Abstract
There have been many reports of the severe clinical disease and pathology seen in young chicks that have been vertically infected with chicken anaemia virus (CAV). The disease is characterized by anaemia, and atrophy of the thymus and bone marrow. However, while it has been suggested that horizontally acquired infections of older birds are common, to date there has been no description in the literature of the pathology of this type of infection. In the present study, 3-week-old and 6-week-old chickens were infected by the oral route, as is likely to occur naturally, and a wide range of tissues were examined immunocytochemically for the presence of CAV antigen. Histological examination was carried out on the thymus, spleen and bone marrow of all birds, and on all other tissue samples in which CAV antigen was found. CAV antigen and associated pathological change were detected in the thymus of both 3-week-old and 6-week-old birds. However, CAV antigen was rarely found in other tissues, which is in contrast to what is found in birds infected when 1-day-old. In particular, very few infected cells were found in the bone marrow. Anaemia and bone marrow atrophy, which are typically found in chicks infected vertically or when 1-day-old, did not develop in the 3-week-old or 6-week-old birds. The findings of this study show that CAV is capable of infecting thymocytes of older birds, in contrast to previous belief, and that it is associated with lymphocyte depletion. There was only limited evidence of viral replication in the other tissues examined.
Le virus de l'anémie infectieuse inoculé par voie orale entraîne une déplétion lymphocytaire au niveau du thymus des poulets âgés de trois et de six semaines
Il existe beaucoup de publications concernant la maladie clinique aiguë et les lésions observées chez les jeunes poussins qui ont été infectés verticalement par le virus de l'anémie du poulet (CAV). La maladie est caractérisée par de l'anémie et l'atrophie du thymus et de la moelle osseuse. Par ailleurs, il a été suggéré que les infections de sujets plus âgés, acquises horizontalement, sont communes. Jusqu'ici il n'existait aucune description dans la littérature des lésions induites par ce type d'infection. Dans cette étude, des poulets de 3 et 6 semaines ont été infectés par voie orale, comme il est probable que cela se fasse dans les conditions naturelles, et la présence de l'antigène CAV a été recherchée par une méthode immunocytochimique à partir de nombreux tissus. Des examens histologiques ont été réalisés à partir du thymus, de la rate et de la moelle osseuse de tous les sujets, et à partir de tous les autres échantillons de tissus dans lesquels l'antigène CAV a été détecté. L'antigène CAV et les lésions associées ont été détectés au niveau du thymus des animaux âgés de 3 et de 6 semaines. Cependant, l'antigène CAV a été rarement retrouvé dans les autres tissus, ce qui est en opposition avec ce qui a été observé chez les poussins infectés à l'âge d'un jour. En particulier, très peu de cellules infectées ont été trouvées dans la moelle osseuse. L'anémie et l'atrophie de la moelle osseuse, qui sont typiquement trouvées chez les poussins infectés verticalement ou à l'âge d'un jour, ne se sont pas développées chez les sujets âgés de 3 ou de 6 semaines. Les résultats de cette étude montrent que le CAV est capable de d'infecter les thymocytes des sujets plus âgés, en opposition avec ce qui était antérieurement admis, et que cette infection est associée à une déplétion lymphocytaire. La réplication virale dans les autres tissus examinés n'a été mise en évidence que de façon très limitée.
Oral inokuliertes Hühner-Anämievirus verursacht Verlust der Lymphozyten im Thymus von drei- und sechswöchigen Küken
Es gibt viele Berichte über schwere klinische Erkrankungen und pathologische Veränderungen in Hühnerküken, die vertikal mit dem Hühner-Anämievirus (CAV) infiziert worden sind. Die Erkrankung ist durch Anämie und Atrophie von Thymus und Knochenmark charakterisiert. Obwohl angenommen wird, dass sich ältere Küken horizontal infizieren, gibt es bis heute in der Literatur keine Beschreibung der Pathologie dieser Infektionsart. In der vorliegenden Untersuchung wurden drei- und sechswöchige Hühnerküken oral, wie es wahrscheinlich natürlicherweise erfolgt, infiziert und anschließend ein breites Spektrum von Geweben immunhistochemisch auf das Vorkommen von CAV-Antigen untersucht. Bei allen Tieren wurden Thymus, Milz und Knochenmark und alle anderen Organe, in denen CAV-Antigen gefunden wurde, einer histologischen Untersuchung unterzogen. Sowohl bei den drei als auch bei den sechs Wochen alten Küken wurde CAV-Antigen in Verbindung mit pathologisch-anatomischen Veränderungen im Thymus nachgewiesen. In anderen Geweben jedoch konnte CAV-Antigen nur sehr selten ermittelt werden, was im Gegensatz zu den Befunden bei am 1. Lebenstag infizierten Küken steht. Insbesondere im Knochenmark wurden nur sehr wenige infizierte Zellen gefunden. Die drei- und sechswöchigen Küken entwickelten weder Anämie noch Knochenmarksatrophie, die typisch sind bei Küken nach einer vertikalen Infektion oder einer Infektion am ersten Lebenstag. Die Ergebnisse dieser Studie belegen, dass im Gegensatz zu bisherigen Ansichten CAV Thymozyten älterer Hühnerküken infizieren kann, was mit einer Lymphozytendepletion verbunden ist. Es gab jedoch nur begrenzte Hinweise auf das Vorkommen einer Virusvermehrung in den anderen untersuchten Organen.
El virus de la Anemia Infecciosa inoculado vía oral en pollos de tres y seis semanas de edad induce depleción linfocitaria en el timo
Se han publicado muchas descripciones de la gravedad de los signos clínicos y de la patología observados en pollos jóvenes que se han infectado verticalmente con el virus de la anemia infecciosa (CAV). La anemia, y la atrofia de timo y médula ósea son lesiones características de esta enfermedad. Sin embargo, pese a que se ha sugerido que la infección horizontal de aves adultas es común, hasta la fecha no hay descripciones en la bibliografía de la patología que produce este tipo de infección. En este estudio, se infectaron pollos de 3 y 6 semanas de vida por vía oral, tal como se supone que ocurre de manera natural, y se examinaron un amplio número de tejidos mediante inmucitoquímica para la presencia del antígeno CAV. Se realizó histología de timo, bazo y médula ósea en todas las aves, y en todos aquellos otros tejidos en que se encontró antígeno CAV. Se detectaron antígeno CAV y cambios patológicos asociados a su presencia en el timo de aves de 3 y 6 semanas de vida. Sin embargo, el antígeno CAV apenas se detectó en otros tejidos, de manera contraria a lo que ocurre en aves infectadas al día de vida. En especial, se encontró muy poca cantidad de células en la médula ósea. No se desarrolló anemia ni atrofia de médula ósea en las aves de 3 ni en las de 6 semanas de vida, contrariamente a lo que ocurre en pollos infectados verticalmente o al día de vida. Los resultados de este estudio muestran que CAV es capaz de infectar timocitos de aves adultas, en contra de lo que se creía previamente, y que esta infección se asocia con depleción linfocitaria. Hay muy pocas evidencias de que ocurra replicación vírica en el resto de tejidos examinados.
Introduction
There have been many reports in the literature of the severe clinical disease and pathology (anaemia and atrophy of the bone marrow and thymus) in young chicks that have been vertically infected with chicken anaemia virus (CAV) (McNulty, Citation1991; Schat, Citation2003). While vertical transmission of CAV to progeny can occur even if the breeding birds have antibody to CAV (Miller et al., Citation2003), severe clinical disease has mostly been seen only after seronegative breeding flocks become infected with CAV during lay (McNulty, Citation1991; Miller & Schat, Citation2004). Since antibodies to CAV are common in chicken breeding flocks (McNulty, Citation1991), clinical disease directly attributable to CAV is relatively rare, and the majority of chicks have maternally derived antibodies (MDA) to CAV. MDA appear to be protective against clinical disease, but are usually no longer detectable by 3 weeks of age (McNulty, Citation1991).
Many broiler flocks examined at the time of slaughter have antibody to CAV (McNulty, Citation1991; Jorgensen et al., Citation1995; Dren et al., Citation1996; Todd, Citation2000). This may be the result of infection acquired horizontally, after maternal antibodies have waned, or it could be due to the emergence of vertically transmitted virus. There have been several field-based studies investigating the effects of such infection of broilers, but these have produced conflicting results. McNulty et al. (1991) compared the performance of broiler flocks that had antibody to CAV at the time of slaughter to equivalent broiler flocks that were seronegative at the time of slaughter. They demonstrated that economic performance was significantly better in the flocks that remained seronegative. De Herdt et al. (Citation2001), in another field study, found that CAV infections in broilers are associated with increased slaughterhouse condemnation rates. However, no adverse effect attributable to CAV infection of broiler flocks was found in other published studies (Goodwin et al., Citation1993; Jorgensen et al., Citation1995; Hagood et al., Citation2000). These different findings may reflect the presence of other factors or conditions in the flocks that could not be controlled, or that are unknown.
The vast majority of naturally acquired CAV infections result in seroconversion in birds from 3 weeks of age upwards, after MDA has waned. While some of these seroconversions may be the result of vertically transmitted virus, it also seems probable that some birds will acquire infection horizontally. There have been several studies to date of the effects of CAV in older birds using intramuscular or intraperitoneal routes of inoculation (Yuasa et al., Citation1983; Toro et al., Citation1997; Dren et al., Citation2000) but, surprisingly, there has only been one controlled experimental study of the effects of CAV in older birds infected by a natural route (McConnell et al., Citation1993). There have been no studies to date of the distribution of viral antigen and associated pathology in older birds infected with CAV by natural routes.
The present paper describes a sequential pathological and immunocytochemical study of birds that were inoculated with CAV when 3 weeks of age by the oral route, which is probably a common route of infection under normal field conditions. A smaller study was also carried out on 6-week-old birds inoculated by the oral route.
Materials and Methods
Birds and housing
For the main experiment (a study of 3-week-old birds), 57 White Leghorn chicks derived from a specific pathogen free (SPF) breeding flock that was free of antibody to CAV (Lohmann, Cuxhaven, Germany) were hatched and reared in three negative-pressure isolators. Progeny of the same parent flock had been tested to confirm susceptibility of this strain of bird to CAV infection, by intramuscular inoculation when 1-day-old (Connor & McNeilly, personal communication). Blood samples were collected from 10 birds per isolator 3 days before inoculation was due to take place, and were examined using an indirect immunofluorescence test for antibody to CAV (McNulty et al., Citation1991).
For the study of 6-week-old birds, 14 White Leghorn chicks derived from a different SPF breeding flock of the same company, and meeting the same criteria as stated earlier, were used. The only reason for use of a different parent flock was that this study was carried out 2 years after the first, when the first breeding flock no longer existed. Blood samples were collected from all birds a few days before inoculation was due to take place, and were examined for antibody to CAV as already described.
Virus inoculation
In both experiments, inoculated chickens each received 0.5 ml CAV (Cux-1 strain) suspension containing 106.6 median tissue culture infective doses by the oral route. Cux-1 virus was supplied by Dr V. von Bulow (Free University of Berlin, Germany) and grown in MDCC-MSB1 cells as described previously (McNulty et al., Citation1990).
Immunostaining for CAV antigen
Sections of formalin-fixed paraffin-embedded sections of tissue samples were stained for CAV antigen using monoclonal antibody (mAb) 2A9 (McNulty et al., Citation1990) followed by one of three detection systems for mouse mAb. For all tissues other than the liver, kidney and bone, the avidin–biotin–peroxidase complex system for detection of mouse primary antibody was used as previously described (Smyth et al., Citation1993). Different detection systems were used for the liver, kidney and bone marrow because non-specific staining of endogenous substances was problematic in these tissues when using the avidin–biotin–peroxidase complex detection system, in spite of the incorporation of blocking procedures (Smyth et al., Citation1993). For sections of the femur, a second immunoperoxidase (IPX) technique, the streptavidin peroxidase detection system for mouse primary antibody was used (Smyth et al., Citation1993). For liver and kidney sections, the alkaline phosphatase anti-alkaline phosphatase detection system (APAAP) (Smyth et al., Citation1993) was used. In tissues where the presence of endogenous substances caused interpretative difficulties, a second of the aforementioned techniques was employed and, for the vast majority of the samples where this occurred, cryostat sections of unfixed tissue were examined by indirect immunofluorescence (IIF) as described in the following.
Cryostat sections of quench frozen unfixed tissues were examined by IIF. Sections were first incubated with 10% normal rabbit serum in 0.005 M Tris-buffered saline (pH 7.6) containing 1% bovine albumin (TBS-BA) for 20 min, followed by overnight incubation at 4°C with mAb 2A9. After two 10-min washes in TBS-BA, sections were incubated in 2.5% flouroscein isothiocyanate-conjugated rabbit anti-mouse antibody (Dako Ltd., High Wycombe, UK) in TBS-BA containing 10% normal chicken serum for 2 h at room temperature. Sections were again washed twice in TBS-BA and mounted in buffered glycerol saline.
Experimental design
Three-week-old bird study
At 3 weeks of age, 39 chicks housed in two isolators were each inoculated with CAV (see earlier) by the oral route. Three of these chickens were killed on each of days 1, 3, 6, 8, 10, 13, 15, 17, 20, 22, 24, 28 and 31 post inoculation (p.i.). Eighteen chickens from the same hatch were kept in a separate isolator and used as negative controls. Two control chickens were killed on each of days 2, 6, 10, 13, 17, 20, 24, 28 and 31 p.i.
The packed cell volume (PCV) was determined on a blood sample collected from each bird before it was killed. Blood samples were also collected from all control birds for examination for antibodies to CAV by IIF (McNulty, Citation1991).
Samples of the following tissues were collected from all birds into 10% neutral buffered formalin for histological and immunocytochemical examination: pharynx, proventriculus, ascending duodenum, jejunum (proximal to Meckel's diverticulum), ileum, caecum, colorectum, a transverse section of the upper jaw taken between the external nares and the eye (sections thus included the walls of the nasal cavity, turbinates and sinuses), trachea, lung, thymus, spleen, bursa of Fabricius, femur, liver, pancreas, kidney, heart, skin and conjunctiva. Femurs were split longitudinally to expose the bone marrow. The samples were fixed for 6 h. Bones and the section of upper jaw were then decalcified in ethylenediamine tetraacetic acid as described by Smyth et al. (Citation1993) for the minimum time necessary. All tissues were processed to paraffin and pairs of sections (adjacent sections) were cut, one for immunocytochemical staining and one for staining with haemotoxylin and eosin. Further sets of adjacent sections were prepared as necessary for control purposes (see later).
With the exception of the femur, for which bone marrow was substituted, a second sample of each tissue from all birds was quench frozen by immersion in isopentane cooled with liquid nitrogen.
Sections of all formalin-fixed paraffin-embedded tissues from each of the inoculated birds and six of the control birds were examined immunocytochemically for the presence of CAV antigen as already described. Cryostat sections were prepared from the bone marrow, thymus and spleen of all inoculated birds and three control birds, and from selected other samples, for IIF staining for CAV antigen.
For negative controls for each technique, in addition to the application of the immunocytochemical techniques to the tissues from the uninoculated birds, selected tissues from inoculated birds were incubated with a mouse primary antibody not expected to bind to tissue, or, alternatively, incubation with the primary antibody was omitted from the technique.
For positive control purposes, sections from known CAV antigen-positive tissues were included with every batch of sections immunostained.
Detailed histological examination was carried out on the thymus, spleen and bone marrow of all birds and on all other antigen-positive tissues.
Six-week-old bird study
Ten birds housed in two isolators were each inoculated with CAV (see earlier) by the oral route. Four birds housed in a separate isolator served as negative controls.
Four inoculated birds were killed on day 11 p.i., and three birds on each of days 13 and 15 p.i. Two control birds were killed on each of days 11 and 15.
The PCV was determined on a blood sample collected from each bird before it was killed. Blood samples were also collected from the control birds for examination for antibodies to CAV by IIF (McNulty, Citation1991).
Based on the results of the study in 3-week-old birds, a reduced range of tissues was collected from all birds into 10% neutral buffered formalin for histological and immunocytochemical examination. The tissues targeted were the thymus, bone marrow, proventriculus, ascending duodenum and lung, since these were considered most likely to contain antigen if infection became generalized, given the distribution of antigen in 1-day-old birds (Smyth et al., Citation1993).
Results
Birds inoculated when 3 weeks old
Packed cell volumes
The PCVs in the control birds ranged from 31 to 36% (mean, 33.5%; standard deviation, 1.59). PCVs in inoculated birds ranged from 24 to 38% (mean, 31%; standard deviation, 2.41).
In the birds inoculated at 3 weeks of age, only one bird had a PCV lower than 27% (the value frequently used to define presence or absence of anaemia in 2-week-old birds). This bird was killed at day 13 p.i. and had a PCV of 24%. Three birds had a PCV of 29%, and all other birds had a PCV ≥30%
Clinical signs
Birds did not show any clinical evidence of disease throughout the period of the experiment.
Macroscopic findings
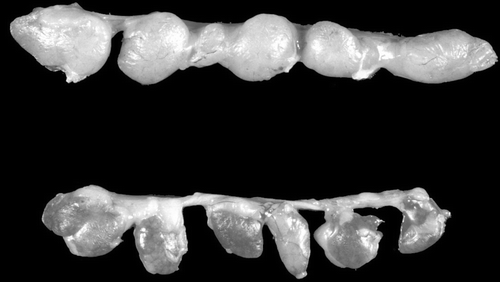
There was reddening and mild atrophy of the thymus of one bird at day 13 () and one bird at day 15 p.i. There was moderate atrophy of the thymus in two birds at day 17 p.i. There were no other significant gross lesions.
Figure 1. Thymic lobules from a control (top) and an infected (bottom) chick at day 13 p.i. with CAV when 3 weeks of age. Note that the lobules of the infected bird are darker in colour (reddened) and are not as plump as those of the uninoculated control. Subsequent histological examination showed loss of lymphocytes in the inoculated birds.
CAV antigen distribution
With the exception of thymus, CAV antigen was rarely detected in tissues using the IPX and APAAP detection system techniques on formalin-fixed paraffin-embedded tissues. For this reason, frozen sections of all bone marrows and spleens were also examined by IIF, which confirmed the results obtained by IPX.
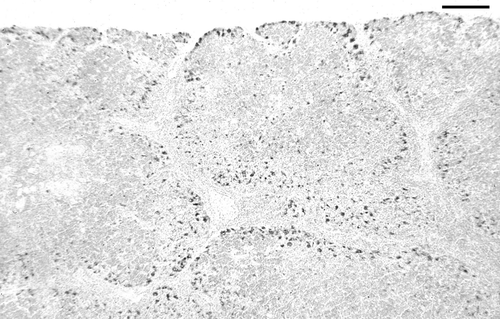
In the thymus, antigen was first detected in small quantities in two of the three birds examined at day 8 p.i. Antigen was then found in thymus of all birds examined on days 10 to 17 p.i., and in the thymus of some birds examined on days 20 and 22 p.i. Antigen was most abundant on days 13 to 17 p.i. () and was distributed predominantly in the outer cortex. Some areas of cortex contained abundant antigen, while other areas of cortex contained little or no antigen. No antigen was detected after day 22. The pattern of antigen staining is described under histopathological findings.
Figure 2. IPX-stained section of thymus showing abundant CAV antigen (dark) located mainly in the outer cortex of lobules. Day 13 p.i., bird inoculated when 3 weeks of age. Bar = 125 µm.
Rare antigen positive cells were detected in other tissues from occasional birds as follows: bone marrow on days 15 and 17 p.i., spleen on days 13 and 17 p.i., lung on days 6 and 8 p.i., and proventriculus on day 15 p.i.
Histopathological findings
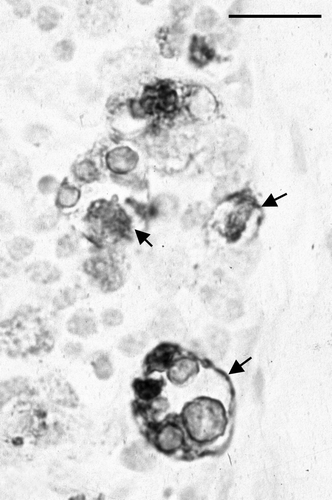
In the thymus, histological changes were first detected in the outer cortex at day 10 p.i., and were focal. In affected areas, there were many markedly enlarged nuclei, often with very pale staining of the chromatin. Some of these nuclei contained small circular eosinophilic inclusions, similar to those previously described by Goryo et al. (Citation1989) and Smyth et al. (Citation1993) (). These markedly enlarged nuclei and the inclusions were positively immunostained by mAb 2A9 (). At day 13 p.i. there was marked lymphocyte depletion of the cortex in all birds (). In two of the three birds, extensive areas of the cortex were almost devoid of recognizable lymphocytes. In subcapsular regions, there were numerous markedly enlarged nuclei, some of which contained small eosinophilic inclusions. Three types of immunostaining were observed in the adjacent tissue sections when stained by IPX: staining of the enlarged nuclei, staining of small structures that resembled apoptotic bodies, and dense staining of the periphery of very large, otherwise unstained, structures, the nature of which was not determined (). At days 15 and 17 p.i. there was marked focally extensive depletion of thymic cortex. Enlarged nuclei with inclusions were less common, and clusters of apoptotic bodies were more evident. Immunostaining was more granular in appearance and sometimes had a dendritic pattern. At days 20 and 22 p.i., the cortex of some birds appeared normal, while in some there were foci of cortical depletion or thinning, and in others clusters of apoptotic bodies were prominent. From day 24 p.i. onwards, there were no obvious abnormalities in the thymus.
Figure 3. Thymic cortex, day 13 p.i. There are numerous very enlarged nuclei (arrows) interspersed among normal sized lymphocytes. Several of the enlarged nuclei contain circular inclusions, the periphery of which stain eosinophilically. Bird inoculated when 3 weeks of age. Haematoxylin and eosin. Bar = 25 µm.
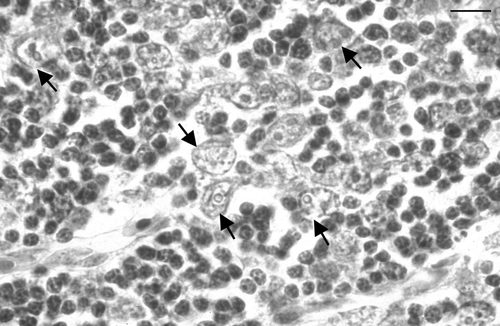
Figure 4. High magnification of an IPX-stained section of thymic cortex. Infected cells are markedly enlarged and circular inclusions are stained in several of the infected cells. Bar = 3 µm.
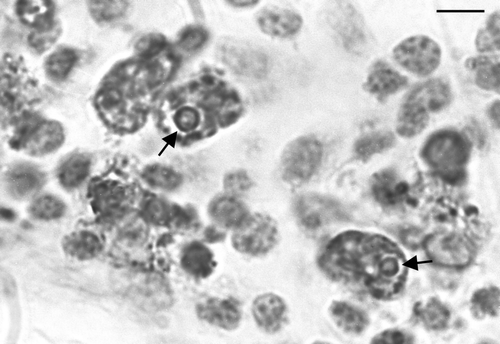
Figure 5. Low magnification of thymic cortex in a bird inoculated with CAV when 3 weeks of age, 13 days p.i. Note marked lymphocyte depletion of the thymic cortex. Haematoxylin and eosin. Bar = 65 µm.
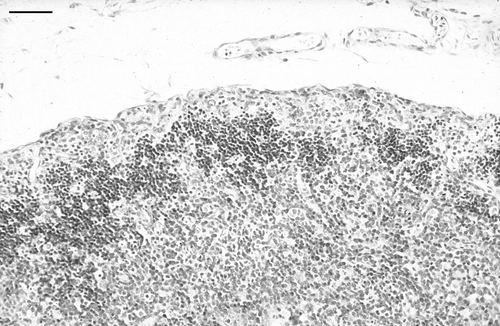
Figure 6. Large IPX-stained structures whose exact nature was not determined (arrows). Day 15 p.i., bird inoculated when 3 weeks of age. Bar = 30 µm.
In other tissues examined, no significant abnormalities were detected.
Birds inoculated when 6 weeks old
In birds inoculated at 6 weeks of age, the mean PCV was 33% (standard deviation, 2.5; range, 30 to 38).
CAV antigen was present in the thymic cortex () of all birds, although it was not uniformly distributed throughout the cortex in all birds. Some areas were heavily infected, while other areas were of normal appearance.
Figure 7. Low magnification of thymic cortex in a bird inoculated with CAV when 6 weeks of age. CAV antigen (dark staining) is located mainly in the outer thymic cortex, similar to that seen in birds inoculated when 3 weeks of age. Bar = 125 µm.
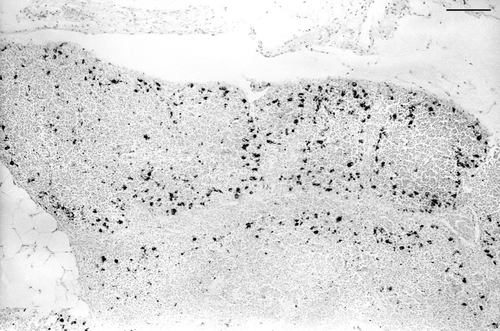
Very occasional cells in the bone marrow and/or lung of some birds were specifically immunostained. Histopathological changes were present in the thymus, and were similar to those seen in the birds inoculated when 3-weeks-old.
Serological testing
Sera collected from birds before experimental infection and the sera from the uninoculated control birds did not contain detectable antibody to CAV.
Discussion
The clinical and pathological features of the disease caused by vertical infection of chicks with CAV are well recognized, and are characterized by anaemia and atrophy of the thymus and bone marrow. While older birds are susceptible to infection (McNulty, Citation1991), chicks are considered to develop resistance to disease caused by CAV by about 2 to 3 weeks of age (Yuasa et al., Citation1980; Rosenberger & Clyde, Citation1989; Schat, Citation2003). Up until this age, most chickens in the field will be protected by MDA (McNulty, Citation1991).
McNulty et al. (1991) described a study of 50 commercial broiler flocks that suggested horizontal infection of broilers with CAV caused economically significant subclinical effects. In support of the findings of McNulty et al., De Herdt et al. (Citation2001) found a higher rate of condemnation of carcases from flocks that had been infected with CAV. However, in other studies of broiler flocks with naturally occurring CAV infections (Goodwin et al., Citation1993; Jorgensen et al., Citation1995) no adverse effect of infection was found. In view of these conflicting findings, experimental studies examining the effects of CAV infection in birds at an age when they are likely to be susceptible to, and could be exposed naturally to, infection by CAV were warranted. However, to our knowledge, there has only been one study of older birds infected by a natural route (McConnell et al., Citation1993) and that did not examine for pathological change or viral distribution.
The study by McConnell et al. (Citation1993) showed that infected birds were transiently but significantly immunosuppressed. However, the mechanism of this effect was not elucidated. In very young birds, immunosuppression is thought to be mainly due to the effects of the virus on thymic lymphoblasts and lymphocytes. However, Jeurissen et al. (Citation1992) suggested that CAV susceptible cells are not present in the thymus after 21 days of age.
The present study examined the outcome of CAV infection by a natural route (oral) in SPF chickens when 3 weeks of age. This showed clearly that, in such birds, CAV replicated in the thymic cortex and was associated with substantial lymphocytic depletion of the thymus. The consequent reduction in thymic size was detectable grossly when compared with controls. The temporal distribution of antigen in the thymus was similar to that seen in birds inoculated by the intramuscular route when 1-day-old, except that there was a longer lag period between inoculation and the appearance of antigen in the thymus, and antigen was detectable for a shorter duration. The finding of lymphocyte depletion of the thymus supports the finding by McConnell et al. (Citation1993) that CAV infections in older birds are immunosuppressive, and indicates that the mechanism of the effect is direct viral damage to cells in the thymic cortex, just as occurs in the very young bird.
The finding of viral antigen in the thymic cortex did not support the suggestion by Jeurrissen et al. (Citation1992) that the cells in the thymic cortex are no longer susceptible to infection after 21 days of age. Our findings are in agreement with others who studied older birds using parenteral routes of inoculation, and who also found viral replication in the thymus. However, to extend our findings and to remove the possibility that infection had occurred just before such resistance developed, we carried out a small study of birds inoculated when 6-weeks-old. Again we found evidence of extensive viral antigen in the thymic cortex, confirming that cells of the thymus remain susceptible to CAV infection.
In a study of birds infected when 1-day-old (Smyth et al., Citation1993), CAV antigen was found in many tissues throughout the body. In contrast, in the birds infected when 3 weeks old, CAV antigen was rarely found in tissues other than thymus. In particular, very few infected cells were found in the bone marrow. The latter is perhaps not surprising as anaemia did not develop in our birds. The possibility that the strain of birds studied had innate resistance to the effects of CAV was excluded, since 1-day-old birds of the same strain were fully susceptible to the effects of the virus.
Because CAV antigenicity is markedly affected by fixation (Smyth et al., Citation1993) IIF was carried out on selected unfixed tissues to ensure that failure to demonstrate antigen was not a fixation artefact.
It has been shown that VP3 of CAV induces nucleosomal laddering characteristic of apoptosis and so it has been suggested that CAV causes cell death by triggering apoptosis in thymocytes (Jeurissen et al., Citation1992; Noteborn et al., Citation1994; Noteborn, Citation2004). Cells undergoing apoptosis exhibit characteristic morphological features such as shrinkage and fragmentation (Anilkumar et al., Citation1992; Cohen, Citation1993; Noteborn & Koch, Citation1995). It is worth noting that, as in our study of CAV infection of 1-day-old birds, we found that the earliest evidence of CAV infection was the presence of markedly enlarged cells with very enlarged nuclei. Such swelling of cells and nuclear enlargement are not features of apoptosis, although it is possible that these enlarged cells eventually become apoptotic.
The findings in the present study support those of McConnell et al. (Citation1993) and McNulty et al. (1991) that horizontally acquired CAV infection has subclinical effects on chickens, and suggest that the mechanism of the effect is immunosuppression caused by viral damage to the thymus. Controlled experimental studies to examine for direct effects of CAV infection on performance (such growth rate and food conversion efficiency) in rapidly growing broilers would be useful, although it seems probable that significant performance effects will be caused by other organisms facilitated by the immunosuppression.
References
- Anilkumar , T.V. , Sarraf , C.E. and Allison , M.R. 1992 . The biology and pathology of programmed cell death (apoptosis) . Veterinary & Human Toxicology , 34 : 251 – 254 .
- Cohen , J.J. 1993 . Apoptosis . Immunology Today , 14 : 126 – 129 .
- De Herdt , P. , Van den Bosch , G. , Ducatelle , R. , Uyttebroek , E. and Schrier , C. 2001 . Epidemiology and significance of chicken infectious anemia virus infections in broilers and broiler parents under nonvaccinated European circumstances . Avian Diseases , 45 : 706 – 708 .
- Dren , C. , Farkas , T. and Nemeth , I. 1996 . Serological survey on the prevalence of chicken anaemia virus infection in Hungarian chicken flocks . Veterinary Microbiology , 50 : 7 – 16 .
- Dren , C.N. , Kant , A. , Roozelaar , D.J. , van Hartog , L. , Noteborn , M.H.M. and Koch , G. 2000 . Studies on the pathogenesis of chicken infectious anaemia virus infection in six-week-old SPF chickens . Acta Veterinaria Hungarica , 48 : 455 – 467 .
- Goodwin , M.A. , Smeltzer , M.A. , Brown , J. , Girschick , T. , McMurray , B.L. and McCarter , S. 1993 . Effect of so-called chicken anemia agent maternal antibody on chick serologic conversion to viruses in the field . Avian Diseases , 37 : 542 – 545 .
- Goryo , M. , Suwa , T. , Umemura , T. , Itakura , C. and Yamashiro , S. 1989 . Histopathology of chicks inoculated with chicken anaemia agent (MSBI-TK5803 strain) . Avian Pathology , 18 : 73 – 89 .
- Hagood , L.T. , Kelly , T.T. , Wright , J.C. and Hoerr , F.J. 2000 . Evaluation of chicken infectious anemia virus and associated risk factors with disease and production losses in broilers . Avian Diseases , 44 : 803 – 808 .
- Jeurissen , S.H.M. , Janse , M.E. , van Rooselaar , D.J. , Koch , G. and de Boer , G.F. 1992 . Susceptibility of thymocytes for infection by chicken anaemia virus is related to pre- and posthatching development . Developmental Immunology , 2 : 123 – 129 .
- Jorgensen , P.H. , Otte , L. , Nielsen , O.L. and Bisgaard , M. 1995 . Influence of subclinical virus infections and other factors on broiler flock performance . British Poultry Science , 36 : 455 – 446 .
- McConnell , C.D.G. , Adair , B.M. and McNulty , M.S. 1993 . Effects of chicken anemia virus on cell-mediated immune function in chickens exposed to the virus by a natural route . Avian Diseases , 37 : 366 – 374 .
- McNulty , M.S. 1991 . Chicken anaemia agent: a review . Avian Pathology , 20 : 187 – 203 .
- McNulty , M.S. , McIlroy , S.G. , Bruce , D.W. and Todd , D. 1991 . Economic effects of subclinical chicken anemia agent infection in broiler chickens . Avian Diseases , 35 : 263 – 268 .
- McNulty , M.S. , Mackie , D.P. , Pollock , D.A. , McNair , J. , Todd , D. , Mawhinney , K.A. , Connor , T.J. and McNeily , F. 1990 . Production and preliminary characterization of monoclonal antibodies to chicken anemia agent . Avian Diseases , 34 : 352 – 358 .
- Miller , M.M. and Schat , K.A. 2004 . Chicken infectious anaemia virus: an example of the ultimate host–parasite relationship . Avian Diseases , 48 : 734 – 745 .
- Miller , M.M. , Ealey , K.A. , Oswald , W.B. and Schat , K.A. 2003 . Detection of chicken anemia virus DNA in embryonal tissues and eggshell membranes . Avian Diseases , 47 : 662 – 671 .
- Noteborn , M.H. 2004 . Chicken anemia virus induced apoptosis: underlying molecular mechanisms . Veterinary Microbiology , 98 : 89 – 94 .
- Noteborn , M.H.M. and Koch , G. 1995 . Chicken anaemia virus infection: molecular basis of pathogenicity . Avian Pathology , 24 : 11 – 31 .
- Noteborn , M.H.M. , Todd , D. , Verschueren , C.A.J. , de Gauw , H.W.F.M. , Curran , W.L. , Veldkamp , S. , Douglas , A.J. , McNulty , M.S. , Van der Eb , A.J. and Koch , G. 1994 . A single chicken anaemia virus protein induces apoptosis . Journal of General Virology , 68 : 346 – 351 .
- Rosenberger , J.K. and Clyde , S.S. 1989 . The effects of age, route of exposure, and coinfection with infectious bursal disease virus on the pathogenicity and transmissibility of chicken anemia agent (CAA) . Avian Diseases , 33 : 753 – 759 .
- Schat , K.A. 2003 . “ Chicken infectious anemia ” . In Diseases of Poultry , 11th edn , Edited by: Saif , Y.M. 182 – 202 . Ames : Iowa State Press .
- Smyth , J.A. , Moffett , D.A. , McNulty , M.S. , Todd , D. and Mackie , D.P. 1993 . A sequential histopathologic and immunocytochemical study of chicken anemia viurs infection at one day of age . Avian Diseases , 37 : 324 – 338 .
- Todd , D. 2000 . Circoviruses: immunosuppressive threats to avian species . Avian Pathology , 29 : 373 – 394 .
- Toro , H. , Ramirez , A.M. and Laurenas , J. 1997 . Pathogenicity of chicken anaemia virus (isolate 10343) for young and older chickens . Avian Pathology , 26 : 485 – 499 .
- Yuasa , N. , Noguchi , T. , Furuta , T. and Yoshida , I . 1980 . Maternal antibody and its effect on the susceptibility of chicks to chicken anemia agent . Avian Diseases , 24 : 197 – 201 .
- Yuasa , N. , Taniguchi , T. , Imada , T. and Hihara , H. 1983 . Distribution of chicken anaemia agent (CAA) and detection of neutralising antibody in chicks experimentally inoculated with CAA . National Institute of Animal Health Quarterly , 23 : 78 – 81 .