Abstract
We evaluated the effects of viral immunodeficiency on the outcome of infectious bronchitis virus (IBV) infection in chickens as a hypothetical cause for failure of adequate protection in vaccinated chickens. Initially, we investigated IBV isolations from cases of respiratory disease in association with the presence of thymic and/or bursal atrophy in 322 submissions during 1997 to 2002. Arkansas (Ark)-type IBV was most frequently isolated in spite of extensive ArkDPI vaccination in the broiler industry. The number of IBV isolations was consistently higher in broilers aged 27 to 43 days, coinciding with lymphocytic depletion of the bursa and/or thymus, providing circumstantial evidence that immunodeficiency and IBV incidence may be linked. S1 gene sequence analyses, antigenic characterizations, and challenge of susceptible chickens demonstrated that the field IBV isolates tested were closely related to vaccine strains and had low pathogenicity for chickens. We experimentally evaluated the effects of immunodeficiency caused by co-infection with chicken anaemia virus and infectious bursal disease virus on the outcome of IBV infection. Clinical signs and histological lesions were more persistent in immunodeficient chickens. Local specific IgA production was delayed and lower levels were achieved in immunodeficient chickens. At the same time, IBV RNA concentrations in tracheas and lachrymal fluids were higher and more persistent in immunodeficient chickens. Collectively, these results indicate that viral immunodeficiency most probably plays a relevant role in the epidemiology and outcome of IBV infection.
Nous avons évalué les effets de l'immunodéficience virale sur le résultat de l'infection par le virus de la bronchite infectieuse aviaire (IBV) chez des poulets comme une cause hypothétique d'échec de protection adéquate chez des poulets vaccinés. Initialement, nous avons investigué les isolements d'IBV de cas de maladie respiratoire en association avec la présence d'atrophie bursale et/ou thymique à partir de 322 soumissions durant les années 1997-2002. L'IBV de type Arkansas (Ark) a été le plus fréquemment isolé en dépit de la vaccination ArkDPI à grande échelle des poulets de chair en élevage industriel. Le nombre d'isolement d'IBV a été nettement supérieur chez les poulets de chair âgés de 27 à 43 jours, coïncidant avec la déplétion lymphocytaire de la bourse de Fabricius et/ou du thymus, fournissant une évidence circonstanciée que l'immunodéficience et l'incidence de l'IBV peuvent être liées. Les analyses de séquence du gène S1 les caractérisations antigéniques, et l'épreuve de poulets sensibles ont démontré que les souches d'IBV isolées sur le terrain étaient très poches des souches vaccinales et présentaient une pathogénicité faible pour les poulets. Nous avons expérimentalement évalué les effets de l'immunodéficience causée par la co-infection avec le virus de l'anémie du poulet et le virus de la bursite infectieuse aviaire (IBDV) sur le résultat de l'infection par l'IBV. Les symptômes et les lésions histologiques ont persisté plus longtemps chez les poulets immunodéficients. La production locale d'IgA spécifique a été retardée et des niveaux inférieurs ont été enregistrés chez les poulets immunodéficients. Au même moment, les concentrations en ARN IBV dans les trachées et les liquides lacrymaux ont été plus élevées et ont persisté plus longtemps chez les poulets immunodéficients. Dans l'ensemble, ces résultats indiquent que l'immunodéficience virale, joue vraisemblablement un rôle important dans l'épidémiologie et le résultat de l'infection par l'IBV.
Wir untersuchten die Auswirkungen einer viral bedingten Immuninsuffizienz auf den Verlauf einer Infektion mit dem infektiösen Bronchitisvirus (IBV) in Hühnern als eine hypothetische Ursache für das Ausbleiben eines adäquaten Impfschutzes in Hühnern. Anfänglich untersuchten wir IBV-Isolate aus Feldfällen von Respirationserkrankungen in Verbindung mit Thymus- und/oder Bursaatrophie in 322 Einsendungen in den Jahren 1997-2002. Trotz umfangreicher Arkansas (Ark) DPI-Vakzinationen in der Broilerindustrie war der Ark-Typ das am häufigsten isolierte IBV. Die Zahl der IBV-Isolate war bei den 27 bis 43 Tage alten Broilern durchweg höher, wobei gleichzeitig in Bursa und/oder Thymus ein Verlust der Lymphozyten festgestellt wurde. Dies belegt, dass die Immuninsuffizienz und das Auftreten einer infektiösen Bronchitis wahrscheinlich im Zusammenhang stehen. Analysen der S1-Gensequenzen, antigenetische Charakterisierungen und Balastungsinfektionen empfänglicher Hühner zeigten, dass die getesteten IBV-Feldstämme mit den Impfstämmen eng verwandt waren und nur eine geringe Pathogenität für Hühner besaßen. Die Effekte einer durch Ko-Infektion mit Hühneranämievirus und dem Virus der infektiösen Bursitis (IBDV) induzierten Immuninsuffizienz auf den Verlauf einer IBV-Infektion untersuchten wir auch experimentell. Die klinischen Symptome und die histologischen Veränderungen waren bei den immunschwachen Tieren persistierender. Ebenso war bei diesen Tieren die lokale spezifische IgA-Produktion verzögert und verringert. Gleichzeitig wiesen die immuninsuffizienten Hühner in Trachea und Tränenflüssigkeit höhere und länger nachweisbare IBV-RNS-Gehalte auf. Insgesamt legen diese Ergebnisse nahe, dass die viral bedingte Immuninsuffizienz höchstwahrscheinlich eine relevante Rolle bei der Epidemiologie und dem Verlauf von IBV-Infektionen spielt.
Evaluamos los efectos de la inmunodeficiencia vírica en el desarrollo de la infección por el virus de la bronquitis infecciosa (IBV) como causa hipotética del fracaso en el establecimiento de una protección adecuada en pollos vacunados. Inicialmente, estudiamos aislamientos de IBV de casos de enfermedad respiratoria asociados a la presencia de atrofia de timo o bolsa de Fabricio en 322 casos remitidos entre 1997-2002. El IBV de tipo Arkansas fue el que se aisló de manera más frecuente pese a la vacunación extensa con ArkDPI en la industria del pollo de engorde. El número de aislamientos de IBV fue consistentemente mayor en pollos de engorde de 27 a 43 días de edad, coincidiendo con depleción linfocitaria de bolsa o timo, proporcionando evidencias circunstanciales de que la inmunodeficiencia y la incidencia de IBV podrían estar relacionadas. El análisis de las secuencias del gen S1, caracterizaciones antigénicas y el desafío de pollos susceptibles demostraron que los aislamientos de campo estaban relacionados estrechamente con las cepas vacunales y que eran poco patógenos para los pollos. Evaluamos experimentalmente los efectos de la inmunodeficiencia causada por la coinfección con virus de la anemia aviar y virus de la bursitis infecciosa aviar (IBDV) en el desarrollo de la infección por IBV. Los signos clínicos y las lesiones histológicas fueron más persistentes en las aves inmunodeficientes. La producción local de IgA específica se retrasó y se produjeron niveles menores en los pollos inmunodeficientes. Al mismo tiempo, las concentraciones de RNA de IBV en las tráqueas y fluidos lacrimales fueron mayores y más persistentes en los pollos inmunodeficientes. En global, estos resultados indican que la inmunodeficiencia vírica probablemente juega un papel relevante en la epidemiología y desarrollo de la infección por IBV.
Introduction
Respiratory disease cases submitted to the Alabama State Veterinary Diagnostic Laboratories have yielded a high frequency of infectious bronchitis virus (IBV) isolations as compared with other respiratory pathogens. The restriction fragment length polymorphism (RFLP) profiles of numerous of these IBV isolates have shown identity with the RFLP of vaccine strains extensively used in this region. For example Arkansas (Ark) serotype isolates have been obtained frequently despite the extensive use of attenuated ArkDPI vaccination in the broiler industry throughout the state. One plausible explanation for this unexpected situation is that minor antigenic changes in the S1 glycoprotein allow escape from vaccinal immunity (Lee & Jackwood, Citation2001). Nix et al. (Citation2000) showed that Ark-type field isolates obtained in Delmarva could be readily distinguished from Ark reference strains, providing evidence for IBV quasispecies that could escape immunity provided by Ark vaccination. Another explanation is the hypothesis that viral immunodeficiency in chickens plays an important role in the epidemiology and outcome of IBV infection. The immunosuppressive agents infectious bursal disease virus (IBDV), affecting principally B-cell responses (Lukert & Saif, Citation2003), and chicken anaemia virus (CAV), affecting immature T cells in the thymus and mature T cells in secondary organs (McNulty, Citation1991), are ubiquitous in the poultry industry. The detrimental effect of concurrent infection with CAV or IBDV with different viruses has been demonstrated by several authors (Lukert & Saif, Citation2003; Schat, Citation2003). The combined infection with CAV and IBDV has been shown to augment the severity of the immunodeficiency in chickens (Yuasa et al., Citation1980; Cloud et al., Citation1992a, Citationb; Imai et al., Citation1999). Little is known of the possible effect of immunodeficiency on susceptibility to IBV and on IBV clinical signs, lesions, and virus shedding (Raj & Jones, Citation1997).
This study was aimed at evaluating the effect of viral immunodeficiency as a possible cause for increased susceptibility to IBV or failure of adequate protection against IBV in vaccinated chickens. We analysed 322 field cases of respiratory disease in broilers with positive IBV isolations and assessed concurrent bursal and/or thymic atrophy. Furthermore, we investigated the genetic, antigenic, and pathogenic characteristics of two locally obtained Ark-type IBV isolates to exclude the possibility of minor antigenic or pathotypic variation as an explanation for outbreaks of disease in the field. Finally, we evaluated experimentally the effects of combined CAV and IBDV infection on the outcome of IBV infection.
Materials and Methods
Field cases of respiratory disease
This study involved 322 diagnostic cases for broiler chickens submitted to the Alabama Department of Agriculture and Industries Laboratories in Boaz, Hanceville, Elba, and Auburn, Alabama, from January 1997 to November 2002. IBV vaccination programmes in Alabama broilers include Massachusetts (Mass), Delaware 072 (DE 072), Connecticut (Conn), and Ark vaccine strains. Most broilers in the state are vaccinated against IBDV with intermediate vaccine strains. Cases were submitted for diagnostic examination by decision of the flock service manager or veterinarian. For this study, case inclusion criteria were the isolation of IBV, the histological examination and scoring of bursa of Fabricius and thymus lesions from representative chickens examined, the age of the broilers was known, and the cases represented flocks in the state of Alabama.
IBV isolation
Trachea, and variably the caecum tonsil, lung and kidney, were collected at necropsy and shipped frozen to the Auburn laboratory. Virus isolation was attempted (Gelb & Jackwood, Citation1998) in 9-day-old to 11-day-old specific pathogen-free (SPF) embryonated chicken eggs (ECE). At 48 to 72 h after inoculation, allantoic fluids were harvested and used for the viral RNA detection by reverse transcriptase-polymerase chain reaction (RT-PCR).
Restriction fragment length polymorphism analysis
Viral RNA was extracted from allantoic fluid by S.N.A.P. Total RNA Isolation Kit (Invitrogen, Inc.) following the manufacturer's protocol. Two-step RT-PCR reactions to amplify the entire IBV S1 gene were carried out using S1OLIGO3′ and NEWS1OLIGO5′ primers (Kwon et al., Citation1993; Jackwood et al., Citation1997). The resulting amplicons were digested with HaeIII, XcmI, and BstYI. The RFLP patterns for each restriction endonuclease were observed following agarose gel (1% agarose and 1% synergel) electrophoresis. The standard strains used in this RFLP study were ArkDPI, Ark99, Ark Georgia Variant-92, Mass 41, DE 072, Conn, Florida, JMK and California 2.
Histopathology
Bursa of Fabricius and thymus lobes were routinely processed for histological examination and application of lesion scores. Lymphocyte depletion was evaluated in both the bursa and thymus; scores for up to eight bursae and thymus lobes were recorded per case. For bursa, the scores were as follows: 1 = normal; 2 = mild depletion of follicular lymphocytes resulting in mild infolding or arborization of mucosa; 3 = moderate to marked depletion of follicular lymphocytes, either as necrosis, or as a mild to modest follicular lymphocytic presence as a sequela to necrosis; and 4 = severe depletion of follicular lymphocytes, either as diffuse, massive necrosis characteristic of acute bursal disease, or as minimal to absent follicular lymphocytic presence as a sequela to necrosis. For thymus, the scores were as follows: 1 = normal; 2 = mild depletion seen as 5 to 20% decrease in cortical lymphocyte density or relative thickness to the medulla; 3 = moderate to marked depletion seen as 25 to 75% decrease in cortical lymphocyte density or relative thickness of the cortex to the medulla; 4 = severe depletion seen as > 75% decrease in cortical lymphocyte density or relative thickness of the cortex to the medulla. Bursal and thymus depletion scores and number of IBV cases versus age were modelled by polynomial regression. IBV RFLP-type frequencies versus age were modelled using a generalized logit approach (Agresti, Citation1990).
Viruses
IBV Ark-type isolates AL/4614/98 and AL/7149/00, obtained in 1998 and 2000, respectively, were used. Both isolates originated from respiratory disease in broiler chickens occurring at 40 and 49 days of age, respectively. IBV isolates were subjected to two passages in SPF ECE before titration. Titres were determined by standard methods (Gelb & Jackwood, Citation1998) in SPF ECE, achieving titres of 106.8 median embryo infective doses (EID50) for isolate AL/4614/98 and 108.3 EID50 for isolate AL/7149/00. A commercial ArkDPI attenuated single-entity vaccine (Shering-Plough Animal Health, Millsboro, Delaware, USA) was used as a reference strain in cross-virus neutralization tests (cross-VNTs).
Antisera
The antiserum to each IBV isolate was prepared during pathogenicity studies (described below). Three chickens of each IBV-challenged group were boosted intravenously and maintained for an additional 2 weeks for antiserum production. Reference ArkDPI antiserum was kindly provided by Dr J. Gelb, University of Delaware, USA. All antisera were inactivated at 56°C for 30 min in a water bath.
Sequencing of Ark-type field isolates
IBV genomic RNA from Ark-type field isolates AL/4614/98 and AL/7149/00 was prepared from allantoic fluids using the High Pure RNA Isolation Kit (Roche Applied Science) following the manufacturer's protocol. cDNA fragments for sequencing were generated by RT-PCR using the OneStep RT-PCR kit (Qiagen) and primers (Kwon et al., Citation1993) designed to amplify IBV S1 sequences of diverse IBV isolates. Each PCR product representing the S1 gene was purified by agarose gel electrophoresis and the QIA quick Gel Extraction Kit (Qiagen, Valencia, California, USA). Nucleotide sequences were determined by automated sequence analysis at the Auburn University DNA Sequencing Facility, using the primers used for PCR. Sequences were aligned and compared by the AlignX program in the Vector NTI Suite of software using ClustalW. For amino acid sequence alignments, the Gonnet matrix, a gap-opening penalty of 15, and a gap extension penalty of 0.3 were used. For nucleotide sequence alignments, the parameters were: swgapdnamt matrix; Gap-opening penalty, 10; and gap extension penalty 0.1.
Antigenic characterization of IBV Ark-type field isolates by cross-VNT
Cross-VNTs were performed between isolates AL/4614/98, AL/7149/00 and the ArkDPI vaccine strain by the α-procedure (constant serum diluted virus method) (Gelb & Jackwood, Citation1998; Thayer & Beard, Citation1998). Antisera were incubated with equal volumes of 10-fold dilutions of the virus isolates and reference strain (10−1 to 10−8) for 1 h and inoculated via the chorioallantoic sac into SPF ECE. The homologous and heterologous neutralization indices were used to calculate the antigenic relatedness between the viruses by the formula of Archetti and Horsfall (Citation1950).
Pathogenicity of IBV isolates
Three groups of 15 3-week-old SPF chickens were used. Chickens of two groups were inoculated individually via the ocular and nasal routes with 200 µl each IBV isolate containing approximately105.5 EID50. The third group served as an uninfected control. Respiratory rales (tracheal or nasal) were assessed by listening (bringing the head of the bird near the examiner's ear) to each individual bird of each group at 4 and 8 days post inoculation (d.p.i.). At 4 and 8 d.p.i., six chickens of each group were humanely killed and necropsied. Samples of trachea were obtained for IBV RNA detection by RT-PCR and for histopathology. Three remaining chickens of each group were reinoculated intravenously with 200 µl each IBV isolate (2 weeks after the first exposure) for specific antiserum production. Antisera were obtained 2 weeks after intravenous inoculation. Sera were separated and stored frozen for use in VNTs. Formalin-fixed sections of trachea were routinely processed, embedded in paraffin, sectioned at 4 to 6 µm, and stained with haematoxylin and eosin for histopathological examination. Histomorphometric data were collected from tracheas using the ImageJ morphometry programme (National Institutes of Health: rsb.info.nih.gov/ij/download.html). The tracheal mucosa at the level of the cranial one-third of the trachea was digitally photographed in longitudinal section at the mid-cartilage at 20x objective. The entire mucosa was outlined, the full length of the digital image, with the polygon tool and the area measured and recorded. Values for each group at 4 and 8 d.p.i. were analysed by one-way analysis of variance with Tukey's post-test.
Experimental design
Three groups of SPF chickens were used. Group 1 (29 chickens) was inoculated intramuscularly at 7 days of age with 0.2 ml CAV isolate 03-4876 (van Santen et al., Citation2004) containing 106.3 median tissue culture infective doses, and orally with 0.5 ml IBDV (APHIS strain) suspension containing 104 median chicken infective doses (IBDV was titrated and kindly provided by Dr J. Giambrone, Auburn University, USA.) The CAV + IBDV-inoculated chickens were subsequently inoculated at day 15 of age via the nasal and ocular routes with 0.1 ml IBV isolate AL/4614/98 containing 105.5 EID50. Group 2 (26 chickens) was inoculated at day 15 with the same dose of IBV AL/4614/98 only. Group 3 (15 chicks) was the uninfected control.
Clinical signs of tracheal and nasal rales were recorded as described above at days 5, 8, 13, and 16 after IBV inoculation from each individual chicken of all groups. Numbers of chickens in each group with rales were compared by Fisher's exact test. Three birds of Group 1 were humanely killed at 15 days of age to confirm bursal and thymic atrophy resulting from CAV and IBDV inoculation at 7 days of age. Five chickens of each of Groups 1 and 2 (IBV inoculated) and three chickens of the uninfected controls were killed on days 9, 14, 19, 23, and 28 after IBV inoculation. Samples of the cranial one-third of the trachea and of the larynx were obtained from all birds for histopathological examination and evaluated without knowledge of treatment groups. Samples of thymus and bursa were obtained to evaluate integrity throughout the experimental period.
Histomorphometric data were collected from these tissues using ImageJ (see above) using a slightly different procedure. The ventral mucosa of the trachea was digitally photographed at 200x objective. Two measurements were performed in the tracheal mucosa: first, the total thickness of the mucosa was measured at five different positions with the line tool; and, second, the area of the lymphocytic tissue in the mucosa was visually selected by adjusting the threshold from 8-bit black-and-white copies of the original digital image and the thickness measured at five different positions. Each bird was also evaluated for severity of tracheal lesions including six types of changes: deciliation, epithelial necrosis, mononuclear cell infiltration, epithelial hyperplasia, Goblet cell hyperplasia or hypertrophy, and attenuation of mucus gland epithelium (flattening of epithelium indicating exhaustion of mucus content). Each type of lesion was graded (1 = normal, 2 = mild lesion, 3 = moderate, and 4 = marked to severe). Therefore, the minimum lesion index for six parameters is 6. For each group, the totals for all birds were added to obtain a tracheal lesion score, and were then divided by the number of birds to obtain a group average. An approximately 1-cm long portion of the centre of the trachea was collected from each chicken and stored at −85°C until RNA isolation for IBV RNA detection. Samples of lachrymal fluid were obtained from 10 birds in each group on days 5, 8, 11, 14, 16, 20, 24, and 28 for IBV-specific IgA antibody determinations by enzyme-linked immunosorbent assay and for IBV genome quantitation.
Statistical analysis
Histomorphometric data were analysed using SAS® PROC GLIMMIX (generalized linear mixed models) with the natural log as the link function (support.sas.com/rnd/app/papers/glimmix.pdf; verified 1 June 2006). Statistical significance of pairwise differences between groups within d.p.i. was evaluated using the PDIFF option in conjunction with the LSMEANS statement. The variance among chickens (d.p.i. x treatment group) was used as the error term.
Preparation of RNA from tracheal samples
Tracheal tissue fragments were homogenized in 0.5 ml tryptose phosphate broth, frozen and thawed three times, then centrifuged at 16 000x g for 5 min at 4°C. RNA was prepared from 0.2 ml supernatant using the High Pure RNA Isolation Kit (Roche Applied Science, Indianapolis, Indiana, USA) and eluted in 50 µl volume. Then 5 µl portions of the prepared RNA were used for conventional RT-PCR (see below).
Preparation of RNA from lachrymal fluids
Lachrymal fluids were collected as described elsewhere (Toro et al., Citation1993) and stored at −20°C. RNA was isolated from 100 µl volumes using the Qiagen QIAamp viral RNA mini kit and eluted in 60 µl volumes. Then 5 µl portions of the prepared RNA were used for conventional RT-PCR and 5 µl portions diluted 1:10 were used for quantitative RT-PCR (see below).
Conventional RT-PCR
Conventional RT-PCR was performed in 50 µl volumes using the Qiagen OneStep RT-PCR kit, 5 µl RNA isolated from trachea or tears, and primers developed by Handberg et al. (Citation1999) that amplify a 453-nucleotide fragment of the IBV nucleocapsid (N) gene. For tracheal samples, 30-fold serial dilutions of template (pooled from five chickens) were used to allow comparison of the relative amount of IBV RNA present in tracheal samples of each group 9 d.p.i. Undiluted tracheal samples from individual chickens from days 14 to 28 after IBV inoculation were tested. Tear samples collected from 11 to 28 d.p.i. were tested undiluted and individually. The reaction programme was: reverse transcription at 50°C for 30 min, 95°C for 15 min; 20 cycles of 94°C for 30 sec, 70–60° C (lowered 0.5°C each cycle) for 30 sec, 72°C for 1 min; then 20 (tracheal samples) or 15 (tear samples) additional cycles of 94°C for 30 sec, 60°C for 30 sec, 72°C for 1 min; followed by 72°C for 7 min. Products were detected after electrophoresis of 5 µl portions of each reaction on a 2% agarose gel containing ethidium bromide.
Fluorescence resonance energy transfer-quantitative RT-PCR
Sequences for primers, modified from UTR11− and UTR41+ (Casais et al., Citation2003), and probes (Qiagen Operon) for fluorescence resonance energy transfer-quantitative RT-PCR are indicated in . The primers shown amplify a 263-nucleotide segment of the 3′ portion of the 3′-untranslated region of the IBV genome. The probes were designed to match a relatively conserved portion of the IBV genome, based on sequences in GenBank. However, the 5′-end-labelled probe differs at one position from the Ark-type vaccine strain sequence used for the RNA and DNA standards and the IBV isolate AL/4614/98, as indicated in . Reactions were performed and monitored in 20 µl volumes in the LightCycler® (Roche). RNA (5 µl) isolated from the trachea or 0.5 µl RNA isolated from tears were used for each assay. Some RNA samples prepared from tears collected 5 days post IBV inoculation were further diluted so that only 0.05 µl were assayed. Buffers, enzymes, primer and probe concentrations, and other reagents were as described by Wang et al. (Citation2004), with the following changes in enzyme concentrations. We used 1.5 u Platinum Taq DNA polymerase (Invitrogen Corp., Carlsbad, California, USA) and 0.028 u Thermoscript® reverse transcriptase (Invitrogen) per reaction. The reaction programme was: reverse transcription at 48°C for 20 min; 95°C for 5 min; 40 cycles of 95°C for 15 sec, 60°C (used during the first five cycles), 58°C (used during the next seven cycles), 56°C (used during the next three cycles), or 50°C (used during the last 25 cycles) for 30 sec, and 72°C for 30 sec. During the last 25 cycles, an additional step of 46°C for 8 sec was inserted between the 95°C and 50°C steps to allow probes to anneal to the PCR product. Fluorescence was measured after the probe annealing step. After the amplification programme, melting point analysis confirmed the specificity of positive reactions. Both DNA (104, 103, 102, and 10 copies of RT-PCR product) and RNA (106, 105, 104, and 103 copies of in vitro-transcribed RNA) standards derived from the ArkDPI vaccine strain as well as negative controls containing only salmon sperm DNA or yeast RNA were included with each set of test samples.
Table 1. Fluorescence resonance energy transfer-quantitative RT-PCR primers and probes
Detection of IBV-specific antibodies
IBV-specific IgA in lachrymal fluid (diluted 1:10) was detected using a commercial IBV ELISA kit (Idexx Lab. Inc., Westbrook, Maine, USA) by substituting horseradish peroxidase-conjugated goat anti-chicken IgA (diluted to 1 µg/ml; Bethyl Lab., Inc., Montgomery, Texas, USA) for the conjugated anti-chicken immunoglobulin of the kit. Data were analysed by Kruskall–Wallis analysis and by Dunn's multiple comparisons test. Tears with absorbance values greater than the mean absorbance for samples from uninfected control chickens + 3 standard deviations were considered to contain detectable IBV-specific antibodies. Numbers of chickens in each group with detectable antibodies in tears were evaluated by Fisher's exact test.
GenBank Accession Numbers
Nucleotide sequences encoding the S1 subunit of isolates AL/4614/98 and AL/7149/00 were deposited in GenBank under the accession numbers DQ458217 and DQ458218, respectively.
Results
Epidemiology of IBV isolations and bursal and thymic atrophy
shows the extent of lymphocytic depletion of the bursa and/or thymus and IBV isolations by age of submission (total of 322 cases). As seen in this figure, broilers aged 20 to 30 days or older consistently showed moderate to severe bursal atrophy. On the other hand, thymus atrophy followed a fourth-order polynomial, with a first increase in damage occurring between days 10 and 20 of age and a second between days 35 and 43 of age. IBV was isolated from broilers from 13 to 54 days of age. The number of IBV isolations was described as a second-order polynomial with a maximum at 30 days of age. RFLP patterns typical of Conn, Mass, Ark, GA 98, and DE 072 serotypes were identified, with variant profiles that did not fully match reference RFLP profiles also found (). The Ark-type represented nearly 60% of all isolates. Differences occurred in the age of the broilers from which the viruses were isolated. Logistic regression analysis of the frequency of distinct serotypes versus broiler age revealed that Ark-type isolations followed a semicircular pattern with most isolates accumulating between 27 and 43 days of age (). Wild strains (Ark99, and variant strains) tended to be isolated from broilers at any age, irrespective of immune status. Georgia 98-like wild strains increased towards the end of the production period, whereas the frequency of Conn and Mass isolations declined steadily with age (not shown).
Figure 1. IBV respiratory cases in Alabama broilers (n = 322) in the period 1997 to 2002 and the extent of thymic or bursal lymphocytic depletion by age of submission. Predicted (pred) bursal or thymic (Tpred) lymphocytic depletion and IBV isolations (IBVpred) by polynomial regression. All terms in the models significant at P < 0.05.
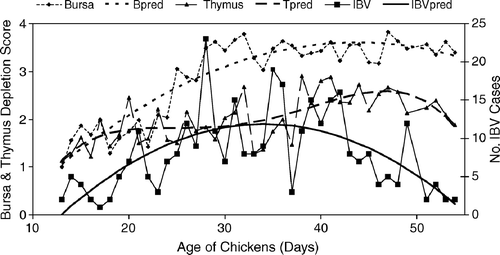
Figure 2. Predicted serotype frequency versus age from a generalized logit model using the Ark 99 strain as the reference or baseline (Agresti, Citation1990). Ark-vaccine-like strains were most frequent between days 27 and 43 of age, coinciding with immunodeficiency (). Wild-type strains such as variant strains or Ark99 (P = 0.01) maintained a similar frequency throughout the production period of the broilers.
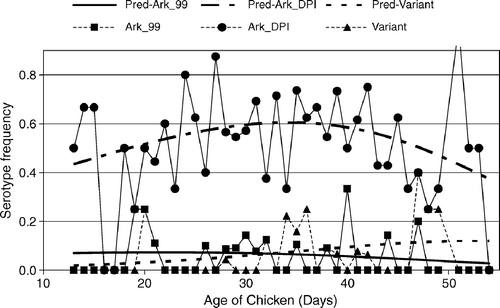
Table 2. Infectious bronchitis viruses isolated from broilers in Alabama, 1997 to 2002
Sequencing of the S1 gene of RFLP Ark-type IBV field isolates
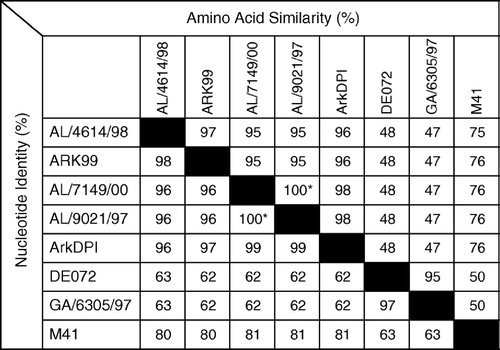
The complete S1 nucleotide and deduced amino acid sequences of two Ark-type isolates were determined and compared with each other and with S1 sequences representative of specific serotypes available at GenBank (). The S1 sequences of isolates AL/4614/98 and AL/7149/00 were closely related with 96% nucleotide identity and 95% amino acid similarity. Both at the nucleotide and at the deduced amino acid levels, isolate AL/7149/00 was most similar to isolate AL/9021/97 (99.9% nucleotide identity, 99.6% amino acid identity) and also closely related to the ArkDPI strain (99% nucleotide and 98% amino acid similarity). Isolate AL/4614/98 was most closely related to Ark99 (98% nucleotide and 97% amino acid similarity) and also closely related to AL/9021/97 and Ark-DPI strains (96% nucleotide and 95 to 96% amino acid similarity). Based on S1 gene sequences, both field isolates were more distant to representatives of the other IBV serotypes frequently isolated in the region.
Figure 3. Nucleotide identities (%) and amino acid similarities (%) of IBV field isolates AL/4614/98 and AL/7149/00 with sequences representative of serotypes or S1 RFLP patterns frequently isolated in Alabama: Ark 99, AL/9021/97 (representative of Alabama isolates with an RFLP pattern similar, but not identical, to ArkDPI), Ark DPI, DE072, GA6305/97 (representative of GA98 RFLP pattern) and M41 (GenBank accession numbers L10384, AY101766, AF006624, AF274435, AF338716, AY561711, respectively). *Alabama isolates AL/7149/00 and AL/9021/97 are not identical. They have two nucleotide differences (99.9% identical), each resulting in an amino acid difference (99.6% identical).
Antigenic characterization of Ark-type IBV isolates by cross-VNT
To evaluate the biological importance of the differences at nucleotide and amino acid levels between the field isolates and the vaccine strain, cross-VNTs were performed. The homologous and heterologous neutralization indices obtained with IBV field isolates AL/4614/98 and AL/7149/00 and reference strain ArkDPI were similar (data not shown). The antigenic relatedness calculated by the formula of Archetti and Horsfall (Citation1950) indicated 100% identity between field isolate AL/7149/00 and the ArkDPI reference strain, and 93.6% similarity to isolate AL/4614/98. Isolate AL/4614/98 showed 92.4% antigenic similarity to the ArkDPI strain. These levels of antigenic similarity indicate that the ArkDPI vaccine strain should protect chickens from challenge with these IBV isolates.
Pathogenicity of IBV isolates
Mild respiratory signs were detected in chickens inoculated with both IBV isolates. Field isolate AL/4614/98 induced audible tracheal or nasal rales in 60% of the chickens at both 4 and 8 d.p.i. Inoculation with isolate AL/7149/00 resulted in only 5% of the birds with respiratory signs at day 4 and 10% on day 8 after inoculation. Histopathological findings in the tracheal and laryngeal mucosa were consistent with the clinical signs, with birds showing mild or moderate hyperplasia of respiratory epithelium and lymphocytic infiltration, loss of cilia, and detachment of epithelium in the tracheal and laryngeal mucosa. The statistical evaluation of histomorphometric data showed a significant increase of tracheal mucosal thickness at day 4 d.p.i. (P < 0.05) only in chickens inoculated with isolate AL/4614/98.
Clinical signs and lesions shown by IBDV + CAV-infected chickens subsequently infected with IBV
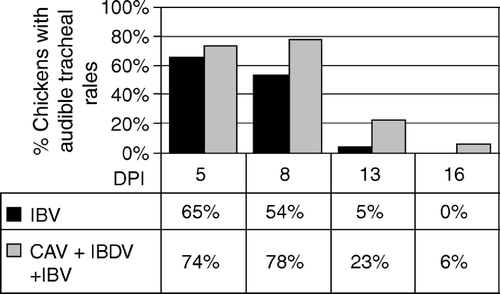
At the time of IBV inoculation, IBDV + CAV-inoculated chickens showed bursal and thymic atrophy. Histomorphometry of samples of thymus and bursa obtained throughout the experimental period showed lymphocytic repopulation of the thymus by day 19 after IBV inoculation, while the bursa maintained significant lymphocyte depletion until day 28 after IBV inoculation. As shown in , a similar percentage of immunodeficient and immunocompetent chickens inoculated with IBV AL/4614/98 showed respiratory signs 5 d.p.i. During the following days after IBV inoculation (8-16 d.p.i.), immunocompetent birds recovered, while a significantly higher number of birds of the immunodeficient group maintained respiratory signs of IBV.
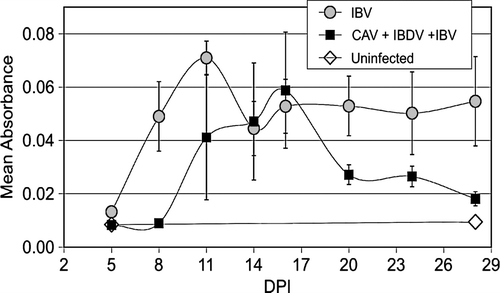
Figure 4. Audible nasal and tracheal rales by day in chickens (26 per group) infected with CAV + IBDV or with IBV alone. DPI = days post IBV inoculation. The total incidence of nasal and tracheal rales from days 8 to 16 was significantly higher (P = 0.04) in CAV + IBDV-infected chickens (Fisher's exact test).
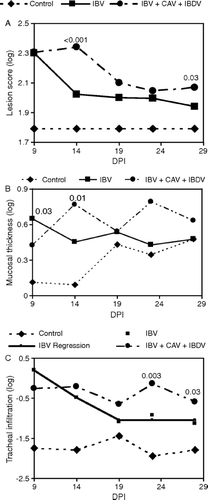
Histopathological evaluation of both IBV-inoculated groups showed lesions in the trachea characteristic of IB. Based on the lesion scores, both IBV-infected groups were significantly different (P < 0.05) from the uninfected controls at all time points (a). The severity of lesions in the trachea was higher in immunodeficient chickens as compared with immunocompetent ones from 14 to 28 d.p.i. This difference was statistically significant (P < 0.05) on 14 and 28 d.p.i. (a). As seen in b, a significant increase (P < 0.05) of the thickness of the tracheal mucosa was detected in immunocompetent chickens at 9 d.p.i., which decreased during the following days until no differences were detected as compared with uninfected controls. It must be noted that the mucosal thickness of control chickens increased with time, consistent with chicken's maturation. At 9 d.p.i. immunodeficient chickens did not show significant changes as compared with the controls, but during the following days these birds increased their mucosal thickness to achieve a significant difference (P < 0.05) compared with the controls (b). Mononuclear cell infiltration in the tracheal mucosa in immunocompetent chickens fitted a non-linear regression; with a linear decline until day 19 followed by a plateau during the following days (c). Immunocompromised chickens never achieved the levels of lymphocytic infiltration exhibited by immunocompetent birds at 9 d.p.i., but maintained a less pronounced infiltration throughout the experimental period with no significant variation (P < 0.05) with time.
Figure 5. Histopathological changes due to IBV in immunodeficient (infected with CAV and IBDV at day 7 of age) and immunocompetent chickens inoculated with IBV at day 15 of age. 5a: Severity of tracheal lesions. Lesions (deciliation, epithelium necrosis, mononuclear cell infiltration, epithelial hyperplasia, Goblet cell hyperplasia or hypertrophy, and attenuation of mucus gland epithelium) graded 1 = normal, 2 = mild lesion, 3 = moderate, and 4 = marked to severe (minimum total score for six parameters = 6). P < 0.05 for IBV versus IBV + CAV + IBDV are shown. IBV + CAV + IBDV different from control (P < 0.01) for all time points. 5b: Mean tracheal mucosal thickness as determined by histomorphometry. Only P < 0.05 for infected versus uninfected controls are shown. 5c: Mononuclear cell infiltration in the tracheal mucosa as determined by histomorphometry. P < 0.05 for IBV versus IBV + CAV + IBDV are shown. IBV + CAV + IBDV different from control (P < 0.01) for all time points. Immunocompetent chickens showed significantly higher (P < 0.05) mononuclear cell infiltration than uninfected chickens at day 9 after IBV infection and declined linearly through day 19 until a plateau. Immunodeficient and control chickens maintained similar values throughout the experimental period.
IBV-specific antibodies
Lachrymal fluid-specific IgA responses were delayed in immunodeficient chickens. As seen in , specific IgA in tears increased significantly as early as 8 d.p.i. This difference compared with the controls was significant (P < 0.05) until day 20 after IBV inoculation. On the other hand, immunodeficient chickens significantly increased their specific IgA levels (P < 0.05) as compared with the controls only at 16 d.p.i. Fisher's exact test indicated that the total number of samples (at all days) with detectable IBV-specific IgA was significantly higher in the group infected with IBV alone than in the group infected with CAV + IBDV + IBV.
IBV RNA in tracheal samples
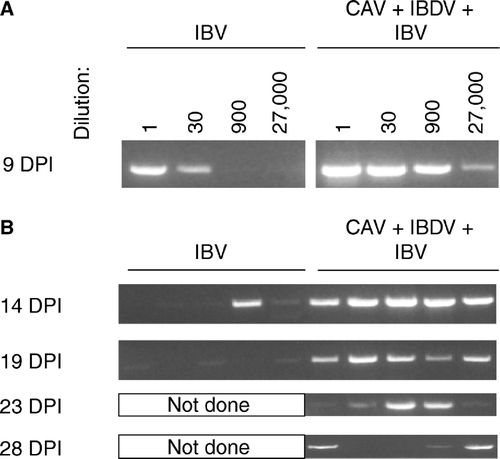
As seen in a, IBV RNA was detected at 9 d.p.i. in pooled samples from tracheas (five chickens) of both immunocompetent and immunodeficient chickens. However, the relative amount of IBV RNA present in the tracheas of immunodeficient chickens greatly surpassed the levels detected in immunocompetent ones; that is, a RT-PCR product was detected in IBDV + CAV-infected chickens at a template dilution of 1:27 000, while immunocompetent chickens showed a clearly-visible band only up to a template dilution of 1:30. Detection of IBV RNA in undiluted tracheal samples obtained from individual chickens at days 14 to 28 showed that immunodeficient chickens maintained high levels of viral RNA longer than immunocompetent birds (b).
Figure 7. Detection of IBV by RT-PCR in samples isolated from trachea of chickens necropsied at the indicated days post IBV inoculation (DPI). All PCR reactions were conducted for 40 cycles using primers recognizing the N gene. 7a: Thirty-fold serial dilutions of template (pooled from five chickens) were used to allow comparison of the relative amount of IBV RNA present in tracheal samples of each group. 7b: Undiluted samples from individual chickens were used as template.
IBV RNA in lachrymal fluid
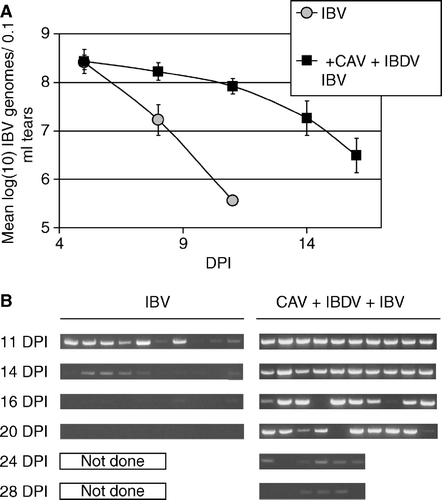
Mean IBV genome values for tear samples assayed individually by fluorescence resonance energy transfer-quantitative RT-PCR are shown in a. The concentration of IBV RNA in tears decreased with time in both IBV-infected groups, but the levels decreased more slowly in chickens also infected with CAV and IBDV. b shows IBV genomes detected in tears collected from individual chickens detected by RT-PCR using primers recognizing the N gene. IBV genomes detected in lachrymal fluids showed a pattern similar to that detected in the tracheas of the infected chickens. In IBV-only-infected chickens, IBV RNA was detected at high levels in a majority of birds (6/10) at day 11 and at lower levels in 50% of the birds (5/10) at day 14 d.p.i. Tear samples were negative for IBV RNA at 16 d.p.i. and 20 d.p.i. in immunocompetent chickens. On the other hand, lachrymal fluids from 100% of immunodeficient chickens (10/10) were positive for high levels of IBV RNA until 14 d.p.i. Eighty per cent of birds (8/10) of this group were positive until 20 d.p.i. and 50% (3/6) of the birds maintained IBV RNA at low levels until day 28 after IBV inoculation.
Figure 8. 8a: IBV genomes detected in tears by fluorescence resonance energy transfer-quantitative RT-PCR. RNA was prepared from 10 chickens of each group at each time point indicated (DPI = days post IBV inoculation) and assayed individually. Samples with IBV RNA levels below the limit of detection of this assay, but with IBV RNA detectable by the non-quantitative RT-PCR shown in (8b), were assigned the value of the lower limit of detection of the qRT-PCR assay. Therefore, the mean value shown is a theoretical maximum mean, and the actual mean is lower. Error bars indicate one standard error of the mean. 8b: IBV genomes detected in tears collected from individual chickens were detected by conventional RT-PCR using primers recognizing the N gene.
Discussion
Typically, outbreaks of IB are associated with immunological escape of antigenically distinct strains that originate from natural selection. Mechanisms for the generation of variation in coronaviruses include nucleotide insertions, deletions, or point mutations in the S1 subunit gene made by the viral polymerase, RNA recombination, and small changes in the S2 subunit that can alter the structure of S1 (Kusters et al., Citation1987; Cavanagh & Davis, Citation1988; Jia et al., Citation1995; Callison et al., Citation1999; Lee & Jackwood, Citation2000), which allow escape from immunity of vaccinated chickens. However, our results indicate that IBV isolates obtained from such outbreaks of respiratory disease in Alabama broilers induced only mild respiratory disease in SPF chickens. Moreover, four additional IBV isolates tested more recently for pathogenicity also induced mild respiratory disease in 5 to 20% of experimentally inoculated SPF chickens (unpublished data). Furthermore, our results indicate that two IBV isolates show a close similarity both at the nucleotide and at the deduced amino acid levels with the vaccine strain ArkDPI routinely used in the region. Because only a few amino acid differences can be responsible for different secondary structure predictions that could create different interactions between the S1 and S2 subunits, which could affect the quaternary structure of the spike glycoprotein, we also analysed the antigenic relatedness between these isolates and the vaccine ArkDPI strain in cross-VNTs. These results confirmed a high degree of antigenic relatedness (and expected cross-protection) between IBV field isolates and the vaccine strain. Collectively, these results demonstrate that the outbreaks of disease caused by these isolates were not attributable to IBV genetic, phenotypic, or pathotypic variations.
The epidemiological data obtained from 2003 to 2004 indicate that most outbreaks of IB have occurred in chickens simultaneously showing bursal and/or thymic atrophy. A few different conditions may cause atrophy of primary immune organs in chickens, but immunodeficiencies due to ubiquitous CAV and/or IBDV are the most common and relevant. CAV transiently causes generalized lymphoid atrophy with a concomitant immunosuppression in 2-week-old to 4-week-old chickens (Adair et al., Citation1991; Cloud et al., Citation1992a; McConnell et al., Citation1993; Bounous et al., Citation1995; Adair, Citation2000). CAV also induces thymic atrophy in older chickens (Toro et al., Citation1997). Specific cytotoxic T lymphocytes have been shown to be important for clearance of IBV infections (Seo & Collisson, Citation1997; Seo et al., Citation1997, Citation2000). CAV infection has been shown to abrogate cytotoxic T lymphocyte responses against other viruses (Markowski-Grimsrud & Schat, Citation2003). In addition, deficiency of T-helper cells in CAV-infected chickens might adversely affect generation of IBV-specific antibodies. On the other hand, IBDV infects primarily the lymphoid tissue in the bursa of Fabricius, causing a prolonged B lymphocyte immunodeficiency. Chicks exposed to IBDV have been shown to be more susceptible to various viruses including IBV (Rosenberger et al., Citation1975; Rosenberger & Gelb, Citation1978; Pejkovski et al., Citation1979; Yuasa et al., Citation1980; Sharma, Citation1984). In addition, IBV infection in bursectomized chickens is longer and more severe than in fully immunocompetent ones (Cook et al., Citation1991), further supporting the suggestion that B-lymphocyte deficiency caused by IBDV infection could affect immune clearance of IBV. Combined infection with CAV and IBDV has been shown to be synergistic with a prolonged acute phase prior to recovery or mortality, significantly lower in vitro lymphocyte responses, and a greater reduction in Newcastle disease virus challenge protection than in chickens infected with each agent alone (Yuasa et al., Citation1980; Cloud et al., Citation1992a, Citationb). As seen here, at 20 to 30 days old, or older, broilers with respiratory disease consistently showed moderate to severe bursal atrophy. On the other hand, thymus atrophy achieved maximum values in broilers aged 30 to 40 days or older. These epidemiological data indicate that birds are most probably becoming infected with IBDV and/or CAV concurrently with specific maternal antibody decay. The number of IBV isolations was consistently higher in broilers aged 30 to 43 days, coinciding with lymphocytic depletion of bursa and/or thymus, providing circumstantial evidence that these findings may be linked. The available information on CAV and IBDV effects discussed above support this epidemiological association.
The results obtained in the in vivo experiment support the hypothesis that viral immunodeficiency influences the outcome of IBV infection. Immunodeficient chickens showed a more prolonged and more severe course of disease than immunocompetent ones as determined by both clinical signs and histopathology. While immunocompetent chickens quickly regained the same mucosal thickness as the controls, immunocompromised birds still showed greater mucosal thickness at day 24 after IBV inoculation. IBV infection in the tracheal mucosa is characterized by massive infiltration of the lamina propria by lymphoid cells and the formation of a large number of germinal centres (Riddell, Citation1987). Our data show that all immunocompetent chickens showed an early (9 d.p.i.) significant mononuclear cell infiltration () as compared with immunocompromised ones. Mononuclear infiltration declined linearly until achieving a plateau in the immunocompetent group. On the other hand, the immunodeficient group showed a less pronounced but more persistent mononuclear infiltration, which is most probably the result of the lymphocytic depletion induced by CAV and IBDV. The delayed specific local IgA response as well as the lower levels of this antibody isotype in lachrymal fluid is consistent with the histopathological findings in the trachea. In chickens, IBV-specific local IgA is produced in lymphocytes of the mucosal-associated lymphoid tissues and in the Harderian gland (Toro et al., Citation1996). We believe that if mononuclear cell infiltration was affected by CAV and IBDV in the trachea, it is likely that the Harderian glands were similarly affected, which would be the cause for reduced specific IgA levels.
IBV replicates in the upper respiratory tract and in the Harderian gland (Toro et al., Citation1996). Thus, we decided to use samples of lachrymal fluid for IBV RNA detection by RT-PCR. Our results indicate that lachrymal fluid is an excellent source for IBV RNA detection, as we were able to detect IBV RNA until day 14 after inoculation in immunocompetent birds and until day 28 after inoculation in immunodeficient birds. We intentionally used a limiting number of PCR cycles to limit the sensitivity of the assay for IBV RNA in tear samples. The high concentrations of IBV RNA both in the trachea and in lachrymal fluids of CAV + IBDV-infected chickens are probably due to reduced viral clearance as consequence of reduced humoral and cellular immune responses (Sharma, Citation1984; Seo & Collisson, Citation1997; Seo et al., Citation1997, Citation2000; Markowski-Grimsrud & Schat, Citation2003).
The results obtained in immunodeficient chickens (i.e. more severe and persistent clinical signs and lesions, a delayed and reduced antibody response, and increased and persisting viral shedding) were predictable, as analogous immunodeficiencies in other species have shown similar results. Moreover, these results support the hypothesis that viral immunodeficiencies may be playing an important role in IBV epidemiology, particularly in outbreaks of disease caused by IBV isolates showing only minor genotypic/phenotypic variation, which would otherwise be efficiently protected against by serotype-specific homologous vaccination. Finally, the fact that IBV is replicating in immunodeficient hosts in the field opens interesting questions on viral evolution. Most investigators attribute the emergence of IBV variants to immune selective pressure. However, in those experiments in which the immune response is effectively removed as a selective host force, viral evolution does not cease, but rather appears to falter or follow different pathways (Kilbourne, Citation1994). Therefore, the hypothesis that IBV persisting for prolong ed periods in immunodeficient hosts might result in the selection of IBV quasispecies with an altered virulence for the immunocompetent host requires attention.
Acknowledgments
The authors acknowledge the assistance of Tami Kelly, Francene Van Sambeek, Joel Cline, Michael Luther, Lloyd Lauerman, Emily Handley, Jeanette Caesar, Bruce McMurtrey, Julia Bright, Leanne Thomas, Scott Helton, Billy Parker, Sara Brown, Melissa Roseman, Ed Lackey, Eddie Jennings, Cassandra Breedlove, and Natalia Petrenko.
References
- Adair , B.M. 2000 . Immunopathogenesis of chicken anaemia virus infection . Developmental and Comparative Immunology , 24 : 247 – 255 .
- Adair , B.M. , McNeilly , F. , McConnell , C.D. , Todd , D. , Nelson , R.T. and McNulty , M.S. 1991 . Effects of chicken anemia agent on lymphokine production and lymphocyte transformation in experimentally infected chickens . Avian Diseases , 35 : 783 – 792 .
- Agresti , A. 1990 . Categorical Data Analysis , New York : John Wiley & Sons .
- Archetti , I. and Horsfall , F.L. 1950 . Persistent antigenic variation of influenza A viruses after incomplete neutralization in ovo with heterologous immune serum . Journal of Experimental Medicine , 92 : 441 – 462 .
- Bounous , D.I. , Goodwin , M.A. , Brooks , R.L. Jr , Lamichhane , C.M. , Campagnoli , R.P. , Brown , J. and Snyder , D.B. 1995 . Immunosuppression and intracellular calcium signalling in splenocytes from chicks infected with chicken anemia virus, CL-1 isolate . Avian Diseases , 39 : 135 – 140 .
- Callison , S.A. , Jackwood , M.W. and Hilt , D.A. 1999 . Infectious bronchitis virus S2 gene sequence variability may affect S1 subunit specific antibody binding . Virus Genes , 19 : 143 – 151 .
- Casais , R. , Dove , B. , Cavanagh , D. and Britton , P. 2003 . Recombinant avian infectious bronchitis virus expressing a heterologous spike gene demonstrates that the spike protein is a determinant of cell tropism . Journal of Virology , 77 : 9084 – 9089 .
- Cavanagh , D. and Davis , P.J. 1988 . Evolution of avian coronavirus IBV: sequence of the matrix glycoprotein gene and intergenic region of several serotypes . Journal of General Virology , 69 : 621 – 629 .
- Cloud , S.S. , Lillehoj , H.S. and Rosenberger , J.K. 1992a . Immune dysfunction following infection with chicken anemia agent and infectious bursal disease virus. I. Kinetic alterations of avian lymphocyte subpopulations . Veterinary Immunology and Immunopathology , 34 : 337 – 352 .
- Cloud , S.S. , Rosenberger , J.K. and Lillehoj , H.S. 1992b . Immune dysfunction following infection with chicken anemia agent and infectious bursal disease virus. II. Alterations of in vitro lymphoproliferation and in vivo immune responses . Veterinary Immunology and Immunopathology , 34 : 353 – 366 .
- Cook , J. , Davison , T. , Huggins , M. and McLaughlan , P. 1991 . Effect of in ovo bursectomy on the course of an infectious bronchitis virus infection in line C White Leghorn chickens . Archives of Virology , 118 : 225 – 234 .
- Gelb , J. Jr and Jackwood , M.W. 1998 . “ Infectious bronchitis ” . In A Laboratory Manual for the Isolation and Identification of Avian Pathogens , 4th edn , Edited by: Glisson , J.R. , Swayne , D.E. , Jackwood , M.W. , Pearson , J.E. and Reed , W.M. 169 – 174 . Kennett Park , PA : American Association of Avian Pathologists .
- Handberg , K.J. , Nielsen , O.L. , Pedersen , M.W. and Jørgensen , P.H. 1999 . Detection and strain differentiation of infectious bronchitis virus in tracheal tissues from experimentally infected chickens by reverse transcription-polymerase chain reaction. Comparison with an immunohistochemical technique . Avian Pathology , 28 : 327 – 335 .
- Imai , K. , Mase , M. , Tsukamoto , K. , Hihara , H. and Yuasa , N. 1999 . Persistent infection with chicken anaemia virus and some effects of highly virulent infectious bursal disease virus infection on its persistency . Research in Veterinary Science , 67 : 233 – 238 .
- Jackwood , M.W. , Yousef , N.M.H. and Hilt , D.A. 1997 . Further development and use of a molecular serotype identification test for infectious bronchitis . Avian Diseases , 41 : 105 – 110 .
- Jia , W. , Karaca , K. , Parrish , C.R. & Naqi , S.A. ( 1995 ). A novel variant of avian infectious bronchitis virus resulting from recombination among three different strains . Archives of Virology , 140 , 259 – 271 .
- Kilbourne , E.D. 1994 . “ Host determination of viral evolution: a variable tautology ” . In The Evolutionary Biology of Viruses , Edited by: Morse , S.S. 253 – 271 . New York : Raven Press, Ltd .
- Kusters , J.G. , Niesters , H.G. , Bleumink-Pluym , N.M. , Davelaar , F.G. , Horzinek , M.C. and van der Zeijst , B.A. 1987 . Molecular epidemiology of infectious bronchitis virus in The Netherlands . Journal of General Virology , 68 : 343 – 352 .
- Kwon , H.M. , Jackwood , M.W. and Gelb , J. Jr . 1993 . Differentiation of infectious bronchitis virus serotypes using polymerase chain reaction and restriction fragment length polymorphism analysis . Avian Diseases , 37 : 194 – 202 .
- Lee , C.W. and Jackwood , M.W. 2000 . Evidence of genetic diversity generated by recombination among avian coronavirus IBV . Archives of Virology , 145 : 2135 – 2148 .
- Lee , C.W. and Jackwood , M.W. 2001 . Origin and evolution of Georgia 98 (GA98), a new serotype of avian infectious bronchitis virus . Virus Research , 80 : 33 – 39 .
- Lukert , P.D. and Saif , Y.M. 2003 . “ Infectious bursal disease ” . In Diseases of Poultry , 10th edn , Edited by: Saif , Y.M. , Barnes , H.J. , Glisson , J.R. , Fadly , A.M. , McDougald , L.R. and Swayne , D.E. 161 – 179 . Ames , IA : Iowa State Press .
- Markowski-Grimsrud , C.J. and Schat , K.A. 2003 . Infection with chicken anaemia virus impairs the generation of pathogen-specific cytotoxic T lymphocytes . Immunology , 109 : 283 – 294 .
- McConnell , C.D.G. , Adair , B.M. and McNulty , M.S. 1993 . Effects of chicken anemia virus on cell-mediated immune function in chickens exposed to the virus by a natural route . Avian Diseases , 37 : 366 – 374 .
- McNulty , M.S. 1991 . Chicken anemia agent: A review . Avian Pathology , 20 : 187 – 203 .
- Nix , W.A. , Troeber , D.S. , Kingham , B.F. , Keeler , C.L. Jr and Gelb , J. Jr . 2000 . Emergence of subtype strains of the Arkansas serotype of infectious bronchitis virus in Delmarva broiler chickens . Avian Diseases , 44 : 568 – 581 .
- Pejkovski , C. , Davelaar , F.G. and Kouwenhouven , B. 1979 . Immunosuppressive effect of infectious bursal disease virus on vaccination against infectious bronchitis . Avian Pathology , 8 : 95 – 106 .
- Raj , G.D. and Jones , R.C. 1997 . Effect of T-cell suppression by cyclosporin on primary and persistent infections of infectious bronchitis virus in chickens . Avian Pathology , 26 : 257 – 276 .
- Riddell , C. 1987 . Avian Histopathology , Kennett Square , PA : American Association of Avian Pathologists .
- Rosenberger , J.K. and Gelb , J. Jr . 1978 . Response to several avian respiratory viruses as affected by infectious bursal disease virus . Avian Diseases , 22 : 95 – 105 .
- Rosenberger , J.K. , Klopp , S. , Eckroade , R.J. and Krauss , W.C. 1975 . The roles of the infectious bursal agent and several avian adenoviruses in the hemorrhagic-aplastic-anemia syndrome and gangrenous dermatitis . Avian Diseases , 19 : 717 – 729 .
- Schat , K.A. 2003 . “ Chicken infectious anemia ” . In Diseases of Poultry , 10th edn , Edited by: Saif , Y.M. , Barnes , H.J. , Glisson , J.R. , Fadly , A.M. , McDougald , L.R. and Swayne , D.E. 182 – 202 . Ames , IA : Iowa State Press .
- Seo , H.S. and Collisson , E.W. 1997 . Specific cytotoxic T lymphocytes are involved in in vivo clearance of infectious bronchitis virus . Journal of Virology , 71 : 5173 – 5177 .
- Seo , S.H. , Wang , L. , Smith , R. and Collisson , E.W. 1997 . The carboxyl-terminal 120-residue polypeptide of infectious bronchitis virus nucleocapsid induces cytotoxic T lymphocytes and protects chickens from acute infection . Journal of Virology , 71 : 7889 – 7894 .
- Seo , S.H. , Pei , J. , Briles , W.E. , Dzielawa , J. and Collisson , E.W. 2000 . Adoptive transfer of infectious bronchitis virus primed alphabeta T cells bearing CD8 antigen protects chicks from acute infection . Virology , 269 : 183 – 189 .
- Sharma , J.M. 1984 . Effect of infectious bursal disease virus on protection against Marek's disease by turkey herpesvirus vaccine . Avian Diseases , 28 : 629 – 640 .
- Thayer , S.G. and Beard , C.W. 1998 . “ Serologic procedures ” . In Manual for the Isolation and Identification of Avian Pathogens , 4th edn , Edited by: Glisso , J.R. , Swayne , D.E. , Jackwood , M.W. , Pearson , J.E. and Reed , W.M. 255 – 266 . Kennett Par , PA : American Association of Avian Pathologists .
- Toro , H. , Lavaud , P. , Vallejos , P. and Ferreira , A. 1993 . Transfer of IgG from serum to lachrimal fluid in chickens . Avian Diseases , 37 : 60 – 66 .
- Toro , H. , Godoy , V. , Larenas , J. , Reyes , E. and Kaleta , E.F. 1996 . Avian infectious bronchitis: viral persistence in the Harderian gland and histological changes after eyedrop vaccination . Avian Diseases , 40 : 114 – 120 .
- Toro , H. , Ramirez , A.M. and Larenas , J. 1997 . Pathogenicity of chicken anemia virus (isolate 10343) for young and older chickens . Avian Pathology , 26 : 485 – 499 .
- van Santen , V.L. , Joiner , K.S. , Murray , C. , Petrenko , N. , Hoerr , F.J. and Toro , H. 2004 . Pathogenesis of chicken anemia virus: comparison of the oral and the intramuscular routes of infection . Avian Diseases , 48 : 494 – 504 .
- Wang , C. , Gao , D. , Vaglenov , A. and Kaltenboeck , B. 2004 . One-step real-time duplex reverse transcription PCRs simultaneously quantify analyte and housekeeping gene mRNAs . Biotechniques , 36 : 508 – 516 .
- Yuasa , N. , Taniguchi , T. , Noguchi , T. and Yoshida , I. 1980 . Effect of infectious bursal disease virus infection on incidence of anemia by chicken anemia agent . Avian Diseases , 24 : 202 – 209 .