Abstract
Nuclear abnormalities in erythrocytes, as micronuclei and nuclear buds (BE), are considered potential biomarkers of genotoxic exposure. We described previously the frequency of spontaneous micronucleated erythrocytes (MNE) in the species Aratinga canicularis. Here, we have used this species to evaluate the induction of MNE and BE by mitomycin-C. Animals were given a single intracoelomic injection of 0, 2, 3 or 4 mg/kg mitomycin-C on two consecutive days. A drop of blood was obtained after 0, 24, 48 and 72 h, and stained smears were used to count micronucleated polychromatic erythrocytes (MNPCE) and polychromatic erythrocytes with buds (BPCE)/1000 polychromatic erythrocytes. The number of MNE and BE in 10 000 total erythrocytes was also counted. MNPCE and BPCE frequencies were elevated at 24, 48, and 72 h after the administration of the lower dose (P<0.03). At a 3 mg/kg dose, the frequency of MNPCE increased at 48 and 72 h (P<0.04) whereas the number of BPCE increased, but not significantly. Administration of 4 mg/kg mitomycin-C increased the number of MNE observed at 72 h (P<0.03), the number of MNPCE at 48 h (P<0.01) and 72 h (P<0.006), the BE frequency at 72 h (P<0.05), and the frequency of BPCE at 48 and 72 h (P<0.001). While mitomycin-C appears to produce a parallel increase in MNPCE and BPCE frequencies, the MNE seemed to be a more sensitive indicator of genotoxicity than the BE. This suggests that evaluating BE and MNE in routine haematological analysis should be considered to evaluate environmental genotoxic exposure.
Anomalies nucléaires des érythrocytes de perroquet (Aratinga canicularis) associées aux lésions génotoxiques
Les anomalies nucléaires des érythrocytes, tels les micronuclei (MN) et les bourgeons nucléaires (BE), sont considérés être des marqueurs potentiels d'exposition génotoxique. Nous avons décrit précédemment la fréquence d'érythrocytes micronucléés spontanés (MNE) dans l'espèce Aratinga canicularis. Ici, nous avons utilisé cette espèce pour évaluer l'induction des MNE et des BE par la mitomycine-C.
Les animaux ont reçu une injection intracoelomique de 0, 2, 3 ou 4 mg/kg de mitomycine-C, deux jours consécutifs. Une goutte de sang a été recueillie après 0, 24, 48 et 72 h, et des frottis colorés ont été utilisés pour compter les érythrocytes polychromatiques micronucléés (MNPCE) et les érythrocytes polychromatiques avec des bourgeons (BPCE)/1.000 érythrocytes polychromatiques. Le nombre de MNE et de BE pour 10.000 érythrocytes a également été compté.
Les fréquences des MNPCE et des BPCE ont été évaluées à 24, 48, et 72 h après l'administration de la plus faible dose (P<0,03). A la dose de 3 mg/kg, la fréquence des MNPCE a augmenté à 48 et 72 h (P<0,04) alors que le nombre des BPCE a augmenté, mais pas significativement. L'administration de 4 mg/kg de mitomycine-C a augmenté le nombre des MNE observé à 72 h (P<0,03), le nombre des MNPCE à 48h (P<0,01) et à 72 h (P<0,006), la fréquence des BE à 72 h (P<0,05), et la fréquence des BPCE à 48 et 72 h (P<0,001).
Alors que la mitomycine-C apparaît produire une augmentation parallèle des fréquences des MNPCE et des BPCE, les MNE paraissent être un indicateur plus sensible de la génotoxicité que les BE. Ceci suggère que l'évaluation des BE et des MNE lors des analyses hématologiques de routine devrait être prise en compte pour évaluer l'exposition génotoxique environnementale.
Kernanomalien in Erythrozyten von Papageien (Aratinga canicularis) im Zusammenhang mit genotoxischer Schädigung
Nukleäre Anoomalien in Erythrozyten wie Mikronuklei (MN) und Kernknospen (-anlagen) (BE) werden als potentielle Biomarker für eine genotoxische Exposition angesehen. Kürzlich haben wir die Häufigkeit spontan auftretender Mikronuklei in Erythrozyten (MNE) in der Spezies (Aratinga canicularis, Orangenbrustsittich) beschrieben. In dieser Studie haben wir diese Spezies verwendet, um die Induktion von MNE und BE durch Mitomycin-C zu untersuchen. Den Tieren wurde an zwei aufeinanderfolgenden Tagen eine Injektion von 0, 2, 3, oder 4 mg/kg Mitomycin-C in die Leibeshöhle gegeben. Nach 0, 24, 48 und 72 h wurde ein Tropfen Blut entnommen, ausgestrichen und gefärbt, um polychromatische Erythrozyten mit Mikronuklei (MNPCE) und polychromatische Erythrozyten mit Knospen (BPCE)/ 1000 polychromatische Erythrozyten auszuzählen. Die Zahl der MNE und BE in insgesamt 10000 Erythrozyten wurde auch bestimmt. 24, 48 und 72 h nach der Verabreichung war bei der niedrigen Dosierung die Häufigkeit von MNPCE und BPCE erhöht (p < 0,03). Bei der Dosis von 3 mg/kg stieg nach 48 und 72 h die Frequenz der MNPCE signifikant (p < 0,04) und der BPCE jedoch nicht signifikant an. Die Gabe von 4 mg/kg Mitomycin-C steigerte die Zahl der MNE nach 72 h (p < 0,03), die Zahl der MNPCE nach 48 (p < 0,01) und 72 h (p < 0,06), die BE-Häufigkeit nach 72 h (p < 0,05 und die BPCE-Frequenz nach 48 und 72 h (p < 0,001). Während Mitomycin-C einen parallelen Anstieg der MNPCE- und BPCE-Häufigkeiten hervorzurufen scheint, sieht es so aus, als ob MNE ein sensitiverer Indikator für eine Genotoxizität als BE ist. Dies suggeriert, dass die Bewertung von BE und MNE in der hämatologischen Routineanalyse zur Bestimmung von genotoxischen Umweltrisiken berücksichtigt werden sollte.
Anormalidades del núcleo de eritrocitos de loros (Aratinga canicularis) relacionadas con daño genotóxico
Las anormalidades nucleares en eritrocitos, como los micronúcleos (MN) y las protuberancias nucleares (BE), se consideran biomarcadores potenciales de exposición genotóxica. Previavemente, hemos descrito la frecuencia espontánea de eritrocitos micronucleados (MNE) en las especies de Aratinga canicularis. En este estudio, hemos utilizado estas mismas especies para valorar la inducción de MNE y BE por mitomicina-C.
Los animales recibieron una única inyección intracelómica de 0, 2, 3 o 4 mg/kg de mitomicina-C en dos días consecutivos. Se obtuvo una gota de sangre tras 0, 24, 48 y 72h, y se utilizaron frotis teñidos para el recuento de eritrocitos policromáticos micronucleados (MNPCE) y eritrocitos policromáticos con protuberancias (BCPE)/1000 eritrocitos policromáticos. También se realizó un recuento del número de MNE y BE en un total de 10,000 eritrocitos.
Las frecuencias de MNPCE y BPCE estaban incrementadas a las 24, 48 y 72h tras la administración de la dosis más baja (P<0.03). A la dosis de 3 mg/kg, la frecuencia de MNPCE aumentó a las 48 y 72 h (P<0.04) mientras que el número de BPCE aumentó, aunque no de manera significativa. La administración de 4 mg/kg de mitomicina-C produjo un incremento del número de MNE observados a las 72 h (P<0.03), del número de MNPCE a las 48 (P<0.01) y 72 h (P<0.006), de la frecuencia de BE a las 72 h (P<0.05), y de la frecuencia de BPCE a las 48 y 72 h (P<0.001).
Mientras que la mitomicina-C induce un incremento paralelo de las frecuencias de MNPCE y BPCE, los MNE pueden ser un indicador más sensible de la genotoxicidad que los BE. Estos resultados sugieren que debería considerarse la valoración de los BE y MNE en los análisis hematológicos rutinarios como indicadores de la exposición genotóxica ambiental.
Introduction
One cytological abnormality that has been studied as an indicator of pathological alterations in certain diseases is the appearance of cells with nuclear buds (BE) (Torres-Bugarín et al., Citation2004). We previously determined the frequency with which spontaneous micronucleated erythrocytes (MNE) appear in the species Aratinga canicularis (Zúñiga-González et al., Citation2000). Interestingly, we observed a high frequency of BE (unpublished data), and, as a result, it is important to determine whether the presence of these abnormal erythrocytes may be inherent to this species or whether a genotoxic agent could have induced the appearance of such structures.
The micronucleus test is a relatively straightforward test to perform in vivo and as such it has been widely used. It produces reproducible results and it is adaptable to different tissues and animal species, including domestic birds on which the assay has been performed in bone marrow cells (Jena & Bhunya, Citation1995). Moreover, internationally accepted guidelines have been established to carry out this assay (Schmid, Citation1975; Hayashi et al., Citation2000; Zúñiga-González et al., Citation2003a). More recently, nuclear protrusions or buds have been described as potential biomarkers of genotoxicity (Serrano-García & Montero-Montoya, Citation2001; Montero et al., Citation2003). In certain species, nuclear buds can be observed in preparations used to evaluate the presence of micronucleus and they could potentially serve as a complementary measure of genotoxicity.
Mitomycin-C is a cytotoxic antineoplastic agent that acts through similar mechanisms to those used by alkylating agents. It is commonly used as a positive control in genotoxicity assays (Jena & Bhunya, Citation1995). In its active form, mitomycin-C selectively inhibits DNA synthesis, and this inhibition is the result of DNA alkylation and cross-linking. Mitomycin-C also inhibits RNA and protein synthesis, albeit to a lesser degree (McVan, Citation1993; Pratt et al., Citation1994).
In the present study, we have used the species A. canicularis to determine whether the frequency of both MNE and BE increases after treatment with the model genotoxin, mitomycin-C, as previously observed in pig and human lymphocyte cultures (Serrano-García & Montero-Montoya, Citation2001).
Materials and Methods
Animals
Twenty-eight birds (A. canicularis) were used in this study. The animals belong to the “Centro para la Conservación e Investigación de la Vida Silvestre de Guadalajara” (The Wildlife Research and Conservation Center, Guadalajara) of the “Dirección General de Vida Silvestre, Secretaría del Medio Ambiente y Recursos Naturales, Jalisco, México” (Wildlife Office, Environment and Natural Resources Department, Jalisco, México), the Mexican federal Department that is in charge of environment and natural resources. This Center is responsible for the preservation of exotic and wild species confiscated from the illegal trafficking. Its aim is to rehabilitate and liberate the animals into their natural environment where possible. However, where the animals do not complete their rehabilitation (often due to causes such as fractures that are not well consolidated and wounds or mutilations) and it is not possible to be liberate them, they are maintained in appropriate conditions of captivity and used for research purposes such as the study carried out here.
The animals used were apparently healthy at the time of sampling upon veterinary examination. Both males and female birds were used and they were of variable weights (mean 75.9±5.9 g). Upon receipt, the birds were identified with a number and registered. After acclimatizing to the laboratory conditions for a minimum of 9 days, the birds were randomly divided into four groups of seven birds each (negative control and three treatment groups; and ). The birds were housed in steel cages (54×30×36 cm, seven birds per cage), and provided with water and food (sunflower seeds) ad libitum.
Table 1. Effect of mitomycin-C on the frequencies of MNE, MNPCE, and PCE in nucleated erythrocytes of treated birds
Table 2. Effect of mitomycin-C on the frequencies of BE, BPCE, and PCE in nucleated erythrocytes of treated birds
Animal treatment
The animals in the control group were given 0.2 ml sterile water intracoelomic and the birds in groups 1 to 3 were given a single intracoelomic injection of 2, 3 or 4 mg mitomycin-C/kg (CAS No. 50-07-7; Sigma, St Louis, Missouri, USA) on two consecutive days. The doses were selected on the basis of the effects described in the literature (Jena & Bhunya, Citation1995). All doses were adjusted to a final volume of 0.2 ml with sterile water. All the animals were handled in accordance with institutional guidelines and National and International Institutes of Health regulations for the humane treatment of research animals.
Blood collection and sample preparation
At 0, 24, 48 and 72 h, a drop of peripheral blood was obtained from the tip of the nail of each bird, and it was smeared on two pre-cleaned and pre-coded microscope slides. The smears were air-dried, fixed in absolute ethanol for 10 min and stained with acridine orange (CAS No. 10127-02-3; Sigma). Acridine orange was used at a concentration of 0.02 mg/ml in phosphate buffer (pH 7.4) (Zúñiga-González et al., Citation2003a).
Sample analysis
The samples were evaluated by the same researcher and they were scored manually using an OLYMPUS CX31 microscope equipped with epifluorescence and an oil-immersion objective (100 x). The number of micronucleated polychromatic erythrocytes (MNPCE) and polychromatic erythrocytes with nuclear buds (BPCE) were determined in 1000 polychromatic erythrocytes (PCE). The numbers of MNE and BE were also determined in 10 000 total erythrocytes and the number of PCE in 1000 total erythrocytes was also counted. Acridine orange stained the PCE red or orange, while normochromatic or mature erythrocytes were stained dark green.
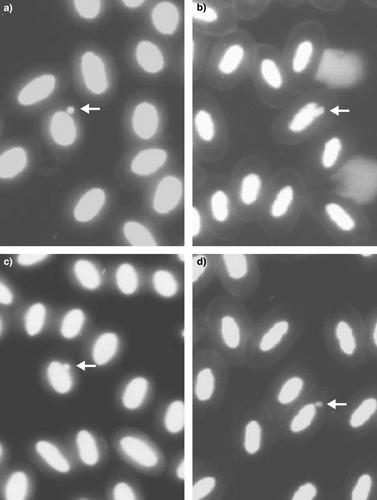
MNE were counted only when the micronucleus was clearly separated from the main nucleus and had a round shape (a). Buds were counted when an elongation from the nucleus originated from the nucleus, partially overlapped the nucleus, or was in the form of drop having an obvious (or presumed) strand connecting it to the nucleus (Figures b to 1d).
Statistical analysis
The results are presented as the means±standard deviation. The data were compared statistically using the SPSS medical software package (version 11.0 for Windows; SPSS, Chicago, Illinois, USA). After establishing the normal distribution of the data, comparisons were made between each treatment group and their respective basal value (0 h) by means of repeated-measures analysis of variance. Subsequently, a least significance difference test was applied to correct the significance values of the multiple post hoc pairwise comparisons. P<0.05 was considered significant.
Results and Discussion
In the present study, the frequencies of MNE, MNPCE, BE and BPCE were measured in mitomycin-C treated parrots to determine whether these parameters increased in a similar manner in vivo as they were previously seen to in pig and human lymphocyte cultures (Serrano-García & Montero-Montoya, Citation2001). No significant differences were observed in the frequencies of MNE and BE from control animals at the different sampling times, indicating that the stress of the manipulation did not affect either of these measures. However, the PCE frequency did increase in control samples 24 h (P < 0.003) and 48 h (P < 0.04) after treatment, and this variation could be related to compensatory cell division in the bone marrow caused by sampling. A similar increase was seen after 24 h in the animals treated with 4 mg/kg mitomycin-C. However, a reduction in PCE frequency was more commonly observed 72 h after exposure to the compound. These decreases in PCE frequency could reflect the bone marrow toxicity of the drug ( and ).
At a dose of 2 mg/kg, mitomycin-C significantly increased the frequency of MNPCE and BPCE at 24, 48, and 72 h (P < 0.03), while MNE and BE frequencies did not increase at any of these sampling times. Exposure to 3 mg/kg compound did not affect the numbers of MNE or BE found at any of the sampling times. However, MNPCE frequencies increased significantly after 48 and 72 h (P < 0.04), and a non-significant increase in BPCE frequencies was observed. After 72 h, the frequency of MNE increased significantly when the animals were exposed to a dose of 4 mg/kg mitomycin-C (P < 0.03), as did the appearance of MNPCE at 48 h (P < 0.01) and 72 h (P < 0.006), that of BE at 72 h (P < 0.05), and the numbers of BPCE at 48 and 72 h (P < 0.001; and ).
Depending on the species, the half-life of bird erythrocytes is approximately 20 to 45 days (Schalm, Citation1964). However, the half-life of an altered erythrocyte, such as a MNE, is shorter than that of a normal erythrocyte. In humans, the half-life of erythrocytes is approximately 120 days in circulation, although normal turnover times can be re-established within 5 to 10 days of exposure to a genotoxin (Heddle et al., Citation1983; MacGregor et al., Citation1987; Hayashi et al., Citation1992, Citation2000; Vanparys et al., Citation1992; Zúñiga-González et al., Citation2003b). Despite the fact that all the birds were maintained under the same conditions before the study, to eliminate the possible influence of a prior genotoxic exposure the parrots used in the present study were also kept under quarantine for a minimum of 9 days. This served as a period of adaptation to the experimental conditions.
The accumulation of MNE and MNPCE are parameters that are related to genotoxicity. An increase in the accumulation of MNPCE results from damage that occurs in the 24 to 48 h period after treatment (Heddle et al., Citation1983) and, as such, MNPCE frequencies allow us to evaluate short periods of exposure (24 or 48 h). In contrast, MNE frequencies can be used to evaluate more chronic exposure, since the significant increases that can initially be observed by determining the MNPCE frequency are reflected 24 h later by changes in the MNE frequency.
The BE frequencies were consistently higher than the MNE frequencies ( and ), suggesting that budding may reflect to a wider spectrum of DNA damage than the formation of micronuclei. The increases observed in this assay suggest that while MNE and BE frequencies generally increase in parallel, MNE may be more sensitive than BE as a biomarker for the genotoxic effects of mitomycin-C. However, the large standard deviations obtained in the study make it difficult to conclude that BE constitute a less sensitive biomarker than MNE. This animal-to-animal variability reduced the overall sensitivity of the assay, and this was especially true when measuring the BE where higher numbers of events were counted. Since the animals were not obtained from a well-controlled animal facility, there are likely to have been differences in the sex and age of the birds. Even though the birds were of a similar weight, they were not necessarily of similar age, and it has been observed that age can influence the capacity of animals to remove MNE from circulation (Zúñiga-González et al., Citation2001a, Citationb).
Parasites can also be found in many domestic avian species, such as doves, with no apparent negative effects on their health. However, infection by the parasite Taenia solium cysticerci is known to induce chromosomal aberrations, sister chromatid exchange and gene mutation, attributed to secretory parasitic molecules (Serrano-García & Montero-Montoya, Citation2001). We found parasites of Haemoproteus sp. in some samples from these birds, although this had no apparent negative effects on their health. It is conceivable that the parasites in these birds could have provoked a series of events that might be responsible for the variability in the MNE and BE frequencies seen here.
Like birds, fishes, amphibians, and reptiles all have large nucleated, ellipsoidal erythrocytes, which could be used to determine environmental genotoxic damage by analysing the BE frequency. In this study, we have demonstrated that the model genotoxin mitomycin-C increases the proportion of BPCE and MNPCE in the nucleated erythrocytes of parrots. Similar increases in nuclear bud frequencies have previously been reported in cell cultures (Serrano-García & Montero-Montoya, Citation2001). Taken together, these results indicate that BE could be an alternative parameter to consider in genotoxicity studies of model species with nucleated erythrocytes, or when it is difficult to establish the spontaneous MNE frequency.
In conclusion, this study demonstrates the relationship between mitomycin-C exposure and an increase in BE. Hence, the scoring of BE in routine haematological analysis should be considered in order to establish normal values for this species and to evaluate environmental genotoxicity exposure.
Acknowledgments
The authors thank Ing. Rogelio Troyo Sanromán for assistance with the statistical analysis.
References
- Hayashi , M. , Kodama , Y. , Awogi , T. , Suzuki , T. , Asita , A.O. and Sofuni , T. 1992 . The micronucleus assay using peripheral blood reticulocytes from mitomycin-C and cyclophosphamide-treated rats . Mutation Research , 278 : 209 – 213 .
- Hayashi , M. , MacGregor , J.T. , Gatehouse , D.G. , Adler , I.D. , Blakey , D.H. , Dertinger , S.D. , Krishna , G. , Morita , T. , Russo , A. and Sutou , S. 2000 . In vivo rodent erythrocyte micronucleus assay. II. Some aspects of protocol design including repeated treatments, integration with toxicity testing, and automated scoring . Environmental and Molecular Mutagenesis , 35 : 234 – 252 .
- Heddle , J.A. , Hite , M. , Kirthart , B.K. , Mavournin , J.T. , MacGregor , G. , Newell , W. and Salamone , M.F. 1983 . The induction of micronuclei as a measure of genotoxicity. A report of the U.S. Environmental Protection Agency Gene-Tox Program . Mutation Research , 123 : 61 – 118 .
- Jena , G.B. and Bhunya , S.P. 1995 . Use of chicks, Gallus domesticus, as an in vivo model for the study of chromosome aberration: a study with mitomycin C and probable location of a ‘hot spot’ . Mutation Research , 334 : 167 – 174 .
- MacGregor , J.T. , Heddle , J.A. , Hite , M. , Margolin , B.H. , Ramel , C. , Salamone , M.F. , Tice , R.R. and Wild , D. 1987 . Guidelines for the conduct of micronucleus assays in mammalian bone marrow erythrocytes . Mutation Research , 189 : 103 – 112 .
- McVan , B.F. 1993 . Physician's Drug Handbook , Pennsylvannia , , USA : Springhouse Corporation .
- Montero , R. , Serrano , L. , Dávila , V. , Segura , Y. , Arrieta , A. , Fuentes , R. , Abad , I. , Valencia , L. , Sierra , P. and Camacho , R. 2003 . Metabolic polymorphisms and the micronucleus frequency in buccal epithelium of adolescents living in an urban environment . Environmental and Molecular Mutagenesis , 42 : 216 – 222 .
- Pratt , W.B. , Ruddon , R.W. , Ensminger , W.D. and Maybaum , J. 1994 . The Anticancer Drugs , 2nd edn , New York : Oxford University Press .
- Schalm , O.W. 1964 . Veterinary Hematology , Philadelphia , PA : Lea and Febiger .
- Schmid , W. 1975 . The micronucleus test . Mutation Research , 31 : 9 – 15 .
- Serrano-García , L. and Montero-Montoya , R. 2001 . Micronuclei and chromatid buds are the result of related genotoxic events . Environmental and Molecular Mutagenesis , 38 : 38 – 45 .
- Torres-Bugarín , O. , Ventura-Aguilar , A. , Zamora-Perez , A. , Gómez-Meda , B.C. , Ramos-Ibarra , M.L. , Morgan-Villela , G. , Gutiérrez-Franco , A. and Zúñiga-González , G. 2004 . Evaluation of cisplatin + 5-FU, carboplatin + 5-FU, and ifosfamide + epirubicine regimens using the micronuclei test and nuclear abnormalities in the buccal mucosa . Mutation Research , 565 : 91 – 101 .
- Vanparys , P. , Deknudt , G. , Vermeiren , F. , Sysmans , M. and Marsboom , R. 1992 . Sampling times in micronucleus testing . Mutation Research , 282 : 191 – 196 .
- Zúñiga-González , G. , Torres-Bugarín , O. , Luna-Aguirre , J. , González-Rodríguez , A. , Zamora-Perez , A. , Gómez-Meda , BC. , Ramos-Ibarra , M.L. , Ramos-Mora , A. , Ortiz , G.G. and Gallegos-Arreola , M.P. 2000 . Spontaneous micronuclei in peripheral blood erythrocytes from 54 animal species . Mutation Research , 467 : 99 – 103 .
- Zúñiga-González , G. , Torres-Bugarín , O. , Ramos-Ibarra , M.L. , Zamora-Perez , A. , Gómez-Meda , B.C. , Ventura-Aguilar , A.J. , Ramos-Mora , A. , Ortiz , G.G. , Álvarez-Moya , C. , González-Rodríguez , A. , Luna-Aguirre , J. and Gallegos-Arreola , M.P. 2001a . Variation of micronucleated erythrocytes in peripheral blood of Sciurus aureo-gaster in relation to age: an increment of micronucleated polychromatic erythrocytes after the administration of colchicine . Environmental and Molecular Mutagenesis , 37 : 173 – 177 .
- Zúñiga-González , G. , Torres-Bugarín , O. , Zamora-Perez , A. , Gómez-Meda , B.C. , Ramos-Ibarra , M.L. , Martínez-González , S. , González-Rodríguez , A. , Luna-Aguirre , J. , Ramos-Mora , A. , Ontiveros-Lira , D. and Gallegos-Arreola , M.P. 2001b . Differences in the number of micronucleated erythrocytes among young and adult animals including humans. Spontaneous micronuclei in 43 species . Mutation Research , 494 : 161 – 167 .
- Zúñiga-González , G. , Gómez-Meda , B.C. , Zamora-Perez , A. , Ramos-Ibarra , M.L. , Batista-González , C.M. , Espinoza-Jiménez , S. , Gallegos-Arreola , M.P. , Álvarez-Moya , C. and Torres-Bugarín , O. 2003a . Induction of micronuclei in proestrus vaginal cells from colchicine- and cyclophosphamide-treated rats . Environmental and Molecular Mutagenesis , 42 : 306 – 310 .
- Zúñiga-González , G.M. , Torres-Bugarín , O. , Zamora-Perez , A. , Gómez-Meda , B.C. , Ramos-Ibarra , M.L. , Gallegos-Arreola , M.P. , Flores-García , A. and López-Uribe , A. 2003b . Induction of micronucleated erythrocytes in mouse peripheral blood after cutaneous application of 5-fluorouracil . Archives of Medical Research , 34 : 141 – 144 .