Abstract
The effect of a live Mycoplasma gallisepticum vaccine on the horizontal transmission of this Mycoplasma species was quantified in an experimental animal transmission model in specific pathogen free White Layers. Two identical trials were performed, each consisting of two experimental groups and one control group. The experimental groups each consisted of 20 birds 21 weeks of age, which were housed following a pair-wise design. One group was vaccinated twice with a commercially available live attenuated M. gallisepticum vaccine, while the other group was not vaccinated. Each pair of the experimental group consisted of a challenged chicken (104 colony-forming units intratracheally) and a susceptible in-contact bird. The control group consisted of 10 twice-vaccinated birds housed in pairs and five individually housed non-vaccinated birds. The infection was monitored by serology, culture and quantitative polymerase chain reaction. The vaccine strain and the challenge strain were distinguished by a specific polymerase chain reaction and by random amplified polymorphic DNA analysis. In both experiments, all non-vaccinated challenged chickens and their in-contact ‘partners’ became infected with M. gallisepticum. In the vaccinated challenged and corresponding in-contact birds, a total of 19 and 13 chickens, respectively, became infected with M. gallisepticum. Analysis of the M. gallisepticum shedding patterns showed a significant effect of vaccination on the shedding levels of the vaccinated in-contact chickens. Moreover, the Cox Proportional Hazard analysis indicated that the rate of M. gallisepticum transmission from challenged to in-contact birds in the vaccinated group was 0.356 times that of the non-vaccinated group. In addition, the overall estimate of R (the average number of secondary cases infected by one typical infectious case) of the vaccinated group (R = 4.3, 95% confidence interval = 1.6 to 49.9) was significantly lower than that of the non-vaccinated group (R = ∞, 95% confidence interval = 9.9 to ∞). However, the overall estimate of R in the vaccinated group still exceeded 1, which indicates that the effect of the vaccination on the horizontal transmission M. gallisepticum is insufficient to stop its spread under these experimental conditions.
L'effet d'un vaccin vivant à Mycoplasma gallisepticum sur la transmission horizontale de cette espèce mycoplasmique a été quantifié dans un modèle animal de transmission expérimentale, utilisant des pondeuses blanches indemnes de microorganismes pathogènes spécifiés. Deux essais identiques ont été réalisés, chacun comprenant deux groupes expérimentaux et un groupe témoin. Chaque groupe expérimental était constitué de 20 oiseaux âgés de 21 semaines qui étaient hébergés par paire. Un groupe était vacciné deux fois avec un vaccin vivant atténué à M. gallisepticum disponible dans le commerce, alors que l'autre groupe n'était pas vacciné. Chaque paire du groupe expérimental était constituée d'un sujet éprouvé (104 unités formant colonie [CFU] par voie intra trachéale) et d'un oiseau contact sensible. Le groupe témoin était constitué de 10 sujets vaccinés, deux fois, hébergés par paire et de 5 animaux non vaccinés hébergés individuellement. L'infection a été suivie par sérologie, culture et par amplification en chaîne par polymérase quantitative (Q-PCR). La souche vaccinale et la souche d'épreuve ont été différenciées par une PCR spécifique et par une amplification génique arbitraire (RAPD). Dans les deux essais tous les sujets non vaccinés éprouvés et leurs "congénères" contacts se sont révélés infectés par M. gallisepticum. Dix-neuf sujets vaccinés et éprouvés ainsi que treize sujets contacts correspondants se sont révélés infectés par M. gallisepticum. L'analyse des profils de portage de M. gallisepticum a montré un effet significatif de la vaccination sur les niveaux d'excrétion des animaux vaccinés contact. De plus, l'analyse de survie par modèle Cox a montré que le taux de transmission par contact de M. gallisepticum à partir des animaux éprouvés dans le groupe vacciné a été 0,356 fois celui du groupe non vacciné. De plus, l'estimation totale de R (le nombre moyen de cas secondaires infectés par un cas infectieux typique) du groupe vacciné (4,3 [95 % IC: 1,6–49,9]) a été significativement inférieur à celui du groupe non vacciné (∞ [95% IC: 9,9–∞]). Cependant, l'estimation totale de R dans le groupe vacciné a encore excédé 1, ce qui indique que l'effet de la vaccination sur la transmission horizontale de M. gallisepticum est encore insuffisant pour stopper sa diffusion dans ces conditions expérimentales.
Der Effekt einer Mycoplasma gallisepticum-Lebendvakzine auf die horizontale Übertragung von Mykoplasmaspezies wurde in einem experimentellen Tierübertragungsmodell mit spezifisch pathogen-freien (SPF) weißen Legehennen quantifiziert. Dazu wurden zwei identische Versuche mit jeweils zwei Versuchs- und einer Kontrollgruppe durchgeführt. Die Versuchsgruppen bestanden jeweils aus 20 Tieren im Alter von 21 Wochen, die paarweise eingestallt wurden. Eine Versuchsgruppe wurde zweimal mit einer kommerziell verfügbaren attenuierten M. gallisepticum-Lebendvakzine geimpft, während die andere Versuchsgruppe ungeimpft blieb. In beiden Versuchsgruppen wurde jeweils eins der paarweise gehaltenen Hühner intratracheal mit 104 Kolonie bildenden Einheiten (CFU) inokuliert und das andere Huhn diente als empfängliches Kontakttier. Die Kontrollgruppe bestand aus 10 paarweise gehaltenen zweifach vakzinierten und fünf einzeln gehaltenen ungeimpften Hühnern. Die Infektion wurde mittels Serologie, Kultivierung und quantitativer Polymerasekettenreaktion (Q-PCR) überprüft. Impf- und Belastungsinfektionsstamm wurden mit einer spezifischen PCR und mit einer “Random amplified polymorphic DNA” (RAPD)-Analyse unterschieden. In beiden Experimenten wiesen alle nicht vakzinierten, inokulierten Hühner sowie ihre Kontakt-Partner eine Infektion mit M. gallisepticum auf. Bei den inokulierten vakzinierten Hühnern und den dazu gehörenden Kontakttieren hatten 19 bzw. 13 Hühner eine M. gallisepticum-Infektion. Die Analyse des M. gallisepticum-Ausscheidungsmusters ließ einen signifikanten Effekt der Impfung auf die Ausscheidungsmenge bei den Kontakttieren der vakzinierten Hühner erkennen. Darüberhinaus wies die Cox Proportional Hazard-Analyse darauf hin, dass die Rate der M. gallisepticum-Übertragung von den inokulierten zu den Kontakttieren nur 0,356 mal so groß war wie bei der nicht vakzinierten Gruppe. Außerdem war der Schätzwert über alles von R (Durchschnittszahl der durch einen typischen Infektionsfall verursachten Sekundärfälle) der vakzinierten Gruppe (4,3 (95% CI:1,6–49,9)) signifikant niedriger als der der nicht vakzinierten Gruppe (∞ (95% CI: 9,9–∞)). Der Schätzwert von R über alles war jedoch in der vakzinierten Gruppe noch größer als 1, was darauf hinweist, dass der Effekt der Vakzination auf die horizontale Übertragung von. M. gallisepticum nicht ausreichend ist, um die Ausbreitung des Erregers unter diesen experimentellen Bedingungen zu verhindern.
Se cuantificó el efecto de una vacuna viva de Mycoplasma gallisepticum en la transmisión horizontal de esta especie de Mycoplasma en un modelo experimental animal de transmisión en Ponedoras Blancas libres de patógenos específicos (SPF). Se llevaron a cabo dos ensayos idénticos y en cada uno de ellos se incluyeron dos grupos experimentales y un grupo control. Los grupos experimentales, cada uno de los cuales estaba formado por 20 aves de 21 semanas de vida, se alojaron siguiendo un diseño por parejas. Un grupo se vacunó dos veces con una vacuna viva atenuada comercial de M. gallisepticum, mientras que el otro grupo no se vacunó. Cada pareja en los grupos experimentales consistía en un pollo infectado (104 unidades formadoras de colonias (CFU) intratraquealmente) y un ave susceptible en contacto. El grupo control estaba formado por 10 aves vacunadas dos veces alojadas en parejas y cinco aves no vacunadas alojadas individualmente. La infección se valoró mediante serología, cultivo y reacción en cadena de la polimerasa cuantitativa (Q-PCR). La cepa vacunal y la cepa de desafío se diferenciaron mediante una PCR específica y análisis del polimorfismo del DNA amplificado al azar (RAPD). En ambos ensayos, todos los pollos no vacunados desafiados así como las respectivas parejas de aves en contacto se infectaron con M. gallisepticum. En las aves vacunadas desafiadas y las respectivas aves en contacto, un total de 19 y 13 pollos respectivamente se infectaron con M. gallisepticum. El análisis de los patrones de excreción de M. gallisepticum mostró un efecto importante de la vacunación sobre los niveles de excreción en los pollos vacunados en contacto. Además, el análisis de Riesgos Proporcionales de Cox indicó que el ritmo de transmisión de M. gallisepticum de las aves desafiadas a las aves en contacto en el grupo vacunado era 0.356 veces el observado en el grupo no vacunado. Además, la estimación global de R (la media del número de casos secundarios infectados por un caso infeccioso típico) del grupo vacunado (4.3(95% CI:1.6-49.9)) era significativamente menor que la del grupo no vacunado (∞ (95% CI:9.9–∞)). Sin embargo, la estimación global de R en el grupo vacunado aún superaba 1 lo cual indica que el efecto de la vacunación en la transmisión horizontal de M. gallisepticum es todavía insuficiente para parar su diseminación bajo estas condiciones experimentales.
Introduction
Live vaccines against Mycoplasma gallisepticum infections in poultry are commonly used in various parts of the world. A number of studies have shown that such vaccines can protect against airsacculitis, egg production losses and vertical transmission (Evans & Hafez, Citation1992; Whithear, Citation1996; Barbour et al., Citation2000; Barbour et al., Citation2002; Kleven & Ley, Citation2003); however, there is limited knowledge about the ability of these products to reduce the horizontal transmission of this Mycoplasma species in a flock.
Some scientists have shown a positive effect of live M. gallisepticum vaccines on reducing the shedding levels of mycoplasmas in individual birds, while others have studied the displacement of field strains by vaccine strains (Whithear, Citation1996; Turner & Kleven, Citation1998; Barbour et al., Citation2000). However, the results are somewhat ambiguous. For instance, two field studies suggested that, after repeated vaccination of an infected chicken flock with the F vaccine strain followed by a series of vaccinations with the ts-11 live vaccine or the latter vaccine alone, the wild M. gallisepticum strain was displaced (Turner & Kleven, Citation1998; Barbour et al., Citation2000). In contrast, a pen trial study failed to demonstrate the displacement of the virulent R strain by either the ts-11 or 6/85 live vaccine strains (Kleven et al., Citation1998).
To stop the spread of M. gallisepticum within a flock it is imperative that the transmission parameter R is less than 1; that is, that each infected chicken on average infects less than one susceptible non-infected flock mate. Recently, an experimental animal model was developed in which the horizontal transmission parameters of M. gallisepticum in chickens were defined (Feberwee et al., Citation2005b). In the study described here this model was used to quantify the effect of a live attenuated M. gallisepticum vaccine on the horizontal transmission of this Mycoplasma species between layer hens. The shedding levels of M. gallisepticum between vaccinated and non-vaccinated birds were also compared.
Materials and methods
Experimental design
The study consisted of two similar experiments (Experiment 1 and Experiment 2), each including a vaccinated group, a non-vaccinated group and a control group. Both the vaccinated and non-vaccinated groups each consisted of 20 specific pathogen free (SPF) White Layers 21 weeks of age, which were divided into 10 pairs of birds and housed in a single cage (size, 100×47×53 cm3). The cages were placed at a distance of 65 cm from each other. At this distance, within the model, the pairs could be regarded as independent observations (Feberwee et al., Citation2005b). The vaccinated groups of both experiments were aerosolized twice with a live M. gallisepticum vaccine at 14 and 18 weeks of age.
Twenty-one days after the second vaccination one chicken of each pair in both groups was removed (D–1) and placed together in an isolator in order to perform an intratracheal challenge. The challenge inoculum consisted of 1.0 ml mycoplasma broth containing 104 colony forming units (CFU) M. gallisepticum F1999/ml (field strain). It was shown previously that this strain induces a positive serological response, even at a low inoculation dose (Feberwee et al., Citation2005a, Citation2005b). Twenty-four hours after challenge the infected chickens were reunited in their cages with the in-contact birds. This moment was regarded as day 0 (D0) of the experiment.
The control groups of both experiments each consisted of 15 chickens, of which 10 were vaccinated twice and also housed in pairs. The remaining five were non-vaccinated birds individually housed. The control birds were of the same breed and age as the other groups. The vaccinated pairs and the individually housed non-vaccinated control chickens were placed at a distance of 178 cm of each other. The vaccinated birds in the control group were included to determine the excretion pattern of the vaccine strain during the experiment. The non-vaccinated birds were included to control the specificity of testing methods and sample handling.
Housing conditions
Climate conditions were the same for all groups. The relative humidity was 60% or less, the ambient temperature was 18 to 20°C and the ventilation rate per room was 1200 m3/h (±5%). The air was expelled through four outlets. Birds were provided with 16 h of light and with feed ad libitum (rearing pellets, production code 2782 CDI, irradiated with 0.9 Mrad; Hope Farm BV, Woerden, The Netherlands). The chickens were housed on a combination of wood shavings and sawdust (Woody Clean BK 10–20; Technilab-BMI, Helmond, The Netherlands).
Vaccination
At the ages of 14 and 18 weeks, birds were vaccinated twice with a live attenuated M. gallisepticum strain (Nobilis Mg 6/85; Intervet), which is not virulent, does not spread and does not induce positive serology in the M. gallisepticum rapid plate agglutination (RPA) test (Evans & Hafez, Citation1992). At 19 weeks old, the birds were assigned to the experimental groups as described earlier until the start of the experiment.
For the first vaccination of Experiments 1 and 2, and for the second vaccination of Experiment 2, the 30 birds were equally divided over three separate negative pressure isolators (10 birds per isolator) with a volume of 1.31 m3 (Beyer & Eggelaar, Utrecht, the Netherlands). However, the second vaccination of Experiment 1 was carried out on groups of 10 chickens using one negative pressure isolator with a volume of 0.79 m3 (design ID Lelystad; Beyer & Eggelaar). Between the vaccine applications, the isolator was ventilated for 10 to 12 min with fresh air at the rate of 25 m3/h.
The aerosol was carried out as described by Landman et al. (Citation2004). Briefly, for each isolator 108 CFU Mg 6/85 suspended in 20 ml phosphate-buffered saline were aerosolized for 3 to 5 min at 2 bar with an air yield of 30 l/min. The bacterial concentrations of the vaccine suspensions were determined before and after aerosolization (ISO 7402). The isolator temperature was 18 to 20°C. For the aerosol application, an air compressor (Mecha Concorde, type 7SAX, 1001, 10 bar/max; SACIM, Verona, Italy) coupled to a spray head (Walther Pilot spray-head with 0.5 mm diameter; Walther Spritz-and Lackiersysteme, Wuppertal, Germany) was used. The ventilation of the isolator was switched off for approximately 30 min (3 to 5 min for the application of the vaccine and 26 min for inhalation of the aerosol). In Experiments 1 and 2, different batches of vaccine were used.
Handling, testing and monitoring
Sampling of birds always started with the control group, followed by the vaccinated challenged group and finally the non-vaccinated challenged group. Within vaccinated and non-vaccinated challenged groups, the order of sampling of pairs was changed daily to prevent the occurrence of systemic errors.
At days 7, 14 and 21 after the first vaccination and at days 7 and 21 after the second vaccination, tracheal samples were taken and tested by quantitative polymerase chain reaction (Q-PCR) (Mekkes & Feberwee, Citation2005) to determine the shedding levels of the vaccine strain. Day 21 after the second vaccination was the day before challenge (D–1).
The M. gallisepticum status of the non-vaccinated and vaccinated chickens was assessed 1 day before challenge (D–1) by serology and Q-PCR. Detection of both the M. gallisepticum challenge and the vaccine strain in the experimental groups after D0 was done by culture, Q-PCR, RPA and haemagglutination inhibition (HI) test as described elsewhere (Feberwee et al., Citation2005a, Citation2005b; Mekkes & Feberwee, Citation2005). For RPA and HI a positive reaction at dilution ≥1:8 in both tests was regarded as positive. All samples that tested positive in the Q-PCR were analysed once in a M. gallisepticum 6/85 Light Cycler (LC) PCR to determine whether shedding was due to the challenge strain. In Experiment 2, these samples were also analysed by the random amplified polymorphic DNA (RAPD) method (Feberwee et al, Citation2005c). The amplification procedure and the analysis of the amplification product was carried out as described by Fan et al. (Citation1995) using the arbitrary primers M16SPCR′, M13F′ and SIOLOGO3′.
At days 2, 3, 4, 5, 7, 9, 11, 14, 21, 25, 28, 31 and 35 after contact, tracheal samples were taken from all chickens for Q-PCR. In Experiment 1 at days 5, 14 and 28, culture was also performed each time using the trachea swab of the same five inoculated chickens of both groups. Serology was performed on blood samples taken at days 7, 14, 21, 28 and 35 after contact.
Mg 6/85 light cycler PCR
The Mg 6/85 LC PCR enabled differentiation of the vaccine and challenge strains. Within the nusG (MGA_0473) and 16S gen (MGAr06) of the M. gallisepticum R strain (Papazisi et al, Citation2003), primers for a M. gallisepticum 6/85-specific fragment of 202 base pairs were selected: 5′-CAGTTGCAAATCCGTAAGG-3′ (forward) and 5′-GATCCCAG-TAATATTAACTGTGTTTC-3′ (reverse). Two fluorescent-labelled probes were designed spanning this region: a donor probe, 5′-ACCCTGGATACATCTACATTAAGATGGAGA-FL; and the acceptor probe, 5′-LC Red640-GAATGAAGCAGCTTGGTTTGCAG-PH. The PCR reactions were performed with the LightCycler 2.0 system using the FastStart DNA Master HybProbe kit (Roche), 2 µl extracted sample DNA, a positive control and H2O (as a negative control), 0.5 µM primers, 0.2 µM probes and 3 mM MgCl2 in a final volume of 20 µl. After 10 min of incubation at 95°C (for activation of the enzyme and denaturation of the template), the samples were amplified by a 40-cycle protocol (95°C for 10 sec, 50°C for 10 sec, and 72°C for 10 sec). The fluorescence signal was captured at every annealing step by a single measurement. These data were used to decide whether a sample was positive or negative. In the case of a positive sample, the probes will hybridize to the M. gallisepticum 6/85-specific internal sequence of the amplified fragment. The emitted fluorescence is measured during the annealing phase.
Specificity and detection limit of the Mg 6/85 LC PCR
The specificity of the Mg 6/85 LC PCR was tested on four M. gallisepticum strains (ATCC 15302, R low, the challenge strain F1999 and the 6/85 vaccine strain). The PCR was also tested on four heterologous Mycoplasma species (Mycoplasma gallinaceum ATCC 33550, Mycoplasma synoviae ATCC 2504, Mycoplasma meleagridis ATCC 25294 and Mycoplasma imitans ATCC 51306). The specificity and sensitivity of the Mg 6/85 LC PCR was also tested on a number of clinical samples derived from 6/85-vaccinated birds (n=48), from strain F1999-inoculated birds (n=34) and from M. gallisepticum-free SPF birds (n=20). Moreover, the specificity of the Mg 6/85 LC PCR was compared with that of the Q-PCR, which had been validated for a number of Mycoplasma species and strains, although not the vaccine strain (Mekkes & Feberwee, Citation2005).
To determine the detection limit of the Mg 6/85 LC PCR, a 10-fold dilution of a concentration of 2.9×107 CFU 6/85 vaccine strain was used. The detection limit was defined as the highest dilution(s) yielding a positive PCR result expressed as CFU equivalents/ml needed for a positive PCR reaction.
Analysis of data
The effect of vaccination on the shedding levels of M. gallisepticum was assessed by calculating the area under the curve (AUC) showing the M. gallisepticum shedding in vaccinated and non-vaccinated birds of both experiments. Since several birds had no excretion, 0.5 was added to the AUC of all birds. A period of 31 days was chosen for this calculation, starting when shedding in the inoculated respectively in-contact animals was first detected. A natural logarithmic (ln) transformation was performed first in order to normalize the data. For analysing ln AUC, the following explanatory factors were included in the statistical model: the experiment (1/2), the vaccination (yes/no), the infection route (challenged/in-contact) and the interaction between these factors. To model the difference within group variances, a variance model with different variances per group (experiment and vaccination) was used. A linear mixed-effects model with the chicken pair as the random variable was performed in the statistical program R, version 2.0.1 (R Development Core Team, Citation2005).
The transmission parameters R (the average number of secondary cases infected by one typical infectious case) and β (the number of new infections that occur due to one infectious animal per unit of time) of the M. gallisepticum inoculum strain were quantified as described earlier (Feberwee et al, Citation2005b). In addition, we tested the hypothesis R vaccinated=R non-vaccinated and tested whether R vaccinated was significantly different from 1. The overall estimate of R was performed under the assumption that the epidemiological process had ended at the end of the experimental period.
Since the statistical model used to quantify β did not fit the data satisfactorily, a Cox Proportional Hazard analysis, which is a parameter-free analysis, was performed. In this analysis the hazard ratio was calculated, representing the difference between vaccinated and non-vaccinated chickens in the time that is needed for the in-contact chickens to start shedding M. gallisepticum. The experiment was used as the cluster variable because chickens in the same experiment are correlated. Where the hazard ratio is 1, then there is no significant difference. The analysis was performed in Program R, version 2.1.1 (R Development Core Team (Citation2005). For a proper handling of ties of the intervals, the Efron method was used.
Results
Specificity and detection limit of the vaccine-specific Mg 6/85 LC PCR
The Mg 6/85 LC PCR detected only the vaccine strain, whereas the Q-PCR detected all tested M. gallisepticum strains including the vaccine and the challenge strain. The results in show a high specificity (100%) of the Mg 6/85 LC PCR for clinical samples originating from SPF chickens infected with the challenge strain. Moreover, a high correlation was found between the results of the M. gallisepticum Q-PCR and the Mg 6/85 LC PCR in 45 out of 48 samples from M. gallisepticum 6/85 live-vaccinated SPF chickens. As for the detection limit, 10 CFU equivalents of Mg 6/85 vaccine strain were still detected by the Mg 6/85 LC PCR.
Table 1. Specificity and sensitivity of Mg 6/85 LC PCR on tracheal swabs from birds vaccinated with the live 6/85 vaccine and from birds challenged with M. gallisepticum F1999, compared with the results of the M. gallisepticum (Mg) Q-PCR
Clinical signs and observation of transmission
No clinical signs were observed after application of either the vaccine or the challenge strain. The excretion in the challenged non-vaccinated and vaccinated group and the in-contact exposed group showed similar patterns in both experiments. Shedding by the challenged non-vaccinated and vaccinated chickens started within 3 days and increased rapidly from day 3 to days 7 to 14. This was followed by a slight decrease of shedding until the end of the experiment (a,b). In the non-vaccinated and vaccinated in-contact chickens, excretion started between days 5 and 9 and increased up to day 21 ending in a plateau (a,b). c and show that in Experiment 1 more vaccinated birds in the control group tested positive for the vaccine strain and at a higher level than in Experiment 2.
Figure 1. Excretion patterns (mean±standard error of the mean) as indicated by Q-PCR of (1a) non-vaccinated and vaccinated challenged and in-contact birds in Experiment 1 after challenge with 104 CFU M. gallisepticum strain F1999, and (b) non-vaccinated and vaccinated challenged and in-contact birds in Experiment 2 after challenge with 104 CFU M. gallisepticum strain F1999. 1c: Shedding of M. gallisepticum 6/85 vaccine strain as indicated by the Q-PCR in the vaccinated control group of Experiments 1 and 2. I, II and III, 7, 14 and 21 days after the first vaccination, respectively; IV, 7 days after the second vaccination; V, 21 days after the second vaccination and the day before challenge(D–1). The other figures indicate the days after first contact between M. gallisepticum challenged and non-challenged susceptible in-contact birds. (Mg = M. gallisepticum)
Table 2. Q-PCR and serology results of the non-vaccinated challenged group, the vaccinated challenged group and the vaccinated non-challenged control group in Experiments 1 and 2
The non-vaccinated birds in the control groups of both experiments were negative in the M. gallisepticum RPA and HI tests and by Q-PCR throughout the experimental period (data not shown), showing that the specificity of the used detection methods was high.
In Experiment 1, the vaccine concentrations before and after the first vaccination were 2.9×107 and 2.2×107 CFU/ml, respectively. For the second vaccination, the vaccine concentrations were 0.7×107 and 3.6×107 CFU/ml, respectively. In Experiment 2, the vaccine concentrations before and after vaccination were 3.1×107 and 5.7×107 CFU/ml, respectively. For the second vaccination they were 0.3×107 and 9.8×107 CFU/ml, respectively.
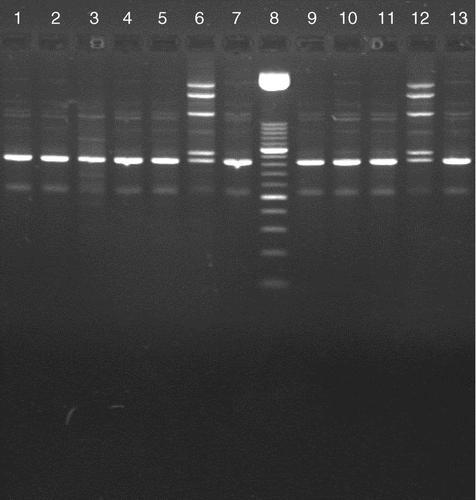
shows that in Experiment 1 and Experiment 2 all non-vaccinated in-contact birds became infected. In both experiments, infection in eight of the 10 birds was also confirmed by positive serology. In Experiment 1, nine birds of the challenged vaccinated group became infected with the challenge strain as shown by positive serology. One bird did not shed M. gallisepticum and remained serologically negative. The presence of viable mycoplasmas in this experiment was shown by the positive culture results (). Five vaccinated in-contact birds were positive in the Q-PCR; however, in only one of these birds the infection was also confirmed by positive serology at 35 days after contact. Moreover, all five birds were negative in the Mg 6/85 LC PCR indicating the presence of the challenge strain in these birds. The other four vaccinated in contact birds showed negative Q-PCR and serology results during the experimental period. In Experiment 2, 10 of the vaccinated challenged birds showed a positive Q-PCR result. Of these chickens, nine also showed a positive serology. Eight in-contact vaccinated birds became infected, of these seven also showed positive RPA and HI titres. All eight birds harboured the challenge strain as shown by the RAPD patterns () and by the negative results of the Mg 6/85 LC PCR ().
Figure 2. RAPD patterns at 35 days after contact of the eight vaccinated in-contact birds that were positive in the Q-PCR. Lanes 1, 2, 3, 4, 5, 9 10, 11, challenge strain originating from eight in-contact birds; lanes 7 and 13, challenge strain (reference); lanes 6 and 12, vaccine strain (reference); lane 8, 50 base pair ladder (DNA Marker XIII; Roche).
Analysis of excretion levels and transmission parameters
Considering the ln AUC, the shedding levels of the challenged vaccinated birds in Experiment 1 and 2 were not significantly lower (−4.21, P=0.101 and −0.16, P = 0.925, respectively) than those of the challenged non-vaccinated birds. However, the excretion levels of the vaccinated in-contact birds in both experiments were significantly lower (−9.35, P<0.001 and −3.27, P=0.065, respectively) than that of the non-vaccinated in-contact birds.
For the estimation of β, and by analogy to previous findings (Feberwee et al., Citation2005b), the best fit was achieved with an excretion threshold of 103 CFU and a latent period of 2 days. The estimates of β for the control group and the vaccinated groups in Experiments 1 and 2 were not significantly different. The overall estimate of β for the vaccinated challenged groups (0.049, 95% confidence interval (CI) = 0.027 to 0.088) was significantly lower than that of the non-vaccinated challenged groups (0.17, 95% CI = 0.108 to 0.265). As the statistical model lacked a satisfactory fit, the Cox Proportional Hazard analysis was used and revealed a hazard ratio of 0.356 (95% CI = 0.239 to 0.528)—meaning that the rate of M. gallisepticum transmission from challenged to in-contact birds in the vaccinated group is 0.356 times that of the non-vaccinated challenged group. It also means that excretion in the vaccinated group started significantly later than in the non-vaccinated challenged group.
The estimated R values for Experiments 1 and 2 in the non-vaccinated and vaccinated challenged groups were not significantly different (R=∞, 95% CI = 4.5 to ∞) for both non-vaccinated groups and (R=2, 95% CI = 0.5 to 12.6 and R=8, 95% CI = 1.6 to 77.3) for the vaccinated groups, respectively. The overall R value (of both experiments taken together) was ∞ (95% CI = 9.9 to ∞) for the non-vaccinated challenged group and 4.3 (95% CI = 1.6 to 49.9) for the vaccinated challenged group. Although the overall R value of the vaccinated challenged group was significantly different to that of the non-vaccinated challenged group (P=0.027), its value in the vaccinated challenged group still significantly exceeded 1.
Discussion
To properly examine the effect of a live attenuated M. gallisepticum vaccine on the horizontal transmission of this bacterium, it was imperative to discriminate between the challenge strain and the vaccine strain. Indeed, in this transmission experiment the Mg 6/85 LC PCR specifically differentiated the 6/85 vaccine from the challenge strain. However, the specificity of this particular PCR should be further validated on a large number of field strains and field samples if it is to be used in the field. Preliminary data suggest that this test is not 100% specific, as a number of Dutch field strains that were isolated before the introduction of the vaccine reacted positively in the Mg 6/85 LC PCR (data not shown).
Analysis of the M. gallisepticum excretion showed that the ln AUC value in the vaccinated challenged in-contact chickens in both experiments was significantly lower than that in the non-vaccinated challenged groups, indicating that there is an effect of vaccination on the shedding levels of the vaccinated in-contact birds. Unfortunately, β could not be estimated satisfactorily and to further investigate whether there was still an effect of vaccination on the rate of transmission the Cox Proportional Hazard analysis, which is a parameter free analysis, was performed. The calculated hazard ratio indicated a difference in transmission between the vaccinated and non-vaccinated challenged groups. However, since R still exceeded 1 it can be expected that vaccinating twice with the live attenuated 6/85 M. gallisepticum vaccine does not halt the spread of M. gallisepticum in a flock. The overall estimation of R was performed under the assumption that the epidemiological process had ended at the termination of the experimental period. However, since R > 1, this assumption—contrary to a situation where R<1—has no critical influence on the calculations that lead to the conclusion that the use of live attenuated M. gallisepticum 6/85 vaccine will not stop the spread of M. gallisepticum.
The fact that β could not be estimated satisfactorily within the statistical model suggests the occurrence of a biological factor that was not included into the model. Despite high vaccine doses, the shedding levels of the vaccine strain in the vaccinated non-challenged control group of Experiment 1 and Experiment 2 differ, indicating a difference in colonization and replication of the vaccine strain. Other workers (Ley et al., Citation1997; Kleven et al., Citation1998) also reported variable colonization of the M. gallisepticum live vaccine strains t-s11 and 6/85, which was related to the apathogenic characteristics of these strains. Arguably, this poor and variable colonization may also be the consequence of genetic heterogeneity of the birds or the variation in vaccination procedure. The birds of both experiments were from the same origin, although from different generations and were not inbred, which may imply differences in disease susceptibility (Zekarias et al., Citation2002). As for the vaccination procedure, the vaccination dose could have been a factor. However, in our experiment the birds were vaccinated with vaccine concentrations of 107 CFU/ml, which should be sufficient to induce protection against M. gallisepticum infections according to Evans & Hafez (Citation1992). Another factor that could elicit variations in the vaccination response is the method of application. For the second vaccination in Experiment 1 the isolator was not decontaminated but was ventilated and had a smaller volume, but this only had a marginal effect on the residual contamination of the isolator and the vaccine concentration as described in detail elsewhere (Landman et al., Citation2004).
It is possible that the design of the experiment itself (i.e. the repeated swabbing of the trachea) may have affected its integrity, thus favouring the colonization and multiplication of the vaccine and challenge strain and subsequently influencing the outcome of R. However, if this were so, more variation would have been expected between the individual chickens in both experiments. Regular swabbing of the trachea is the only available sampling method for accurate establishment of M. gallisepticum excretion patterns. Reliance on serology alone would lead to an underestimation of R as the number of serologically positive birds was lower than the number of birds shedding the M. gallisepticum challenge strain at 35 days after contact.
In conclusion, our data show that vaccination with a live attenuated M. gallisepticum vaccine significantly reduced the shedding levels in in-contact birds and the horizontal transmission of a M. gallisepticum challenge strain under experimental circumstances. However, the effect on the horizontal transmission was insufficient to stop its spread within a flock. This supports the findings of other authors (Kleven et al., Citation1998), who failed to demonstrate the displacement of the virulent R strain by both the attenuated ts-11 and 6/85 live vaccines in a pen floor study. Nevertheless, the virulent F vaccine strain readily displaced the R strain in the same study. Furthermore, field studies have also confirmed that continuous vaccination of replacement pullets with the F strain has the potential to displace the original field strain. However, the F strain was able to maintain itself on multiple-age farms after vaccination was discontinued (Cummings & Kleven, Citation1986; Ley et al., Citation1993; Ley et al., Citation1997; Turner & Kleven, Citation1998). Turner & Kleven (Citation1998) showed that eradication of the live F vaccine strain could finally be achieved using a less virulent vaccine strain. These results suggest that for the eradication of a virulent M. gallisepticum field strain, it may be necessary to vaccinate with the F strain first for one or more production cycles prior to switching to a more attenuated vaccine strain (Levisohn & Kleven, Citation2000).
Acknowledgments
This research was supported by a grant from the Dutch Commodity Board for Poultry and Eggs. The authors thank Wies de Haan (Internet International) for her contribution to the development of the M. gallisepticum 6/85 PCR.
References
- Barbour , E.K. , Hamadeh , S.K. and Eidt , A. 2000 . Infection and immunity in broiler chicken breeders vaccinated with a temperature-sensitive mutant of Mycoplasma gallisepticum and impact on performance of offspring . Poultry Science , 79 : 1730 – 1735 .
- Branton , S.L. , Bearson , S.M. , Bearson , B. , Lott , B.D. , Maslin , W.R. , Collier , S.D. , Pharr , G.T. and Boykin , D.L. 2002 . The effects of 6/85 live Mycoplasma gallisepticum vaccine in commercial layer hens over a 43-week laying cycle on egg production, selected egg quality parameters, and egg size distribution when challenged before beginning of lay . Avian Diseases , 46 : 423 – 428 .
- Cummings , T.S. and Kleven , S.H. 1986 . Evaluation of protection against Mycoplasma gallisepticum infection in chickens vaccinated with the F-strain of M. gallisepticum . Avian Diseases , 30 : 169 – 171 .
- Evans , R.D. and Hafez , Y.S. 1992 . Evaluation of Mycoplasma gallisepticum strain exhibiting reduced virulence for prevention and control of poultry mycoplasmosis . Avian Diseases , 36 : 197 – 201 .
- Fan , H.H. , Kleven , S.H. and Jackwood , M.W. 1995 . Application of polymerase chain reaction with arbitrary primers to strain identification of Mycoplasma gallisepticum . Avian Diseases , 39 : 729 – 735 .
- Feberwee , A. , Mekkes , D.R. , De Wit , J.J. , Hartman , E.G. and Pijpers , A. 2005a . Comparison of culture, PCR and different serological tests for detection of Mycoplasma gallisepticum and Mycoplasma synoviae infections . Avian Diseases , 49 : 260 – 268 .
- Feberwee , A. , Mekkes , D.R. , Klinkenberg , D. , Vernooij , J.C.M. , Gielkens , A.L.J. and Stegeman , J.A. 2005b . An experimental model to quantify horizontal transmission of Mycoplasma gallisepticum . Avian Pathology , 34 : 355 – 361 .
- Feberwee , A. , Dijkstra , J.R. , von Banniseht-Wysmuller , T.E. , Gielkens , A.L.J. and Wagenaar , J.A. 2005c . Genotyping of Mycoplasma gallisepticum and M. synoviae by amplified fragment length polymorphism (AFLP) analysis and digitalized random amplified polymorphic DNA (RAPD) analysis . Veterinary Microbiology , 111 : 125 – 131 .
- Kleven , S.H. Ley D. ( 2003 ) Diseases of Poultry 11th edn (pp. 719 – 744 , 756–766 ).
- Kleven , S.H. , Fan , H.H. and Turner , K.S. 1998 . Pentrial studies on the use of live vaccines to displace virulent Mycoplasma gallisepticum in chickens . Avian Diseases , 42 : 300 – 306 .
- Landman , W.J.M. , Corbanie , E.A. , Feberwee , A. and van Eck , J.H.H. 2004 . Aerosolization of Mycoplasma synoviae compared with Mycoplasma gallisepticum and Enterococcus faecalis . Avian Pathology , 33 : 210 – 215 .
- Levisohn , S. and Kleven , S.H. 2000 . Avian mycoplasmosis (Mycoplasma gallisepticum) . Revue scientifique et technique (International Office of Epizootics) , 19 : 425 – 442 .
- Ley , D.H. , Avakian , A.P. and Berkhoff , J.E. 1993 . Clinical Mycoplasma gallisepticum infection in multiplier breeder and meat turkeys caused by F strain: identification by sodium dodecyl sulphate-polyacrylamide gel electrophoresis, restriction endonuclease analysis and polymerase chain reaction . Avian Diseases , 37 : 854 – 862 .
- Ley , D.H. , McLaren , J.M. , Miles , A.M. , Barnes , J.H. , Miller , S.H. and Franz , G. 1997 . Transmissibility of Live Mycoplasma gallisepticum strains ts-11 and 6/85 from vaccinated Layer pullets to sentinel poultry . Avian Diseases , 41 : 187 – 194 .
- Mekkes , D.R. and Feberwee , A. 2005 . Real-Time PCR for the qualitative and quantitativedetection of Mycoplasma gallisepticum . Avian Pathology , 34 : 348 – 354 .
- Papazisi , L. , Gorton , T.S. , Kutish , G. , Markham , P.F. , Browning , G.F. , Nguyen , D.K. , Swartzell , S. , Madan , A. , Mahaireas , G. and Geary , S. J. 2003 . The complete genome sequence of the avian pathogen Mycoplasma gallisepticum strain R(low) . Microbiology , 149 : 2307 – 2316 .
- R Development Core Team . ( 2005 ). R: A Language and Environment for Statistical Computing . Vienna , , Austria : R Foundation for Statistical Computing . Available online at : http://www.R-project.org
- Turner , K.S. and Kleven , S.H. 1998 . Eradication of live F strain Mycoplasma gallisepticum vaccine using live ts-11 on a multi age commercial layer farm . Avian Diseases , 42 : 404 – 407 .
- Whithear , K.G. 1996 . Control of avian mycoplasmosis by vaccination . Revue scientifique et technique (International Office of Epizootics) , 15 : 1527 – 1553 .
- Zekerias , B. , ter Huurne , A.H.M. , Landman , W.J.M. , Rebel , M.J. , Pol , J.M.A. and Gruys , E. 2002 . Immunological basis of differences in diseases resistance in the chicken . Veterinary Research , 33 : 109 – 125 .