these flagellates are either non-pathogenic or pathogenic under particular conditions (Pecka, Citation1973; Reynaud & Chauve, Citation1987). Likewise, field studies reveal that clinical enteritis does not increase, even though these parasites proliferated after Dimetridazole was replaced by water acidification (Dernburg et al., Citation2005). The hypothesis of H. meleagridis being linked with other flagellates in typhlo-hepatitis on duck farms needs to be confirmed by field analysis at the time of the outbreaks.
To attempt to explain the disparity between clinical signs observed in different species, it is essential to investigate the parasite dissemination in the host body. Indeed, it seems that in ducks, in guinea fowl (Lund & Chute, Citation1972b) and sometimes in chickens (Homer & Butcher, Citation1991), the parasite remains in the caecal lumen and never invades the liver, contrary to what is usually observed in turkeys. The biological meaning of this difference underlines the need for further studies using other methods of investigation.
Finally, H. meleagridis alone probably does not represent a substantial danger for duck production, but we established that it is able to multiply in domestic ducks and its passage in ducks does not alter its virulence in turkeys. So, as a precaution, sharing the same building or land should be banned for ducks and turkeys, as for chickens and turkeys, all the more since it has now been proven that lateral transmission of the parasite without H. gallinarum is possible (Hu & McDougald, Citation2003). On the other hand, we have to take into account the epidemiological role of wild ducks as a carrier and/or reservoir of H. meleagridis for other poultry production, as for other pathogens (Takakuwa et al., Citation1998). It would now be interesting to investigate the question of blackhead's transmission from ducks to galliforms and the potential role of H. gallinarum in this transmission, by further experiments.
Acknowledgments
The authors acknowledge the valuable technical assistance of Mrs S. Balleydier and Dr P. Belli in preparing histological smears, and of Mr C. Dang for animal maintenance. They would like to thank Mrs C. Farmer for help in translating the manuscript.
References
- BonDurant , R.H. and Wakenell , P.S. 1994 . “ Histomonas meleagridis and relatives ” . In Parastic Protozoa , 2nd edn , Edited by: Kreier , J. Vol. 9 , 189 – 206 . San Diego, CA : Academic Press .
- Callait , M.P. , Granier , C. , Chauve , C. and Zenner , L. 2002 . In vitro activity of therapeutic drugs against Histomonas meleagridis (Smith, 1895) . Poultry Science , 81 : 1122 – 1127 .
- Dernburg , A. , Rogier-Saderne , M.C. , Chauve , C. and Zenner , L. 2005 . Consequences of the withdrawal of dimetridazole on intestinal parasitism in ducks . Veterinary Record , 156 : 148 – 150 .
- Gibbs , B.J. 1962 . The occurrence of the protozoan parasite Histomonas meleagridis in the adult and eggs of the cecal worm Heterakis gallinae . Journal of Protozoology , 9 : 288 – 293 .
- Homer , B.L. and Butcher , G.D. 1991 . Histomoniasis in Leghorn pullets on a Florida farm . Avian Diseases , 35 : 621 – 624 .
- Hu , J. and McDougald , L.R. 2003 . Direct lateral transmission of Histomonas meleagridis in turkeys . Avian Diseases , 47 : 489 – 492 .
- Hu , J. , Fuller , L. and McDougald , LR. 2004 . Infection of turkeys with Histomonas meleagridis by the cloacal drop method . Avian Diseases , 48 : 746 – 750 .
- Lee , D.L. 1969 . The structure and development of Histomonas meleagridis (Masticamoebidae: Protozoa) in the female reproductive tract of its host, Heterakis gallinae (Nematoda) . Parasitology , 59 : 877 – 884 .
- Leibovitz , L. 1973 . Necrotic enteritis of breeder ducks . American Journal of Veterinary Research , 34 : 1053 – 1061 .
- Lund , E.E. and Chute , A.M. 1972a . Reciprocal responses of eight species of galliform birds and three parasites: Heterakis gallinarum, Histomonas meleagridis and Parahistomonas wenrichi . Journal of Parasitology , 58 : 940 – 945 .
- Lund , E.E. and Chute , A.M. 1972b . Experimental Histomoniasis in the Guinea Fowl, Numida meleagris . Journal of Protozoology , 19 : 639 – 643 .
- Lund , E.E. , Chute , A.M. and Vernon , E.L. 1974 . Experimental infections with Histomonas meleagridis and Heterakis gallinarum in ducks and geese . Journal of Parasitology , 60 : 683 – 686 .
- McDougald , L.R. 2003 . “ Other protozoan diseases of the intestinal tract—Histomoniasis (Blackhead) ” . In Diseases of Poultry , 11th edn , Edited by: Saif , Y.H. , Barnes , H.J. , Glisson , J.R. , Fadly , A.M. and McDougald , L.R. 1001 – 1006 . Ames : Iowa State University Press .
- Pecka , Z. 1973 . Pathogenicity of Tetratrichomonas gallinarum . Veterinarni medicina (Praha) , 36 : 183 – 188 .
- Reynaud , M.C. and Chauve , C. 1987 . Etude d'un tetratrichomonas parasite des caecums du “canard mulard”. Note I. Isolement et description . Bulletin de la Société Française de Parasitologie , 5 : 167 – 174 .
- Ruff , M.D. , Mc Dougald , L.R. and Hansen , M.F. 1970 . Isolation of Histomonas meleagridis from embryonated eggs of the Heterakis gallinarum . Journal of Protozoology , 17 : 10 – 11 .
- Stepkowski , S. & Kilmont , S. (1979) . Obserwacje nad hodowla in vitro Histomonas meleagridis (Smith, 1895) . Medycyna Weterynaryjna , 35 , 502 – 505 .
- Takakuwa , H. , Ito , T. , Takada , A. , Okazaki , K. and Kida , H. 1998 . Potentially virulent Newcastle disease viruses are maintained in migratory waterfowl populations . Japan Journal of Veterinary Research , 45 : 207 – 215 .
- Toma , B. , Dufour , B. , Sanaa , M. , Benet , J.J. , Moutou , F. , Louza , A. & Ellis , P. (1999) . Applied veterinary epidemiology and the control of diseases in populations ( 536 pp). Maisons-Alfort : AEEMA ed .