Abstract
The purpose of this study was to investigate the influence of the cytostatic drug, 5-fluorouracil (5-FU), which causes depletion of heterophil granulocytes, on clinical symptoms and histological lesions during the progress of infectious bursal disease virus (IBDV) infection in chickens. The aim was to disclose the mechanism behind the clinical disease symptoms. Three groups of specific pathogen free chickens were used for the experiment. Chickens in groups 1 and 3 were pretreated with 5-FU, while chickens in group 2 were treated with a placebo. After 5 days, the chickens in groups 2 and 3 were inoculated with the classical IBDV strain F52/70. Bursae of Fabricius were sampled at fixed intervals, and the progress of the infection was monitored by various histological techniques and reverse transcriptase-polymerase chain reaction (RT-PCR). We found correlation between histological observations and RT-PCR results. In the 5-FU pretreated chickens, IBDV caused only mild clinical symptoms, even though histological alterations similar to alterations caused by IBDV were still observed. The 5-FU pretreatment resulted in severe heterophil granulocyte depletion by days 2 and 3 after infection (post inoculation) and increased numbers of bursal secretory dendritic cells in the medulla of the follicles. IBDV infection seemed to induce fusion of secretory dendritic cells, resulting in formation of multinucleated giant cells, loaded with apoptotic B cells and virus particles associated with granules of bursal secretory dendritic cells. Our results indicate that the heterophil granulocytes together with the bursal secretory dendritic cells contribute to the outbreak and/or progress of clinical symptoms.
Impact de la déplétion des granulocytes hétérophiles causée par le 5-fluorouracil sur l'infection par le virus de la bursite infectieuse aviaire (IBDV) chez des poulets SPF
L'objectif de cette étude a été d'investiguer l'influence de la molécule cytostatique 5-fluorouracil (5-FU), entraînant une déplétion des granulocytes hétérophiles, sur les symptômes et les lésions histologiques durant l'évolution de l'infection par le virus de la bursite infectieuse aviaire (IBDV) chez le poulet. Le but était de montrer le mécanisme sous-tendant les symptômes de la maladie. Trois groupes de poulets exempts de microorganismes pathogènes spécifiés (SPF) ont été utilisés dans cette expérimentation. Les poussins des groupes 1 et 3 ont reçu le 5-FU, alors que les poulets du groupe 2 ont reçu un placebo. Après cinq jours, les poulets des groupes 2 et 3 ont été inoculés avec la souche classique F52/70 d'IBVD. Les bourses de Fabricius ont été prélevées et fixées par intervalles et l'évolution de l'infection a été suivie par des techniques différentes d'histologie et par RT-PCR. Il a été trouvé une corrélation entre les observations histologiques et les résultats de RT-PCR. Chez les poulets ayant reçu le 5-FU, l'IBDV a entraîné des symptômes légers, alors même que des lésions histologiques similaires à celles causées par l'IBDV étaient encore observées. L'administration du 5-FU a entraîné une déplétion sévère des granulocytes hétérophiles les 2ème et 3ème jours après l'infection (p.i.) et une augmentation du nombre des cellules dendritiques sécrétrices dans la medulla des follicules de la bourse. Il semble que l'infection par l'IBDV a induit la fusion des cellules dendritiques sécrétrices, entraînant la formation de cellules géantes multinuclées, chargées de cellules B apoptiques et des particules virales associées à des granules de cellules dendritiques sécrétrices de la bourse. Nos résultats indiquent que les granulocytes hétérophiles avec les cellules dendritiques sécrétrices de la bourse contribuent à la maladie et/ou à l'évolution des symptômes.
Bedeutung des Verlusts der heterophilen Granulozyten durch 5-Fluorouracil für eine IBDV-Infektion bei SPF-Hühnern
Gegenstand dieser Studie war es, den Einfluss des zytostatischen Arzneimittels 5-Fluorouracil (5-FU), das den Verlust der heterophilen Granulozyten verursacht, auf die klinischen Symptome und pathohistologischen Veränderungen im Verlauf einer Infektion mit dem Virus der infektiösen Bursitis (IBDV) bei Hühnern zu untersuchen. Dabei war das Ziel die Aufdeckung des Mechanismus hinter den klinischen Krankheitssymptomen. Für dieses Experiment wurden drei Gruppen spezifiziert pathogenfreier (SPF) Hühner verwendet. Die Hühner in den Gruppen 1 und 3 wurden mit 5-FU vorbehandelt, während die Hühner in der Gruppe 2 ein Plazebo erhielten. Nach fünf Tagen wurden die Tiere in den Gruppen 2 und 3 mit dem klassischen IBDV-Stamm F52/70 inokuliert. Zu bestimmten Zeitpunkten wurden die Bursae Fabricii entnommen und der Verlauf der Infektion wurde mittels verschiedener histologischer Techniken und der RT-PCR verfolgt. Wir fanden eine Korrelation zwischen den pathohistologischen Befunden und den RT-PCR-Ergebnissen. Bei den mit 5-FU vorbehandelten Hühnern führte das IBDV nur zu geringgradigen klinischen Symptomen, obwohl die pathohistologischen Veränderungen noch denen bei einer IBDV-Infektion entsprachen. Die 5-FU-Vorbehandlung verursachte am 2. und 3. Tag nach der Infektion (p.i.) einen hochgradigen Verlust der heterophilen Granulozyten und erhöhte die Zahl der sekretorischen dendritischen Bursazellen in der Follikelmedulla. Die IBDV-Infektion schien eine Fusion der sekretorischen dendritischen Zellen zu induzieren, was zur Bildung von vielkernigen Riesenzellen führte, die mit apoptotischen B-Zellen und Viruspartikeln verbunden mit den Granula der sekretorischen dendritischen Bursazellen beladen waren. Unsere Ergebnisse zeigen, dass die heterophilen Granulozyten zusammen mit den sekretorischen dendritischen Bursazellen am Ausbruch und Verlauf der klinischen Symptome bei einer IBDV-Infektion beteiligt sind.
Efecto de la depleción de granulocitos heterófilos causada por 5-fluorouracil en la infección por IBDV en pollos SPF
El objetivo de este estudio fue estudiar la influencia del fármaco citostático, 5-fluorouracil (5-FU), que produce depleción de granulocitos heterófilos, en los síntomas clínicos y lesiones histológicas que tienen lugar durante el proceso de infección del virus de la bursitis infecciosa aviar (IBDV) en pollos. El objetivo era revelar los mecanismos responsables de los síntomas clínicos de la enfermedad. Se utilizaron para esta prueba tres grupos de pollos libres de patógenos específicos. Las aves en los grupos 1 y 3 se pretrataron con 5-FU, mientras que las aves en el grupo 2 se trataron con placebo. Tras cinco días, los pollos de los grupos 2 y 3 se infectaron con la cepa clásica de IBDV F52/70. Se tomaron muestras de bolsa de Fabricio a intervalos fijados, y se monitorizó el desarrollo de la enfermedad mediante diversas técnicas histológicas y RT-PCR. Hallamos una correlación entre las observaciones histológicas y los resultados de RT-PCR. En los pollos pretratados con 5-FU, IBDV sólo causó signos clínicos leves, aunque se siguieron observando lesiones histológicas, similares a las lesiones causadas por IBDV. El pretratamiento con 5-FU produjo depleción intensa de granulocitos heterófilos a los 2 y 3 días post infección (p.i) y un incremento del número de células dendríticas secretoras de la bolsa en la médula de los folículos. Parece que la infección por IBDV induce la fusión de células dendríticas secretoras, lo que produce la formación de células gigantes multinucleadas, cargadas de células B apoptóticas y partículas víricas asociadas a gránulos de células dendríticas secretoras de la bolsa. Nuestros resultados indican que los granulocitos heterófilos junto con las células dendríticas secretoras de la bolsa contribuyen a la aparición y/o progreso de los síntomas clínicos.
Introduction
Infectious bursal disease virus (IBDV) is the prototype of the genus Avibirnavirus belonging to the family Birnaviridae (Fauquet et al., Citation2005). Two serotypes are recognized, serotypes 1 and 2 (McFerran et al., Citation1980), but only serotype 1 is pathogenic to chickens. Serotype 1 strains may cause severe clinical symptoms in young chickens, result in immunosuppression and increase flock mortality to 80% (Bumstead et al., Citation1993; Sharma et al., Citation2000; van den Berg, Citation2000). The chicken's susceptibility depends on several factors, including breed (Nielsen et al., Citation1998) and age (Allan et al., Citation1972). Domesticated chickens at the age of 3 to 6 weeks are most susceptible. Clinical symptoms seem to be related to the development of the primary target organ, the bursa of Fabricius (Okoye & Uzoukwu, Citation1990), which reaches its maximum size at the age of 3 to 6 weeks (Hoffmann & Lade, Citation1972). Chemical or surgical bursectomy prevents chickens from responding with clinical disease to IBDV infection (Okoye & Uzoukwu, Citation1990; Okoye et al., Citation1992; Hiraga et al., Citation1994). In bursa-intact chickens, viral particles replicate in lymphoid cells of the bursa causing lymphocyte depletion (Nieper & Muller, Citation1996), and possibly in macrophages and other cells (Khatri et al., Citation2005), but the exact mechanism by which the virus causes disease is not yet known. Young chickens, less than 1 week old, do not respond to infection with clinical signs, but the immune response is compromised (Saif, Citation1991; Sivanandan & Maheswaran, Citation1980; Rosenberger & Gelb, Citation1978). At this age, the function of the innate immune system is hampered by the functionally immature heterophil granulocytes (Seto, Citation1981; Kogut et al., Citation1994; Wells et al., Citation1998). Heterophil granulocytes belong to the polymorphonuclear leucocytes circulating in the blood and involved in elimination of pathogens. Granulocytes and macrophages have a common myeloid progenitor (Inaba et al., Citation1993), and both are capable of phagocytosis and degranulation (Lam, Citation1998). In the blood of normal adult chickens, 15 to 40% of the white blood cells are heterophil granulocytes (Jain, Citation1993). Probably activated by T cells, granulocytes cause release of cytokines or oxidative burst (Kogut et al., Citation2001 Citation2003). Release of cytokines and nitric oxide has been suggested as part of the infectious bursal disease syndrome resembling septic shock (van den Berg, Citation2000).
Epithelial cells of endodermal origin form the fine, supporting structure of the follicular medulla in the bursa of Fabricius (Oláh & Glick, Citation1992). In addition to epithelial cells, four different cell types are normally present in the follicles: B cells, T cells, dendritic cells and macrophages. Dendritic or secretory cells (BSDCs) are present in mature and immature forms (Oláh & Glick, Citation1987). Contrary to B cells, BSDCs express vimentin intermediate filaments (Oláh et al., Citation1992; Oláh & Glick, Citation1995). During IBDV infection, the normal cell population changes, as B cells are depleted (Ramm et al., Citation1991), the number of macrophages decreases (Khatri et al., Citation2005), the BSDCs leave the medulla (Oláh et al., Citation1997) and the number of heterophils and T cells is increased (Sharma et al., Citation1989; Tanimura & Sharma, Citation1997).
A model for heterophil depletion of chickens by treatment with the cytostatic drug 5-flurouracil (5-FU) has been described (Kogut et al., Citation1993) and used for investigation of the influence of heterophil granulocytes on bacterial (Bojesen et al., Citation2004) and viral (Raj et al., Citation1997) infections in chickens. In a previous, unpublished experiment, chickens treated with the recommended dose of 5-fluorouraci (5-FU) and subsequently infected with IBDV showed mild clinical symptoms, but survived the infection. Based on this study, the present experiment was designed to make infectious bursal disease viraemia occur at the time when blood contents of heterophile granulocytes were at its lowest level.
Aiming at obtaining basic information on the role of heterophile granulocytes in the complex disease manifestations of infectious bursal disease, we investigated the impact of 5-FU-induced heterophil depletion on clinical symptoms and bursa lesions during IBDV infection. The distribution of B cells, T cells, dendritic cells and virus particles in the bursa of Fabricius were investigated by immunocytochemistry, and light and transmission electron microscopy. In addition, the presence of virus RNA was monitored by reverse transcriptase-polymerase chain reaction (RT-PCR).
Materials and Methods
Chickens
White Lohmann chickens from specific pathogen free (SPF) eggs (Lohmann Tierzucht, Cuxhaven, Germany) were hatched at the laboratory. One-day-old chickens were reared in isolators (HM 1500; Andersen BV, Sevenum, The Netherlands) with a filtered airflow. The chickens had unlimited access to water and commercial chicken feed. Light supply was programmed to 8 h of darkness every 24 h after the first week. All chickens, including the terminally ill, were humanly killed in accordance with Article 2(1) in Directive 86/609/European Economic Community of 24 November 1986.
Experimental design
Three groups of 15 chickens were used in the experiment. Before treatment, heparin-stabilized blood samples were collected from the ulnar vein of each chicken. Chickens in groups 1 and 3 were treated with 5-FU at the age of 15 days, while chickens in group 2 were given placebo. Five days later, at 20 days old, groups 2 and 3 were inoculated with IBDV, while group 1 was similarly inoculated with placebo. Three chickens from each group were killed on days 20, 21, 22, 23 and 30, corresponding to 0, 1, 2, 3 and 10 days after IBDV infection.
Before the chickens were killed, heparin-stabilized blood samples were collected from the ulnar vein. Autopsy was performed immediately. Each bursa of Fabricius was sampled and divided into four pieces, which were frozen for RT-PCR, placed in 4% formaldehyde for evaluation of bursa lesions, or in 4% buffered glutaraldehyde for light and transmission microscopy, and snap-frozen in liquid nitrogen for immunohistochemistry, respectively.
Virus
The classical IBDV strain, Faragher 52/70 (F52/70) (Lohmann Tierzucht) was obtained from bursa tissues from previously infected chickens. The bursa tissues were homogenized in an equal weight of sterile sand using a sterile mortar and pestle. The homogenate was eluted in phosphate-buffered saline (PBS) containing 10 000 U/ml penicillin, 10 000 µg/ml streptomycin, 250 µg/ml gentamycin, 500 U/ml nystatin and 5% foetal bovine serum (Invitrogen, Carlsbad, California, USA). This eluate was centrifuged for 15 min at 1912 ×g. The resulting supernatant was diluted in Hank's buffered saline solution (Gibco™, Paisley, UK) to 10−1, 10−2, 10−3, 10−4 and 10−5, and inoculated via the chorio-allantoic membrane into embryonated hen's eggs to determine virus titres (Busby et al., Citation1964). The inoculate had a titre of 104.2 embryo lethal dose, median. Experimental chickens were inoculated with 0.2 ml dilution as drops in the eyes, beak and nostrils.
Heterophile depletion
The cytostatic drug 5-FU (Sigma, Copenhagen, Denmark) is used in cancer treatment. The effect of 5-FU is related to its replacement of uracil in RNA (Ardalan & Glazer, Citation1981). A dose of 200 mg/kg was injected once into the vena ulnaris (Kogut et al., Citation1993). Sterile saline was used as placebo.
Blood
Heparinized blood samples were collected from the ulnar vein and refrigerated. One drop from each sample was smeared onto a glass slide, air dried and stained with Haemacolor (Malinckrodt Baker B.V., Deventer, The Netherlands). One hundred cells were counted under a microscope. All polymorphonuclear cells were regarded as heterophil granulocytes when the percentage of the total blood cell count was calculated.
Light and electron microscopy
Bursa tissue samples were fixed in formaldehyde overnight and embedded in paraffin wax. The paraffin-embedded bursa tissue samples were cut into 2 µm sections, mounted on Super Frost Plus slides (Hounisen, Risskov, Denmark), stained by haematoxylin and eosin (Swayne et al., Citation2000) and studied under a Leica DMRB microscope. Bursa lesions were evaluated quantitatively according to the lesion scoring system recommended by a working group under COST839 (Mundt, Citation2002).
Bursa tissue samples were fixed in glutaraldehyde overnight. After washing in PBS, the tissue samples were postfixed in 1% OsO4 for 2 h. Graded alcohol was used for dehydration of the tissue blocks prior to embedding in Polybed® Araldite mixture (Polysciences Inc. Eppelheim, Germany). These samples were cut into semithin 1-µm sections and thin sections. The 1-µm semithin sections were stained with toluidin blue for light microscopy, while thin sections were contrasted by 1% uranyl acetate and lead citrate and studied by Hitachi type H-7600 electron microscope.
Immunohistochemistry
A bursa tissue sample was completely enveloped in liver tissue to protect from the damage caused by sudden freezing. This “package” was placed on a small piece of carton and frozen on polystyrene, floating in liquid nitrogen, and stored at −80°C. Immunostaining was performed on 8-µm cryostat sections, which were collected on poly-l-lysine-coated slides (Sigma-Aldrich Kft., Budapest, Hungary). Briefly, the acetone-fixed and air-dried sections were re-hydrated in PBS and incubated with primary antibodies for 45 min. The primary antibodies were: Clone Lu5 anticytokeratin mAb (Boehringer-Mannheim GmbH, Indianapolis, USA), Clone 5-11G2 (Mouse IgG1) Bu-1b for B cell staining (Southern Biotechnology Associates Inc., Birmingham, USA), rabbit anti-human T cell, CD3 (rabbit IgG) for T-cell staining (Dako Cytomation, Aarhus, Denmark), Clone Amf-b17 (mouse IgG1), vimentin intermediate filament for staining of avian dendritic cells and heterophil granulocytes (DSHB, Iowa, USA), Clone 74.3 staining intracellular antigen in the BSDCs and follicular dendritic cells (FDCs) (Jeurissen et al., Citation1992), Clone CG-106 (mouse IgG1) surface IgG staining BSDCs (Sigma-Aldrich Kft.), rabbit polyclonal serum (rabbit IgG) virion staining IBDV (produced in our laboratory), and 5A-10 (mouse IgM) VP2 staining IBDV (gift from Dr Palya, Ceva-Phylaxia Veterinary, Hungary). Biotinylated horse anti-mouse IgG, biotinylated horse anti-mouse IgM and biotinylated horse anti-rabbit IgG (Vector Laboratories, Burlingame, California, USA) were followed by quenching of endogenous peroxidase activity by 1.5% hydrogen peroxide (Sigma-Aldrich Kft.) for 10 min. After applying the avidin–biotin–peroxidase complex (Vectastain Elite ABC Kit, Burlingame, California) the binding sites of the primary antibodies were visualized by 4-chloro-1-naphthol (Sigma Aldrich Kft.).
Reverse transcriptase-polymerase chain reaction
RNA was extracted as previously explained (Kabell et al., Citation2005). The QIAGEN OneStep RT-PCR Kit (Qiagen GmbH, Hilden, Germany) was used according to the manufacturer's instructions. Briefly, 10 µl of a 5 × reaction buffer, 2 µl dNTP mix, 2 µl enzyme mixture, 100 pmol each oligonucleotide and 5 µl RNA were mixed with RNase-free water to a final volume of 50 µl. All ingredients were kept on ice during handling.
The PCR reaction was performed in a Biometra T3 Thermocycler (Biometra GmbH, Goettingen, Germany) as follows: 30 min at 50°C (RT reaction), 94°C for 15 min (initial PCR activation), 39 three-step cycles of 94°C for 30 sec, 58°C for 1 min and 68°C for 2 min, and 68°C for 7 min (final extension).
Results
Clinical signs
The only 5-FU-treated chickens (group 1) did not show any signs of disease, but 10 days after 5-FU treatment they began to shed more feathers than normally expected. The virus inoculated chickens (group 2) developed severe clinical symptoms on day 3 after virus inoculation, including anorrhexia, depression, diarrhoea and prostration. Consequently, remaining birds were euthanized. The 5-FU-treated and virus-inoculated chickens (group 3) showed mild signs of depression 3 to 4 days after virus inoculation, and they shed feathers like group 1.
Blood
Chickens from group 1 showed relatively high percentages of heterophil granulocytes in the blood before treatment, decreasing on the seventh day after 5-FU treatment, remaining low during the rest of the experiment, as shown in . Heterophil granulocytes from the non-5-FU-treated group 2 remained within the value range of 12 to 50% during the experiment. The average number of heterophil granulocytes from groups 2 and 3 were still comparable 5 days after 5-FU treatment. The next day, 1 day post inoculation (p.i.), the number of heterophil granulocytes in the 5-FU-treated and virus-inoculated group 3 increased to an average of 33%, and 2 and 3 days after infection it decreased to subnormal levels, to increase again 10 days after virus inoculation.
Table 1. Heterophile granulocytes in blood and bursa lesion score
Histology of the bursa of Fabricius
Five days after 5-FU treatment, the size of the bursal follicles from groups 1 and 3 was moderately decreased, and the centres of the follicles seemed mildly depleted. The overall picture of the effect of 5-FU treatment was a mild histological alteration of the bursa at the time of the IBDV inoculation. From 2 days p.i., lesions observed in samples from the 5-FU-treated and IBDV-inoculated group 3 included lymphocyte depletion, as seen by haematoxylin and eosin staining (data not shown) and folding of the interfollicular epithelium, illustrated by anticytokeratin immunostaining of epithelial cells (a), comparable with the lesions seen in the only IBDV-inoculated group 2 (). Cell alterations, observed during the first 3 days of the IBDV infection, are summarized in . By day 2, staining by Bu-1b in groups 2 and 3 showed that the B-cell depletion was significant in the centre of the medulla and close to the cortico-medullary border of the cortex (b). One day later, B-cell staining of the medulla appeared as a non-homogeneous mass, encircled by a strongly depleted cortical ring. The non-homogeneous B-cell staining in the medulla consisted of irregularly shaped bodies 40 to 50 µm in size.
Figure 1. Reactions to IBDV infection in 5-FU-pretreated and non-pretreated chickens. 1a: 5-FU and IBDV inoculation (group 3): 3 days after IBDV inoculation (p.i.), folded surface epithelium was observed (arrows); the medullary epithelial reticular cells form “empty holes”. Anticytokeratin immunostaining. Magnification, 40×. 1b: IBDV inoculation (group 2): 2 days p.i., B-cell depletion. Depletion started in the centre of the medulla and inner part of the cortex. Bu-1b staining. Magnification, 35×. 1c: IBDV inoculation (group 2): 2 days p.i., the follicular cortex flooded by heterophil granulocytes (arrows). Semithin section stained with toluidin blue. Magnification, 500×. 1d: 5-FU and IBDV inoculation (group 3): 2 days p.i., reduction in the number of heterophil granulocytes (arrows). Compare with 1c. Semithin section stained with toluidin blue. Magnification, 500×. 1e: IBDV inoculation (group 2): 2 days p.i., huge numbers of vimentin-positive cells in the medulla and surface epithelium of the follicles. Magnification, 140×. 1f: 5-FU and IBDV inoculation (group 3): 2 days p.i., the number of vimentin-positive cells in the medulla reduced as compared with group 2 (see 1e). Magnification, 140×.
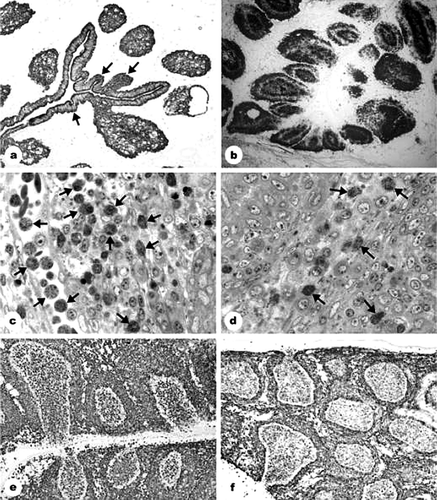
Table 2. Histological development in the bursa of Fabricius 5 to 8 days after 5-FU treatment (0 to 3 days after IBDV inoculation)
Heterophile granulocytes were observed in the toulouidin-blue-stained sections, being present in large numbers in the vessels and follicles of group 2, contrary to group 3 (c,d). Although it is difficult to make a quantitative estimation on immunohistochemical and histological slides, sections stained with toulouidin blue as well as sections stained with anti-vimentin showed that the number of granulocytes was clearly decreased on day 2 p.i. in the 5-FU-treated and IBDV-inoculated group 3, remaining low during the experiment, as compared with the only IBDV-inoculated group 2 (c,d and e,f). On day 3, vimentin-positive cells in group 3 were few, but the majority was small and heavily stained and proved to be heterophil granulocytes, while enlarged, pale-stained and ring-shaped cells were identified as giant cells. Transmission electron micrographs did not show virus particles in the heterophile granulocytes. However, the transmission electron micrographs indicated that cell clusters described earlier as inhomogeneous B-cell staining represented single-nucleated or multi-nucleated giant cells, which were loaded with virus and cell debris (a,b).
Figure 2. BSDCs and T cells during IBDV infection. 2a: 5-FU and IBDV inoculation (group 3): 2 days after IBDV infection. The cell contains apoptotic lymphocytes (A) and two demarcated bodies, in which the virus particles are associated with an electron-dense substance (arrow). Part of one of the demarcated bodies is outlined and shown in 2b. Lipid droplets (L) in the cytoplasm. Magnification, 10 000×. 2b: Detail from 2a. In the demarcated body, the virus particles are intermingled with electron dense substance. Magnification, 40 000×. 2c: IBDV inoculation (group 2): 3 days p.i., CD3-positive T cells aggregate in the follicular cortex. Medulla contains only scattered T cells. Magnification, 140×. 2d: IBDV inoculation (group 2): 2 days p.i., the 74.3-positive BSDCs aggregate along the cortico-medullary border in several follicles. Magnification, 160?×. 2e: IBDV inoculation (group 2): 3 days p.i., the 74.3-positive BSDCs seem to be completely aggregated in the medulla. Magnification, 140×. 2f: IBDV inoculation (group 2): 2 days p.i., the anti-IgG staining confirm observations obtained by 74.3 monoclonal antibodies. The IgG-positive BSDCs aggregate along the cortico-medullary border. Compare with 2d. Magnification, 70×.
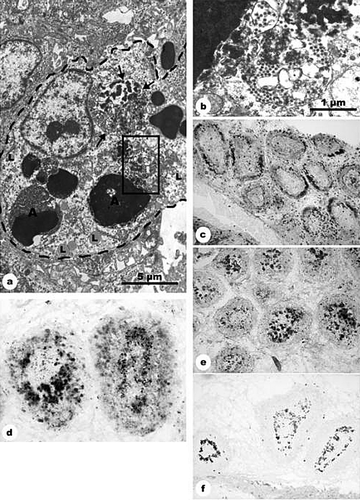
CD3 staining showed that the number of T cells increased in the follicular cortex of both groups of IBDV-inoculated chickens, while the medulla contained only scattered T cells (c).
Besides the granulocytes, the number of BSDCs differed in the IBDV-inoculated groups. The number of BSDCs seemed to increase in both groups at day 2 p.i., as evaluated by the touluidin blue staining. This elevation may not be absolute, possibly relative, as B cells were depleted. In group 3, the shape of the BSDCs was bulky, not elongated as generally seen in mature BSDCs, their cytoplasm contained only few granules around the Golgi zone, and lipid droplets were scattered over the cytoplasm. Mature forms of BSDC(s) were seen infrequently in the 5-FU pretreated and IBDV inoculated group 3, unlike the only IBDV-inoculated group 2. Two days p.i., the 74.3 monoclonal antibody staining revealed follicles of highly variable appearance. In most follicles, the 74.3 positive BSDCs were aggregated close to the inner surface of the cortico-medullary border (d). The 74.3 monoclonal antibody staining revealed a similar staining pattern as the B-cell staining 3 days p.i., but the size of the bodies appeared smaller (e). IgG staining confirmed that the observed rearrangement was due to BSDCs (f).
Progress of the IBDV infection was monitored by polyclonal anti-IBDV and monoclonal anti-virus protein, VP2. Two days p.i. the virus and the virus protein in both virus-inoculated groups were concentrated mainly in the follicular medulla, while 1 day later the cortex was also heavily loaded with virus. Virus particles were packed in the cytoplasm of large bulky cells in both the medulla and the cortex (data not shown).
Reverse transcriptase-polymerase chain reaction
All bursa tissue samples from IBDV-inoculated chickens were positive, and all bursa tissue samples from uninoculated chickens were negative.
Discussion
The aim of this study was to disclose a possible impact of 5-FU depletion of heterophil granulocytes on the complex pathogenesis and onset of clinical symptoms of IBDV infection. The 5-FU-pretreated and IBDV-inoculated group 3 showed only mild clinical disease symptoms, and the chickens survived the infection, contrary to the placebo-pretreated and IBDV-inoculated group 2, in spite of similar histological lesions in tissues from the bursa of Fabricius. These observations are in accordance with Raj et al. (Citation1997), who reported reduced clinical response to bronchitis virus infection after 5-FU pretreatment. The shedding of feathers, observed in both 5-FU-treated groups of chickens 10 days after treatment, might indicate an impact of 5-FU possibly similar to alopecia in humans during cancer treatment.
The blood contents of heterophil granulocytes from 5-FU-treated and placebo-treated chickens were within the normal range 5 days after 5-FU treatment, when the IBDV inoculation took place. After 1 day, the heterophil counts rose inexplicably in the only 5-FU-treated group 1, while the two other groups showed normal values. However, the number of heterophil granulocytes was drastically reduced in both treated groups 7 and 8 days after 5-FU treatment, 2 and 3 days after virus inoculation, when viraemia was expected. The results of the blood cell counts were too few to be statistically evaluated, but the effect of 5-FU on blood cells seemed already discussed in detail by Kogut et al. (Citation1993) and Bojesen et al. (Citation2004). The histological studies of bursa tissues confirmed the results of lymphocyte counts, revealing cellular depletion 5 days after 5-FU treatment. Fifteen days after 5-FU treatment, a marked difference in blood contents of heterophils between groups 1 and 3 was observed. The increase in the number of heterophil granulocytes in group 3 was probably stimulated by the IBDV infection, while the non-infected chickens may require more time to restore the normal blood content of heterophil granulocytes.
According to the bursa lesion scores, 5-FU seemed to moderately reduce the number of B cells. However, the histological alterations 2 and 3 days p.i. corresponded to the general picture characterizing IBDV infections, and the 5-FU treatment did not seem to influence the development of pathological lesions. The IBDV infection reportedly stimulates the heterophil granulocytes entering the bursa (Sharma et al., Citation1989; Lam, Citation1998), but this reaction was markedly decreased after 5-FU treatment. Except for the low number of heterophil granulocytes, and an increased number of BSDCs in group 3, the histological alterations of the bursa caused by IBDV were comparable between groups 2 and 3. The observed histological differences may indicate that the heterophil granulocytes and the BSDCs play crucial roles in the onset of clinical symptoms. In the 5-FU-pretreated group 3, the cytological features of the BSDCs indicated their immature condition, as compared with the mature BSDCs that have two long cytoplasmic processes, one of which is occupied by cytoplasmic granules (Oláh & Glick, Citation1987; Nagy et al., Citation2001). The immature, newly formed BSDCs were lined up along the cortico-medullary border, while the mature BSDCs were scattered over the medulla. When the 5-FU pretreatment reduced the heterophil invasion, it may have turned the disease from acute to subacute. This induced, subacute form of the IBDV infection did not impair the BSDCs as severely as the acute form, resulting in an increase in the total number of BSDCs.
Phagocytosis of the cell debris and engulfing of virus particles was evidenced by light and transmission microscopy and by immunohistochemistry. In the medulla of the follicles, only the BSDCs may be charged with phagocytosis (Nagy et al., Citation2004). In group 2, the BSDCs disappeared, as shown by a decreased number of vimentin-positive cells in the follicular medulla, substantiated by the faded IgG staining. However, transmission microscopy showed that their cytoplasmic granules seemed to fuse with virus particles. The virus attack on the BSDCs may transform them rapidly to phagocytic cells. Under physiological conditions, the turnover of the BSDCs is slow. The senescent BSDCs enter the follicle-associated epithelium, and are eliminated in the lumen of the bursa (Nagy et al., Citation2004). This turnover is highly facilitated by IBDV (Oláh et al., Citation1997), and the BSDCs may leave the medulla not only towards the follicle-associated epithelium, but also through the cortico-medullary border into the cortex, carrying the virus and the digested apoptotic cells. Thus, the terminal maturation of the BSDC might be a phagocyte, distinct for the bursa of Fabricius (Nagy et al., Citation2004). The multinucleated giant cells appeared first in the medulla and about 1 day later in the cortex. The light and electron microscopy and immunocytochemistry revealed only epithelial reticular cells, lymphocytes and BSDCs in the medulla. We propose that BSDCs form the multinucleated giant cells.
In groups 2 and 3, T cells were recognized in the cortex of the follicles and in the interfollicular connective tissue from the second day after IBDV inoculation. T cells may reportedly influence the development of bursa lesions (Rautenschlein et al., Citation2002). According to the bursa lesion score evaluation, major differences were not observed between bursa lesions after IBDV infection in groups 2 and 3, suggesting that T-cell influence was not compromised by 5-FU.
In conclusion, the impact of 5-FU, appearing as a depletion of blood heterophil granulocytes, did not seem to alter the course of the IBDV infection in the bursa even though clinical symptoms were drastically reduced. We suggest that the decrease of heterophil granulocytes induced a subacute form of infection that maintained an increased number of BSDCs. The reactions of the heterophil granulocytes, and possibly the BSDCs, may be crucial for the development of clinical disease. As both cells are known to produce cytokines (Oláh & Glick, Citation1993; Kogut et al., Citation2003; Swaggerty et al., Citation2004), these observations add credibility to theories on cytokine involvement in clinical symptoms (Sharma et al., Citation2000). Further investigations into the function of heterophil granulocytes and BSDCs may add information to explanations of how IBDV causes clinical disease.
Acknowledgments
This work was partially supported by a grant of Hungarian Scientific Research Foundation OTKA-T42558, and partly by the Danish Institute for Food and Veterinary Research.
In addition, the authors wish to thank laboratory attendants Gitte Bach and Zsuzsa Vidra for valuable assistance.
References
- Allan , W.H. , Cullen , G.A. and Faragher , J.T. 1972 . Immunosuppression by infectious bursal agent in chickens immunized against Newcastle disease . Veterinary Record , 90 : 511 – 512 .
- Ardalan , B. and Glazer , R. 1981 . An update on the biochemistry of 5-Fluorouracil . Cancer Treatment Reviews , 8 : 157 – 167 .
- Bojesen , A.M. , Nielsen , O.L. , Christensen , J.P. and Bisgaard , M. 2004 . In vivo studies of Gallibacterium anatis infection in chickens . Avian Pathology , 33 : 145 – 152 .
- Bumstead , N. , Reece , R.L. and Cook , J.K.A. 1993 . Genetic differences in susceptibility of chicken lines to infection with infectious bursal disease virus . Poultry Science , 72 : 403 – 410 .
- Busby , D.W.G. , House , W. and MacDonald , J.R. 1964 . “ Neutralisation ” . In Virological Technique , 1st edn , Edited by: Busby , D.W.G. , House , W. and MacDonald , J.R. 143 – 146 . London : J. & A. Churchill .
- Fauquet , C.M. , Mayo , M.A. , Maniloff , J. , Desselberger , U. & Ball , L.A. (Eds.) (2005) . Virus Taxonomy. Classification and nomenclature of viruses Eighth Report of the International Committee on Taxonomy of Viruses (ICTV) (1217 pp.) . London : Academic Press, Elsevier .
- Hiraga , M. , Nunoya , T. , Otaki , Y. , Tajima , M. , Saito , T. and Nakamura , T. 1994 . Pathogenesis of highly virulent infectious bursal disease virus infection in intact and bursectomized chickens . Journal of Veterinary Medical Science , 56 : 1057 – 1063 .
- Hoffmann , G. and Lade , R . 1972 . Post-hatching development and involution of bursa Fabricii in chicken (Gallus-Domesticus) . Zeitschrift fur Zellforschung und Mikroskopische Anatomie , 124 : 406 – 412 .
- Inaba , K. , Inaba , M. , Deguchi , M. , Hagi , K. , Yasumizu , R. , Ikehara , S. , Muramatsu , S. and Steinman , R.M. 1993 . Granulocytes, macrophages and dendritic cells arise from a common major histocompatability complex class-II-negative progenitor in mouse bone marrow . Proceedings of the National Academy of Sciences , 90 : 3038 – 3042 .
- Jain , N.C. (1993) . Chapter 3: Comparative haematologic features of some avian and mammalian species . In Essentials of veterinary hematology 1st edn (pp. 54 – 71 ). Philadelphia , PA : Lea & Febiger .
- Jeurissen , S.H. , Claassen , E. and Janse , E.M. 1992 . Histological and functional differentiation of non-lymphoid cells in the chicken spleen . Immunology , 77 : 75 – 80 .
- Kabell , S. , Handberg , K.J. , Kusk , M. and Bisgaard , M. 2005 . Detection of infectious bursal disease virus in various lymphoid tissues of experimentally infected specific pathogen free chickens by different reverse transcription polymerase chain reaction assays . Avian Diseases , 49 : 534 – 539 .
- Khatri , K. , Palmquist , J.M. , Cha , R.M. and Sharma , J. 2005 . Infection and activation of bursal macrophages by virulent infectious bursal disease virus . Virus Research , 113 : 44 – 50 .
- Kogut , M.H. , Tellez , G.I. , Hargis , B.M. , Corrier , D.E. and Deloach , J.R. 1993 . The effect of 5-Fluorouracil treatment of chicks—a cell depletion model for the study of avian polymorphonuclear leukocytes and natural host defenses . Poultry Science , 72 : 1873 – 1880 .
- Kogut , M.H. , Tellez , G.I. , Mcgruder , E.D. , Hargis , B.M. , Williams , J.D. , Corrier , D.E. and Deloach , J.R. 1994 . Heterophils are decisive components in the early responses of chickens to Salmonella-Enteritidis infections . Microbial Pathogenesis , 16 : 141 – 151 .
- Kogut , M.H. , Genovese , K.J. and Lowry , V.K. 2001 . Differential activation of signal transduction pathways mediating phagocytosis, oxidative burst, and degranulation by chicken heterophils in response to stimulation with opsonized Salmonella enteritidis . Inflammation , 25 : 7 – 15 .
- Kogut , M.H. , Rothwell , L. and Kaiser , P. 2003 . Differential regulation of cytokine gene expression by avian heterophils during receptor-mediated phagocytosis of opsinized and nonopsonized Salmonella enteritidis . Journal of Interferon and Cytokine Research , 23 : 319 – 327 .
- Lam , K.M. 1998 . Alteration of chicken heterophil and macrophage functions by the infectious bursal disease virus . Microbial Pathogenesis , 25 : 147 – 155 .
- McFerran , J.B. , Mcnulty , M.S. , Mckillop , F.R. , Connor , T.J. , Mccracken , R.M. , Collins , D.S. and Allan , G.M. 1980 . Isolation and serological studies with infectious bursal disease viruses from fowl, turkeys and ducks — demonstration of a second serotype . Avian Pathology , 9 : 395 – 404 .
- Mundt , E. (2002) . First ringtest on histopathological scoring of bursal section of IBDV infected chicken . In T. van den Berg (Ed.) , Immunosuppressive viral diseases in poultry. Proceedings and Annual Report 2002 European Communities, COST action 839 (pp. 227 – 238 ) European Commission, Directorate-General for research , Brussels .
- Nagy , N. , Magyar , A. , David , C. , Gumati , M.K. and Olah , I. 2001 . Development of the follicle-associated epithelium and the secretory dendritic cell in the bursa of Fabricius of the guinea fowl (Numida Meleagris) studied by novel monoclonal antibodies . Anatomical Record , 262 : 279 – 292 .
- Nagy , N. , Magyar , A. , Tóth , M. and Oláh , I. 2004 . Quail as the chimeric counterpart of the chicken: morphology and ontogeny of the bursa of Fabricius . Journal of Morphology , 259 : 328 – 339 .
- Nielsen , O.L. , Sorensen , P. , Hedemand , J.E. , Laursen , S.B. and Jorgensen , P.H. 1998 . Inflammatory response of different chicken lines and B haplotypes to infection with infectious bursal disease virus . Avian Pathology , 27 : 181 – 189 .
- Nieper , H. and Muller , H. 1996 . Susceptibility of chicken lymphoid cells to infectious bursal disease virus does not correlate with the presence of specific binding sites . Journal of General Virology , 77 : 1229 – 1237 .
- Okoye , J.O.A. and Uzoukwu , M. 1990 . Pathogenesis of infectious bursal disease in embryonally bursectomized chickens . Avian Pathology , 19 : 555 – 570 .
- Okoye , J.O.A. , Nwoush , C. , Onwujiobi , C.B.O. , Onuoha , A.S. and Okonkwo , P.U. 1992 . Pathogenesis of infectious bursal disease in cyclophosphamide-treated chickens . Avian Pathology , 21 : 615 – 620 .
- Oláh , I. and Glick , B. 1987 . Bursal secretory cells: an electron microscope study . Anatomical Record , 219 : 268 – 274 .
- Oláh , I. and Glick , B. 1992 . Follicle-associated epithelium and medullary epithelial tissue of the bursa of Fabricius are two different compartments . Anatomical Record , 233 : 577 – 587 .
- Oláh , I. and Glick , B. 1993 . Bursal secretory dendritic-like cells: a microenvironmntal issue . Poultry Science , 72 : 1262 – 1266 .
- Oláh , I. and Glick , B. 1995 . Dendritic cells in the bursal follicles and germinal centers of the chicken's caecal tonsil express vimentin but not desmin . Anatomical Record , 243 : 384 – 389 .
- Oláh , I. , Kendall , C. and Glick , B. 1992 . Anti-vimentin monoclonal antibody recognizes a cell with dendritic appearance in the chicken's bursa of Fabricius . Anatomical Record , 232 : 121 – 125 .
- Oláh , I. , Horvath , E. , Magyar , A. , Nagy , N. , Toth , A. , Minko , K. and Gumati . 1997 . Effect of infectious bursal disease virus (IBDV) on the bursal secretory dendritic cell. Abstracts of the 7th Congress of the ISDCI . Developmental and Comparative Immunology , 21 : 96
- Raj , G.D. , Savage , C.E. and Jones , R.C. 1997 . Effect of heterophil depletion by 5-fluorouracil on infectious bronchitis virus infection in chickens . Avian Pathology , 26 : 427 – 432 .
- Ramm , H.C. , Wilson , T.J. , Boyd , R.L. , Ward , H.A. , Mitrangas , K. and Fahey , K.J. 1991 . The effect of infectious bursal disease virus on B lymphocytes and bursal stromal components in specific pathogen-free (SPF) White Leghorn chickens . Developmental and Comparative Immunology , 15 : 369 – 381 .
- Rautenschlein , S. , Yeh , H.Y. , Njenga , M.K. and Sharma , J.M. 2002 . Role of intrabursal T cells in infectious bursal disease virus (IBDV) infection: T cells promote viral clearance but delay follicular recovery . Archives of Virology , 147 : 285 – 304 .
- Rosenberger , J.K. and Gelb , J. 1978 . Response to several avian respiratory viruses as affected by infectious bursal disease virus . Avian Diseases , 22 : 95 – 105 .
- Saif , Y.M. 1991 . Immunosuppression induced by infectious bursal disease virus . Veterinary Immunology and Immunopathology , 30 : 45 – 50 .
- Seto , F. 1981 . Early development of the avian immune system . Poultry Science , 60 : 1981 – 1995 .
- Sharma , J. M. , Dohms , J.E. and Metz , A.L. 1989 . Comparative pathogenesis of serotype 1 and variant serotype 1 isolates of infectious bursal disease virus and their effect on humoral and cellular immune competence of specific-pathogen-free chickens . Avian Diseases , 33 : 112 – 124 .
- Sharma , J.M. , Kim , I.J. , Rautenschlein , S. and Yeh , H.Y. 2000 . Infectious bursal disease virus of chickens: pathogenesis and immunosuppression . Developmental and Comparative Immunology , 24 : 223 – 235 .
- Sivanandan , V. and Maheswaran , S.K. 1980 . Immune profile of infectious bursal disease: I. Effect of infectious bursal disease virus on peripheral blood T and B lymphocytes of chicken . Avian Diseases , 24 : 715 – 725 .
- Swaggerty , C.L. , Kogut , M.H. , Ferro , P.J. , Rothwell , L. , Pevzner , I.Y. and Kaiser , P. 2004 . Differential cytokine mRNA expression in heterophils isolated from Salmonella-resistant and -susceptible chickens . Immunology , 113 : 139 – 148 .
- Swayne , D.E. , Glisson , J.R. , Jackwood , M.W. and Pearson , J.E.R. 2000 . A Laboratory Manual for the Isolation and Identification of Avian Pathogens , 4th edn , Pensylvania : University of Pensylvania .
- Tanimura , N. and Sharma , J.M. 1997 . Appearance of T cells in the bursa of Fabricius and cecal tonsils during the acute phase of infectious bursal disease virus infection in chickens . Avian Diseases , 41 : 638 – 645 .
- van den Berg , T.P. 2000 . Acute infectious bursal disease in poultry: a review . Avian Pathology , 29 : 175 – 194 .
- Wells , L.L. , Lowry , V.K. , DeLoach , J.R. and Kogut , M.H. 1998 . Age dependent fagocytosis and bactericidal activities of the chicken heterophil . Developmental and Comparative Immunology , 22 : 103 – 109 .