Abstract
The clinical efficacy of drinking-water administration of enrofloxacin for 3 and 5 days, amoxicillin for 5 days and florfenicol for 5 days for the treatment of respiratory disease induced by an experimental Ornithobacterium rhinotracheale infection in turkeys pre-infected with avian pneumovirus (APV) was assessed based on clinical, bacteriological and histopathological examinations. Experimental groups of 15 susceptible 3-week-old turkeys were each inoculated oculonasally with APV subtype A and 3 days later with susceptible O. rhinotracheale bacteria. Antimicrobial treatment started 1 day after O. rhinotracheale inoculation. After infection, the birds were examined and scored for clinical signs, swabbed daily and weighed at different times. Five birds were euthanized and examined for macroscopic lesions at necropsy at 5 days post bacterial inoculation, and the remainder at 15 days post bacterial inoculation. Samples of the turbinates, trachea, lungs, air sacs, heart and pericardium were collected for bacteriological and/or histological examination. Recovery from respiratory disease caused by an APV/O. rhinotracheale dual infection was most successful after enrofloxacin treatment, irrespective of treatment duration, followed by florfenicol. Amoxicillin treatment was not efficacious. Clinical signs and the number of O. rhinotracheale organisms re-isolated from the trachea and the different respiratory organs were significantly reduced by enrofloxacin treatment for 3 and 5 days. O. rhinotracheale bacteria were not re-isolated from the tracheas of the birds treated with enrofloxacin except for one bird in the 5-day group, as early as 1 day after medication onset. In the group treated with enrofloxacin for 5 days, O. rhinotracheale organisms with a higher minimal inhibitory concentration value (×8) were isolated starting 2 days following treatment onset, initially from a single turkey and subsequently from the other animals.
Comparaison de l'efficacité de quatre traitements antibiotiques vis-à-vis d'une infection expérimentale à Ornithobacterium rhinotracheale chez des dindonneaux pré infectés par un pneumovirus aviaire
L'efficacité clinique de l'administration dans l'eau de boisson, de l'enrofloxacine pendant 3 et 5 jours, de l'amoxicilline pendant 5 jours et du florfénicol pendant 5 jours pour le traitement d'une maladie respiratoire induite par une infection expérimentale à Ornithobacterium rhinotracheale (ORT) chez des dindes pré infectées avec un pneumovirus aviaire (AVP) a été évalué à l'aide d'examens cliniques bactériologiques et histopathologiques.
Des groupes de 15 dindes sensibles, âgées de 3 semaines, ont été inoculées par voie oculonasale avec un AVP de type A et 3 jours plus tard avec un ORT sensible. Le traitement antibiotique a démarré le 1er jour après l'inoculation de l' ORT. Après l'infection, les symptômes ont été enregistrés et des écouvillons ont été réalisées tous les jours, de plus les animaux ont été pesés à différents moments. Cinq jours après l'inoculation de la bactérie (dpbi) 5 animaux ont été euthanasiés puis autopsiés et les lésons macroscopiques ont été recherchées. Il en a été de même sur les survivants 15 dpbi. Les échantillons de cornets nasaux, trachée, poumons, sacs aériens, cæur, et de péricarde ont été prélevés pour des examens bactériologiques et/ou histologiques.
Suite à la maladie respiratoire causée par une double infection à APV/ORT, le traitement ayant donné les meilleurs résultats de guérison a été l'enrofloxacine, quelle que soit la durée du traitement, suivi par le florefénicol. Le traitement à l'amoxicilline n'a pas été efficace. Les symptômes et le nombre de germes d'ORT ré isolés de la trachée et des différents organes respiratoires ont été significativement réduits après les traitements à l'enrofloxacine pendant 3 et 5 jours. ORT n'a pas été réisolé des trachées des oiseaux traités à l'enrofloxacine à l'exception d'un sujet dans le groupe traité durant 5 jours, juste le jour après le début du traitement. Dans le groupe traité à l'enrofloxacine durant 5 jours, des germes d'ORT présentant des valeurs de concentration minimale inhibitrice plus élevées (x 8) ont été isolés à partir du deuxième jour après le début du traitement, à partir d'un seul sujet puis ensuite à partir des autres sujets.
Vergleich der Wirksamkeit von vier antimikrobiellen Behandlungsschemata gegen eine experimentelle Infektion mit Ornithobacterium rhinotracheale von Putenküken nach vorheriger Infektion mit aviärem Pneumovirus
Die klinische Wirksamkeit einer Medikation mit Enrofloxacin für 3 oder 5 Tage, Amoxycillin für 5 Tage und Florfenicol für 5 Tage über das Trinkwasser als Behandlung einer durch eine experimentelle Infektion mit Ornithobacterium rhinotracheale (ORT) verursachte Respirationserkrankung in Puten, die vorher mit aviärem Pneumovirus (APV) infiziert worden waren, wurde durch klinische, bakteriologische und histopathologische Untersuchungen ermittelt. Versuchsgruppen mit jeweils 15 empfänglichen dreiwöchigen Putenküken wurden okulonasal mit APV-Subtyp A und drei Tage später mit empfindlichen ORT-Bakterien infiziert. Die antimikrobielle Behandlung begann einen Tag nach der ORT-Inokulation. Nach der Infektion wurden die Küken auf klinische Veränderungen untersucht, täglich mittels Tupferabstrichen beprobt und in bestimmten Abständen gewogen. Fünf Tiere pro Gruppe wurden 5 Tage nach der bakteriellen Infektion (dpbi) und die restlichen Tiere 15 dpbi euthanasiert und seziert. Für die bakteriologische und/oder histologische Untersuchung wurden Proben von Nasenmuscheln, Trachea, Lunge, Luftsäcke, Herz und Perikard entnommen. Eine Genesung von der durch eine Doppelinfektion mit APV/ORT verursachten Respirationserkrankung trat am schnellsten nach der Enrofloxacin-Behandlung unabhängig von der Behandlungsdauer ein, gefolgt von Florfenicol. Die Amoxicillin-Behandlung war nicht erfolgreich. Die klinischen Symptome und die Zahl der ORT-Organismen, die aus der Trachea und den verschiedenen Respirationgsorganen isoliert wurden, konnten durch die drei- oder fünftägige Enrofloxacin-Behandlung signifikant reduziert werden. ORT-Bakterien wurden mit einer Ausnahme in der 5-Tage-Gruppe am ersten Behandlungstag nicht aus den Tracheen der Enrofloxacin-behandelten Putenküken reisoliert. In der Gruppe, die 5 Tage lang mit Enrofloxacin behandelt wurde, konnten ab dem zweiten. Behandlungstag anfangs aus einer und nachfolgend auch aus den anderen Küken ORT-Organismen mit einem 8-fach höheren minimalen Hemmkonzentration-Wert isoliert werden.
Comparación de la eficacia de cuatro tratamientos antibióticos frente a la infección experimental con Ornithobacterium rhinotracheale en pavos previamente infectados con pneumovirus aviar
Se evaluó la eficacia clínica de la administración de enrofloxacina durante 3 y 5 días, amoxicilina durante 5 días y florfenicol durante 5 días en agua de bebida como tratamientos de la enfermedad respiratoria causada por la infección experimental de Ornithobacterium rhinotracheale (ORT) en pavos previamente infectados con pneumovirus aviar (APV) en base a observaciones clínicas, bacteriológicas e histopatológicas. Los grupos experimentales formados por 15 pavos susceptibles de 3 semanas de edad se inocularon vía oculonasal con APV del subtipo A y tres días más tarde con bacteria ORT susceptible. Los tratamientos antibióticos se iniciaron un día después de la inoculación de ORT. Tras la infección, las aves se examinaron, se valoraron sus signos clínicos y se tomaron hisopos diariamente y se pesaron en distintas ocasiones. Se sacrificaron y se examinaron las lesiones macroscópicas en la necropsia de 5 aves a los 5 días post inoculación bacteriana (dpbi) y de las restantes a los 15 dpbi. Se tomaron muestras de cornetes nasales, tráquea, pulmones, sacos aéreos, corazón y pericardio para estudios bacteriológico y/o histológico. La recuperación de la enfermedad respiratoria causada por la infección mixta de APV/ORT fue más satisfactoria tras el tratamiento con enrofloxacina, independientemente de la duración de este tratamiento, seguido por el tratamiento con cloranfenicol. El tratamiento con amoxicilina no fue eficaz. Los signos clínicos y el número de organismos ORT reaislados de la tráquea y de distintos órganos respiratorios se redujo de manera significativa con el tratamiento con enrofloxacina durante 3 y 5 días. No se reaislaron bacterias ORT de tráqueas de aves tratadas con enrofloxacina incluso en períodos tan breves de medicación como un día, a excepción de un ave en el grupo de 5 días. A partir de los dos días de tratamiento, en el grupo tratado con enrofloxacina, solamente se aislaron organismos ORT con mayores (x8) concentraciones mínimas inhibitorias (MIC) en un pavo, y posteriormente en el resto de los animales.
Introduction
Viral and bacterial respiratory tract infections frequently occur in diseased turkeys of all ages and may cause considerable financial losses due to reduced growth, an increased mortality rate, high medication costs and a higher number of condemnations at processing (van Empel & Hafez, Citation1999).
Ornithobacterium rhinotracheale is an infectious pathogen that has been ascribed an aetiologic role in the respiratory disease complex in turkeys. This Gram-negative bacterium is mostly regarded as a facultatively pathogenic organism, and in field cases simultaneous isolation of O. rhinotracheale, respiratory viruses and/or other bacteria is frequently encountered. One of the viruses judged to have a triggering role is avian pneumovirus (genus Metapneumovirus) (APV) (van Empel et al., Citation1996). Recently, in a longitudinal study performed by Van Loock et al. (Citation2005), it was shown that both APV and O. rhinotracheale infections often occur between hatch and slaughter on Belgian turkey farms. In a previous study using an optimized experimental in vivo model, it was demonstrated that O. rhinotracheale following APV inoculation in 3-week-old turkeys has a synergistic effect on the course of respiratory disease (Marien et al., Citation2005). Dual infection with APV and O. rhinotracheale indeed resulted in more severe clinical signs, macroscopic and histological findings, and a longer persistence of O. rhinotracheale in the respiratory tract, compared with the single infections.
Disease caused by O. rhinotracheale may be reduced by preventing predisposing factors including inadequate ventilation, high ammonia levels, too high or too low relative humidity and infection with additional pathogenic agents (van Empel & Hafez, Citation1999). In practice, however, O. rhinotracheale infections are mostly dealt with using different antibiotics such as amoxicillin, ampicillin, doxycycline, tetracycline, trimethoprim/sulphonamide, enrofloxacin and florfenicol. Hitherto, the actual in vivo efficacy of antibiotics for the treatment of O. rhinotracheale infections in poultry has not yet been investigated. This is to a great extent rooted in the fact that, until only very recently (Marien et al., Citation2005), no suitable infection model was available. The objectives of the present study are to compare the efficacy of enrofloxacin, amoxicillin and florfenicol for treatment of respiratory disease due to experimental O. rhinotracheale infection in 3-week-old turkeys following APV challenge. The efficacy was evaluated on the basis of several parameters; that is, clinical signs, histopathological findings, re-isolation and titration of the bacterium, and weight gain.
Materials and Methods
Turkeys
Seventy-five specific pathogen free turkeys (AFSSA, Ploufragan, France) were used in this study. The turkeys were hatched in our facilities. The birds were housed on litter in separate isolation rooms with HEPA-filtered air, had free access to food and water, and received 16 h of light per day. At 2 weeks of age the birds were shown to be free from maternally-derived antibodies to O. rhinotracheale and APV by means of an enzyme-linked immunosorbent assay available commercially (Biochek, Gouda, The Netherlands) and an in-house serum neutralization test, respectively. Neutralization was tested on Vero cells in 96-well cell-culture microplates using standard procedures. Briefly, serial twofold dilutions of the sera were made and incubated for 1 h at 37°C with an equal volume of virus suspension (subtype A), containing tissue culture infectious dose with a 50% endpoint (TCID50) APV. The reading was based on the absence or presence of a cytopathic effect over 7 days. The serum neutralization titres were the reciprocal of the highest serum dilution that inhibited the cytopathic effect in 50% of the wells.
Virus
The APV strain A/T6/96 (subtype A) was used. The strain was isolated during a respiratory outbreak on a Belgian turkey farm (Van de Zande et al., Citation1998). The virus stock had a titre of 5.0 log10 50% ciliostatic dose (CD50)/ml after the third passage in tracheal organ cultures.
Bacterium
The O. rhinotracheale type strain LMG 9086T was used, which was originally isolated from a turkey with a respiratory tract infection. The strain was serotyped as type A in an agar gel precipitation test (Hafez & Sting, Citation1999) performed by Prof. H. M. Hafez (Institute of Poultry Diseases, Free University of Berlin, Germany). The strain was stored at −70°C. The organism was cultured for 48 h at 37°C on Columbia agar (Oxoid LTD, Basingstoke, UK) with 5% sheep blood in a 5% CO2 atmosphere. The O. rhinotracheale bacteria were transferred into brain heart infusion broth (Oxoid) for 24 h at 37°C with agitation. The bacterial challenge inoculum was prepared by washing the cultured bacteria twice in phosphate-buffered saline (PBS) followed each time by 5 min of centrifugation at 3000 r.p.m. 2000×g at 4°C. The resulting pellet was re-suspended in PBS to obtain a final concentration of 8.6 log10 colony-forming units (cfu)/ml. Confirmation of the number of cfu/ml was done by inoculating 10-fold dilutions in PBS on Columbia agar with 5% sheep blood and counting the number of colonies. The minimal inhibitory concentrations (MICs) of amoxicillin, florfenicol and enrofloxacin for this challenge strain were 2, 1 and ≤0.03 µg/ml, respectively, as determined according to Devriese et al. (Citation2001).
Antimicrobial agents
Three antimicrobial agents were used in this study: enrofloxacin (Baytril® 10% oral solution; Bayer, Leverkusen, Germany), amoxicillin (powder form) (Suramox 50®, soluble powder; Virbac S.A., Carros, France) and florfenicol (soluble form) (Nuflor®; Schering-Plough S.A., Xochimilco, Mexico) with manufacturer-recommended doses of 10 mg/kg for enrofloxacin and 20 mg/kg for amoxicillin and florfenicol.
Experimental design
Seventy-five specific pathogen free turkeys were randomly divided into five groups of 15 birds at 1 day of age.
In all groups, turkeys first received APV, and subsequently received O. rhinotracheale 3 days later. Each bird was inoculated with APV by the oculonasal route at a dose of 4.4 log10 CD50. O. rhinotracheale was likewise administered oculonasally with a dosage of 8 log10 cfu. For inoculation with APV and O. rhinotracheale, a total of 250 µl was divided equally over the nares and eyes.
Four groups received antibiotic treatment: enrofloxacin 10 mg/kg for 3 days (group E3), enrofloxacin 10 mg/kg for 5 days (group E5), florfenicol 20 mg/kg for 5 days (group F), and amoxicillin 20 mg/kg for 5 days (group A). Starting at 24 h post bacterial inoculation, the drinking water was medicated with the appropriate antimicrobial agent. The birds were continuously dosed and received their daily medication over a 24-h period. To enable correct dosing, the daily water uptake and mean group body weights were determined. All turkeys were weighed immediately before APV inoculation, before O. rhinotracheale inoculation, at day 5 after O. rhinotracheale inoculation, and finally at the end of the experiment (15 days post bacterial inoculation (dpbi)). The concentration of each antibiotic to be administered in the water could be calculated accurately on the basis of the water consumption and body weight data. The fifth group was included as an untreated control group (group C).
All birds were clinically examined on a daily basis throughout the experiment. The clinical signs were scored as described in Van de Zande et al. (Citation2001). Briefly, the clinical condition of each bird was assigned a score from 0 (absence of clinical signs) to 7 (nasal exudate with extremely swollen sinuses and frothy eyes, poor general condition and anorexia). The mean clinical score was calculated for each experimental group.
Tracheal swabs were collected from the animals in all groups at 3 days post viral inoculation to confirm infection with APV, and daily until 11 dpbi for O. rhinotracheale titration. The tracheal swabs were taken using cotton-tipped aluminium-shafted swabs (Copan Diagnostics Inc., Corona, USA) and placed in 1 ml PBS supplemented with Ca2 + and Mg2 + and in the case of virus titration an additional 10% foetal calf serum (Gibco, Invitrogen Corporation, Merelbeke, Belgium), penicillin (1000 U/ml) (Biopharma, Rome, Italy) and kanamycin (0.5 mg/ml) (Gibco). Processing occurred as described in the following.
Five birds of each group were randomly selected and were sacrificed at 5 dpbi. The remaining 10 birds were sacrificed at 15 dpbi. The birds were necropsied and examined for gross lesions. Samples of the turbinates, trachea, lungs, air sacs, heart and pericardium were collected for bacterial isolation and processed immediately as described later. Finally, samples from the turbinates, trachea and lungs were taken and fixed in 10% neutral buffered formalin for histopathological examination.
Virological and bacteriological titration of tracheal swabs
The viral titre in log10 CD50 per gram of mucus and the number of cfu of O. rhinotracheale per gram of mucus were determined. This was done using the procedures described in Marien et al. (Citation2005).
Bacteriological titration of tissue suspensions and swabs
Samples of the turbinates, trachea and lungs were titrated for O. rhinotracheale from the 25 birds sacrificed at 5 dpbi. The number of cfu of O. rhinotracheale per gram mucus was determined as described in Marien et al. (Citation2005).
The swabs taken from the heart, pericardium and air sacs were inoculated onto 5% sheep blood agar supplemented with 5 µg/ml gentamicin and 5 µg/ml polymyxin. After 24 to 48 h of incubation at 37°C in 5% CO2 atmosphere, the agar was examined for presence of O. rhinotracheale. The same procedure was followed for samples from the turbinates, trachea, lungs, heart, pericardium and air sacs from the birds sacrificed at 15 dpbi.
Histopathology
Following fixation in formalin, the tissues were embedded in paraffin, sectioned at 4 µm, mounted on glass slides and stained with haematoxylin and eosin using standard procedures. Tissues from the turbinates and trachea were additionally stained by the Periodic Acid Schiff reaction in order to visualize the mucus layer. The degree of severity of the various histological lesions in the trachea and turbinates was assessed, including infiltration of heterophils and/or monomorphonuclear cells, degeneration of the mucous glands in the respiratory epithelium and damage to the ciliated epithelial cells and cilia). Each histological parameter was assigned a score from 0 (absence of particular lesion) to 4 (abundant presence of particular lesion).
MIC of the parent and re-isolated O. rhinotracheale isolates
The MIC values of 124 O. rhinotracheale isolates retrieved from the tracheal swabs before, during and after antibiotic treatment were determined, specifically for the isolates from enrofloxacin groups E3 and E5, for amoxicillin group A and, finally, for florfenicol group F.
Determination of the MIC values was performed as described by Devriese et al. (Citation2001), although modified slightly in that in this study MIC values were determined on Mueller Hinton II agar (BBL, Sparks, Maryland, USA) supplemented with 5% sheep blood. The antibiotics used for the antimicrobial susceptibility test were enrofloxacin (Baytril® 10% oral solution; Bayer), amoxicillin (Suramox 50®; Virbac S.A.) and florfenicol (Nuflor®; Schering-Plough S.A.). Final concentrations ranging from 0.03 to 128 µg/ml (serial doubling dilutions) were tested.
Statistical analyses
The clinical scores and tracheal swabbing data of all animals were first analysed by a fixed-effects model with the area under the curve between 0 and 5 dpbi as the response variable, and the treatment group as a fixed effect, to study the early effects of the infection based on all treated animals.
Next, the repeated clinical scores between 0 and 9 dpbi, using only animals that were not euthanized at 5 dpbi, were analysed by a mixed proportional odds model with the clinical score as the response variable, the animal as a random effect and the treatment group and time as categorical fixed effects.
Tracheal swabbing values between 0 and 9 dpbi, involving only animals that were not euthanized at 5 dpbi, were analysed by a mixed model with log10cfu/g as the response variable, the animal as a random effect, and the treatment group and time as categorical fixed effects. O. rhinotracheale titres from the different organs were analysed by a fixed-effects model, and weight by a mixed model with the animal as a random effect and the time, treatment group and initial weight as fixed effects. Finally, the histopathological scores were analysed by the Kruskal–Wallis test.
All tests were performed at a global 5% significance level, and the seven relevant pairwise comparisons (C versus E3, C versus E5, E3 versus E5, E5 versus A, E5 versus F, E3 versus A, E3 versus F) were tested at a significance level adjusted using Bonferroni's technique. Only these seven comparisons will be considered throughout the paper.
Results
During the experiment, mortalities did not take place in any of the experimental groups.
Clinical signs
The mean clinical score for each group is shown in . Respiratory signs were seen in all APV-inoculated groups starting from 3 days post viral inoculation. From 10 dpbi onwards, clinical signs were not detected in any of the animals in the different experimental groups.
Figure 1. Mean clinical scores in turkeys inoculated with APV and O. rhinotracheale and subsequently treated with different antimicrobial agents: ▪, group E3, 3 days of enrofloxacin (10 mg/kg); •, group E5, 5 days of enrofloxacin (10 mg/kg); ▴, group A, 5 days of amoxicillin (20 mg/kg); ×, group F, 5 days of florfenicol (20 mg/kg); ♦, no treatment, control group. Arrow indicates first day of antibiotic treatment.
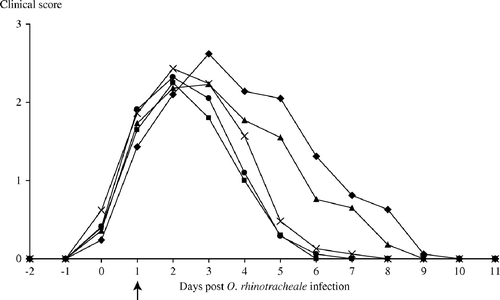
Using the area under the curve from 0 to 5 dpbi, there is a significant difference between the five treatments (P=0.0007). Group C has the highest total score (10.33), followed by groups A, F, E5 and E3 (7). Significant differences are presented in . In the proportional odds model, both a significant change in time (P<0.0001) and a significant difference (P<0.0001) between the treatments were detected. Group E3 had the lowest odds ratio, followed by groups E5, F, A and C.
Table 1. Statistical analyses of clinical scores, tracheal swabbing, isolation of O. rhinotracheale from organs and weight of turkeys inoculated with APV and O. rhinotracheale with an interval of 3 days and treated with different antibiotic therapies
Thus, compared with the non-treated control group, clinical signs were significantly reduced by the enrofloxacin treatments, irrespective of treatment duration, but not by treatment with florfenicol or amoxicillin.
Virological titration of tracheal swabs
APV was recovered from tracheal swabs from every individual bird. The mean titres for groups C, E3, E5, A and F were 6.1, 5.9, 6.2, 5.8 and 6.1 log10CD50/g mucus, respectively.
Bacteriological titration of tracheal swabs
The results of the O. rhinotracheale titrations of the tracheal swabs are shown in . Mean titres as well as titres for each individual bird are depicted. From 11 dpbi onwards, O. rhinotracheale bacteria were not isolated in any of the animals in the different experimental groups.
Figure 2. Bacterial titres (log10 cfu/g mucus) in tracheal mucus collected at different times after O. rhinotracheale inoculation in APV/O. rhinotracheale dually infected turkeys receiving different antibiotic treatments: ▪, group E3, 3 days of enrofloxacin (10 mg/kg); •, group E5, 5 days of enrofloxacin (10 mg/kg); ▴, group A, 5 days of amoxicillin (20 mg/kg); ×, group F, 5 days of florfenicol (20 mg/kg); ♦, no treatment, (control group). Individual values are indicated with small symbols; means are indicated with larger symbols. Arrow indicates first day of antibiotic treatment.
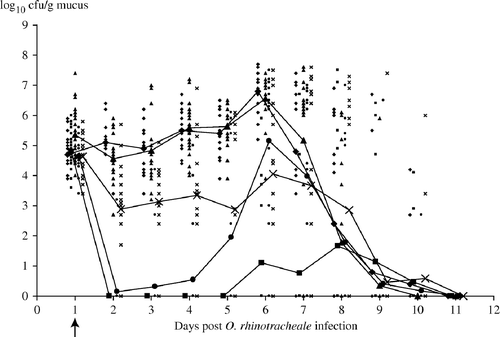
Using the area under the curve in the fixed-effects model, there is a significant difference between the five treatments (P<0.0001), and in the mixed model both a significant change in time (P<0.0001) and a significant difference between treatments (P<0.0001) were detected. In both analyses, group C has the highest total score followed by groups A, F, E5 and E3, and all seven pairwise comparisons (C versus E3, C versus E5, E3 versus E5, E5 versus A, E5 versus F, E3 versus A, E3 versus F) are significant as indicated in .
Compared with the non-treated control group, the number of O. rhinotracheale organisms in the tracheal mucus was hence significantly reduced by both enrofloxacin treatments (3-day and 5-day treatment) and by the 5-day florfenicol treatment. Compared with the non-treated group, treatment with amoxicillin did not cause a significant reduction in the O. rhinotracheale titres in the trachea.
Bacteriological titration of tissue samples
The results of O. rhinotracheale titrations in the various organs sampled at 5 dpbi are presented in . It should be noted that the clinical signs were very similar in terms of severity in all five euthanized birds of one group. For results from the turbinates, trachea and lungs, there were significant differences between the treatments (P<0.0001). Pairwise significant differences are presented in . Compared with the non-treated control group, the number of O. rhinotracheale in the trachea and lungs was significantly reduced by the enrofloxacin treatments and by the florfenicol treatment, as opposed to treatment with amoxicillin. Furthermore, the number of O. rhinotracheale isolated from the turbinates was significantly reduced by the 3-day enrofloxacin treatment. For the air sacs, pericardium and heart, very few positive samples were encountered (in group C and group A), and significant differences between the treatments did not occur.
Table 2. Bacterial titre (log10 cfu/g tissue) of O. rhinotracheale in different organs of turkeys inoculated with APV and O. rhinotracheale with an interval of 3 days and treated with different antibiotic therapies
O. rhinotracheale was recovered from none of the organs (turbinates, trachea, lungs, pericardium, and air sacs) of the birds in the five groups swabbed at 15 dpbi.
Histopathology
Similar microscopic changes were encountered in all experiment groups. In the mucosa of the turbinates and the trachea, a mixed inflammatory reaction was seen. Furthermore, focal loss of cilia and/or necrosis of the epithelium, as well as degeneration of mucous glands, were found in the turbinates and trachea. In the lung samples, focal areas of bronchitis and bronchiolitis were observed.
No significant difference between the five treatments was found for any of the different parameters considered.
Weight
Significant differences occurred between the different treatments (P<0.0001). Weight evolves differently over time from treatment to treatment; that is, there is a significant interaction between time and treatment (P<0.0001). Pairwise significant differences (0.7%) are presented in .
Antimicrobial therapy
The theoretically consumed dose of antibiotics per group of birds was very close (within 10%) to the target dose of 10 mg/kg for enrofloxacin and 20 mg/kg for amoxicillin and florfenicol. The mean actual daily dose (mg/kg) for the total medication period was 10.2 (range, 9.1 to 11.3) for group E3, 10.2 (range, 9.4 to 11.9) for group E5, 20.4 (range, 19.0 to 22.5) for group F, and 19.9 (range, 17.5 to 22.7) for group A.
MIC of the parent and re-isolated O. rhinotracheale isolates
In the O. rhinotracheale bacteria re-isolated from birds in groups C, E3, A and F, no changes in MIC values were observed compared with the challenge strain. However, 2 days after initiation of enrofloxacin treatment onwards, the MICs of the O. rhinotracheale bacteria isolated from tracheal swabs from group E5 had values that increased from 0.03 to 0.25 µg/ml.
Discussion
This study is the first to experimentally investigate the clinical efficacy of different antimicrobial therapies for treatment of dual APV/O. rhinotracheale infection in turkeys. Statistical analysis of the obtained results revealed that, under the circumstances used in this study, recovery from respiratory disease caused by APV/O. rhinotracheale dual infection in 3-week-old turkeys was most successful overall after enrofloxacin treatment (3 or 5 days of treatment), followed by florfenicol treatment. Clinical signs as well as the number of O. rhinotracheale bacteria recovered from the trachea and different respiratory organs (turbinates, trachea and lungs), were significantly reduced by enrofloxacin treatment for 3 or 5 days, compared with the non-treated group. Compared with the non-treated group, 5-day treatment with amoxicillin did not cause a significant reduction in any of the aforementioned parameters and, although 5-day florfenicol treatment significantly diminished the amount of O. rhinotracheale isolated out of the trachea and lungs, this did not seem to result in a significant reduction in clinical symptoms. These results are in accordance with data presented by Froyman & Cooper (Citation2003), who demonstrated that enrofloxacin treatment was most efficacious for the treatment of colisepticaemia in chickens, followed by florfenicol, and that amoxicillin was not effective.
A possible explanation for the observed differences between antimicrobials relates to their different pharmacokinetic and pharmacodynamic properties. An important pharmacokinetic parameter for assessing the ability of antibiotic agents to distribute throughout the tissues is the apparent volume of distribution at steady state (Vd (ss) ). The Vd (ss) value gives an indication of the diffusion of the active antimicrobial compound in the body tissues. A relatively low Vd (ss) value indicates a drug that is less extensively distributed in extravascular tissues. Different pharmacokinetic studies with enrofloxacin, florfenicol and amoxicillin in either turkeys or chickens have been performed (Carceles et al., Citation1995; Anadón et al., Citation1996; Rios et al., Citation1997; Bugyei et al., Citation1999; Knoll et al., Citation1999; Shen et al., Citation2003; Cox et al., Citation2004; Switala et al., Citation2004; Dimitrova et al., Citation2006). From these studies, it may be concluded that the Vd (ss) value of amoxicillin (0.042 to 1.52 l/kg) is generally lower than those of florfenicol (1.5 to 4.99 l/kg) and enrofloxacin (2.92 to 3.9 l/kg).
Another perhaps influential parameter may be the elimination half-life of the different antibiotics. When comparing half-life values determined in different experiments, it may be concluded that enrofloxacin has long half-life values (Brown, Citation1996; Bugyei et al., Citation1999; Garcia Ovando et al., Citation1999; Knoll et al., Citation1999; Dimitrova et al., Citation2006 and that the values for florfenicol are generally lower (Afifi & El-Sooud, Citation1997; Shen et al., Citation2002 Citation2003), with the lowest half-life values noted for amoxicillin (Lashev & Pashov, Citation1992; Carceles et al., Citation1995; Anadon et al., Citation1996). Although both enrofloxacin and amoxicillin have good bactericidal activity, as opposed to florfenicol, which is bacteriostatic, for example, for Escherichia coli and Salmonella spp. (Graham et al., Citation1988), it seems that the bactericidal effect of amoxicillin is impaired because it is less efficiently distributed throughout the tissues and in addition is eliminated quickly (Goren et al., Citation1981). The prominent results for enrofloxacin may be explained by its rapid bactericidal activity at relatively low concentrations (Brown, Citation1996) and its excellent distribution throughout tissues for longer periods of time (Brown, Citation1996; McKellar, Citation1996).
The Clinical and Laboratory Standards Institute (CLSI, formerly NCCLS) breakpoints for amoxicillin vary greatly according to the bacteria involved. For instance, enterobacteriaceae are considered resistant when they have MIC values as high as 32 µg/ml or more, whereas staphylococci are already designated as resistant with MIC values of 0.5 µg/ml or more (CLSI Guidelines, Citation2002). No CLSI breakpoints have been established for O. rhinotracheale, but from the results of the present investigation it can be concluded that an infection caused by O. rhinotracheale strains with an MIC of 2 µg/ml, does not seem to respond to antibiotic treatment with amoxicillin. According to Devriese et al. (Citation2001), who tested 45 O. rhinotracheale strains from poultry, MIC values for ampicillin generally varied between 1 and 8 µg/ml. According to Bush (Citation2003), the antibacterial spectrum of amoxicillin is identical to that of ampicillin and there are few differences in antibacterial activity, especially against Gram-negative bacteria.
No increase in the amoxicillin or florfenicol MIC value for the bacteria re-isolated from the respective groups was noted. Likewise, no change in antibiotic sensitivity was observed in O. rhinotracheale bacteria reisolated from group E3. In group E5, no O. rhinotracheale could be isolated after the first day after onset of treatment, but on the second day of the 5-day treatment course O. rhinotracheale organisms with an eight-fold higher MIC value (from ≤0.03 to 0.25 µg/ml) were isolated, first from one turkey and then successively from the other penmates. This increase in enrofloxacin MIC value concurred with a slight O. rhinotracheale re-excretion in the trachea, also reflected in the higher counts in different organs (turbinates and lungs) at 5 dpbi, compared with the almost negative O. rhinotracheale counts post-treatment in the turkeys treated for 3 days.
The re-emergence of O. rhinotracheale isolates with increased MIC value was first seen in one animal on day 2 of antibiotic treatment, and subsequently, each following day, isolates with increased MIC were found in more birds (data not shown). This may suggest that the less susceptible O. rhinotracheale isolate might have spread from one turkey to other penmates, although further epidemiological studies need to be performed to confirm this. Since O. rhinotracheale isolates with a rise in MIC value were already found after 2 days of enrofloxacin treatment, this suggests that this rise in MIC may also have occurred in the 3-day treatment group.
Although the MIC of the re-isolated bacteria increased from ≤0.03 to 0.25 µg/ml, the O. rhinotracheale isolates are not to be considered resistant to enrofloxacin (Devriese et al., Citation2001; CLSI Guidelines, Citation2002). The term “reduced susceptibility” is more appropriate to characterize the antibiotic sensitivity status of the isolates with an eight-fold increase in MIC value. It should be noted that in the present study, this emergence of decreased sensitivity did not lead to a clinical relapse. Experimental infection studies, including the fully susceptible O. rhinotracheale strain and the O. rhinotracheale strain with slightly higher MIC value, would be necessary to be able to conclude anything about the impact of this MIC rise on the in vivo efficacy of enrofloxacin. Froyman & Cooper (Citation2003) reported that in chickens enrofloxacin was less efficacious for the treatment of disease caused by an E. coli strain with reduced sensitivity (MIC of 0.5 µg/ml) when compared with the treatment of disease caused by a fully sensitive E. coli strain (MIC of 0.015 µg/ml).
Acknowledgments
This work was supported by a grant from Bayer HealthCare AG, Animal Health. The authors would like to express their appreciation to Venessa Eeckhaut, Arlette Van de Kerckhove, Carine Boone and Christian Puttevils for their skilled technical assistance. Furthermore, the authors would like to thank Dr M. Hafez (Institute of Poultry Diseases, Free University of Berlin, Germany) for serotyping the O. rhinotracheale strain used and Mr B. Van Dam (BioChek) for supplying the enzyme-linked immunosorbent assay.
References
- Afifi , N.A. and El-Sooud , K.A. 1997 . Tissue concentrations and pharmacokinetics of florfenicol in broiler chickens . British Poultry Science , 38 : 425 – 428 .
- Anadón , A. , Martinez-Larrañaga , M.R. , Diaz , M.J. , Bringas , P. , Fernandez , M.C. , Martinez , M.A. and Fernandez-Cruz , M.L. 1996 . Pharmacokinetics of amoxicillin in broiler chickens . Avian Pathology , 25 : 449 – 458 .
- Brown , S.A. 1996 . Fluoroquinolones in animal health . Journal of Veterinary Pharmacology and Therapeutics , 19 : 1 – 14 .
- Bugyei , K. , Black , W.D. and McEwen , S. 1999 . Pharmacokinetics of enrofloxacin given by the oral, intravenous and intramuscular routes in broiler chickens . Canadian Journal of Veterinary Research , 63 : 193 – 200 .
- Bush , K. 2003 . “ β-Lactam antibiotics: penicillins ” . In Antibiotic and Chemotherapy , 8th edn , Edited by: Finch , R.G. , Greenwood , D. , Norrby , S.R. and Whitley , R.J. New York : Churchill Livingstone .
- Carceles , C.M. , Vicente , M.S. and Escudero , E. 1995 . Pharmacokinetics of amoxicillin-clavulanic acid combination after intravenous and intramuscular administration to turkeys and chickens . Avian Pathology , 24 : 643 – 952 .
- CLSI Guidelines (2002) . Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals; Approved Standard-Second Edition NCCLS document M31-A2 (pp. 55 – 59 ). Pennsylvannia , , USA : NCCLS .
- Cox , S.K. , Cottrell , M.B. , Smith , L. , Papich , M.G. , Frazier , D.L. and Bartges , J. 2004 . Allometric analysis of ciprofloxacin and enrofloxacin pharmacokinetics across species . Journal of Veterinary Pharmacology and Therapeutics , 27 : 139 – 146 .
- Devriese , L.A. , De Herdt , P. and Haesebrouck , F. 2001 . Antibiotic sensitivity and resistance in Ornithobacterium rhinotracheale strains from Belgian broiler chickens . Avian Pathology , 30 : 197 – 200 .
- Dimitrova , D.J. , Lashev , L.D. , Janev , St.G. and Pandova , V.D. 2006 . Pharmacokinetics of enrofloxacin and its metabolite ciprofloxacin in male and female turkeys following intravenous and oral administration . Veterinary Research Communications , 30 ( 4 ) : 415 – 422 .
- Froyman , R. & Cooper , J. (2003) . Assessment of the efficacy of fluoroquinolones and other antimicrobials against respiratory colibacillosis and septicemia in chickens under standardized challenge conditions . In Proceedings of the XIII Congress of the WPVA (p. 84 ). Denver, CO , USA .
- Garcia Ovando , H. , Gorla , N. , Luders , C. , Poloni , G. , Errecalde , C. , Prieto , G. & Puelles , I. (1999) . Comparative pharmacokinetics of enrofloxacin and ciprofloxacin in chickens . Journal of Veterinary Pharmacology and Therapeutics , 22 , 209 – 212 .
- Goren , E. , De Jong , W.A. and Van Solkema , A. 1981 . Some pharmacokinetical aspects of ampicillin trihydrate and its therapeutic efficacy in experimental Escherichia coli infection in poultry . Avian Pathology , 10 : 43 – 55 .
- Graham , R. , Palmer , D. , Pratt , B.C. and Hart , C.A. 1988 . In vitro activity of florphenicol . European Journal of Clinical Microbiology and Infectious Diseases , 7 : 691 – 694 .
- Hafez , H.M. and Sting , R. 1999 . Investigations on different Ornithobacterium rhinotracheale “ORT” isolates . Avian Diseases , 34 ( 1 ) : 1 – 7 .
- Knoll , U. , Glünder , G. and Kietzmann , M. 1999 . Comparative study of the plasma pharmacokinetics and tissue concentrations of danofloxacin and enrofloxacin in broiler chickens . Journal of Veterinary Pharmacology and Therapeutics , 22 : 239 – 246 .
- Lashev , L.D. and Pashov , D.A. 1992 . Interspecies variations in plasma half-life of ampicillin, amoxicillin, sulphadimidine and sulphacetamide related to variations in body mass . Research in Veterinary Science , 53 : 160 – 164 .
- Marien , M. , Decostere , A. , Martel , A. , Chiers , K. , Froyman , R. and Nauwynck , H. 2005 . Synergy between avian pneumovirus and Ornithobacterium rhinotracheale in turkeys . Avian Pathology , 34 ( 3 ) : 204 – 211 .
- McKellar , Q.A. 1996 . Clinical relevance of the pharmacologic properties of fluoroquinolones . Compendium on Continuing Education for the Practicing Veterinarian , 18 ( 2 ) : 492 – 497 .
- Rios , A. , Martínez-Larrañaga , M.R. and Anadón , A. 1997 . Plasma disposition of florfenicol in broiler chickens following intravenous administration . Journal of Veterinary Pharmacology and Therapeutics , 20 ( Suppl. 1 ) : 182
- Shen , J. , Wu , X. , Hu , D. and Jizng , H. 2002 . Pharmacokinetics of florfenicol in healthy and Escherichia coli-infected broiler chickens . Research in Veterinary Science , 73 : 137 – 140 .
- Shen , J. , Hu , D. , Wu , X. and Coats , J.R. 2003 . Bioavailability and pharmacokinetics of florfenicol in broiler chickens . Journal of Veterinary Pharmacology and Therapeutics , 26 : 337 – 341 .
- Switala , M. , Okoniewski , P. , Smutkiewicz , A. , Jaworski , K. & Hrynyk , R. (2004) . Pharmacokinetics of florfenicol and thiamphenicol in turkeys . In Proceedings of the Second International Conference on Antimicrobial Agents in Veterinary Medicine (AAVM) (p. 99 ). Ottawa , Canada .
- Van de Zande , S. , Nauwynck , H. , Cavanagh , D. and Pensaert , M. 1998 . Infections and reinfections with avian pneumovirus subtype A and B on Belgian turkey farms and relation to respiratory problems . Journal of Veterinary Medicine Series B , 45 ( 10 ) : 621 – 626 .
- Van de Zande , S. , Nauwynck , H. and Pensaert , M. 2001 . The clinical, pathological and microbiological outcome of an Escherichia coli O2:K1 infection in avian pneumovirus infection in turkeys . Veterinary Microbiology , 81 : 353 – 365 .
- van Empel , P.C.M. and Hafez , H.M. 1999 . Ornithobacterium rhinotracheale: a review . Avian Pathology , 28 : 217 – 227 .
- van Empel , P. , van den Bosch , H. , Goovaerts , D. and Storm , P. 1996 . Experimental infection in turkeys and chickens with Ornithobacterium rhinotracheale . Avian Diseases , 40 : 858 – 864 .
- Van Loock , M. , Geens , T. , De Smit , L. , Nauwynck , H. , van Empel , P. , Naylor , C. , Hafez , H.M. , Goddeeris , B.M. and Vanrompay , D. 2005 . Key role of Chlamydophila psittaci on Belgian turkey farms in association with other respiratory pathogens . Veterinary Microbiology , 107 : 91 – 101 .