Abstract
Apoptosis plays an important role in pathogenesis of many viral infections. Infection of chicken with avian reovirus S1133 causes tissue injury related to virus-induced apoptosis. To determine whether avian reovirus (ARV) induced apoptosis in chicken tissues, six 3-week-old specific pathogen free White Leghorn chicks were inoculated with ARV S1133. Tissues were dual-labelled for the simultaneous detection of viral antigen containing and apoptotic cells. DNA laddering was detected in ARV-infected but not mock-infected chicken tissues. Dual-labelling assay revealed that the majority of antigen-expressing cells were not apoptotic. Surprisingly, some apoptotic but non-antigen-expressing cells were frequently located in the vicinity of antigen-expressing cells. Syncytium formation in ARV-infected chicken tissues undergoing apoptosis was apparent, suggesting a correlation between virus replication and apoptosis in chicken tissues.
Apoptose induite par un réovirus aviaire associée à une lésion de tissu
L'apoptose joue un rôle important dans la pathogénie de nombreuses infections virales. L'infection des poulets par le réovirus aviaire S1133 entraîne une lésion de tissu associée à une apoptose induite par le virus. Pour déterminer si un réovirus aviaire (ARV) induit une apoptose dans les tissus de poulet, six poussins White Leghorn, exempts de microorganismes pathogènes spécifiés, âgés de 3 semaines ont été inoculés avec l'ARV S1133. Les tissus ont été soumis à un double marquage pour la détection simultanée de l'antigène viral et des cellules apoptotiques. La fragmentation de l'ADN a été détectée dans l'ARV- mais pas dans les tissus de poulet infecté par un placebo. Ce double marquage a révélé que la majorité des cellules exprimant l'antigène n’était pas apoptotique. De façon surprenante, quelques cellules apoptotiques qui n'exprimaient pas l'antigène étaient souvent localisées dans le voisinage des cellules exprimant l'antigène. Des formations syncytiales ont été mises en évidence dans les tissus de poulet infectés par l'ARV subissant l'apoptose, suggérant une corrélation entre la réplication virale et l'apoptose dans les tissus de poulet.
Mit Gewebsschädigung in Zusammenhang stehende durch aviäres Reovirus induzierte Apoptose
Apoptose spielt in der Pathogenese vieler Virusinfektionen eine bedeutsame Rolle. Die Infektion von Hühnern mit dem aviären Reovirus S1133 verursacht Gewebsschädigung, die mit der virusinduzierten Apoptose in Beziehung stehen. Um zu untersuchen, ob aviäres Reovirus (ARV) in Geweben von Hühnern Apoptose induziert, wurden sechs dreiwöchige spezifisch pathogenfreie (SPF)- weiße Leghornküken mit ARV S1133 inokuliert. Die Gewebe wurden für den gleichzeitigen Nachweis von viralem Antigen und apoptotischen Zellen doppelt markiert. DNS-Laddering wurde in ARV aber nicht in den schein-infizierten Hühnergeweben nachgewiesen. Der Doppel-Markierungstest zeigte, dass die Mehrzahl der antigenhaltigen Zellen nicht apoptotisch war. Erstaunlicherweise befanden sich apoptotische, aber antigenfreie Zellen häufig in der Nachbarschaft zu Antigen exprimierenden Zellen. Aufgrund der Synzytienbildung in den ARV-infizierten Hühnergeweben, in denen Apoptose auftritt, wird eine Korrelation zwischen der Virusreplikation und der Apoptose vermutet.
La apoptosis inducida por reovirus aviar está relacionada con daño tisular
La apoptosis juega un papel importante en la patogenia de muchas infecciones víricas. La infección de pollos con el reovirus aviar S1133 produce daño tisular relacionado con apoptosis inducida por virus. Para determinar si el reovirus aviar (ARV) induce apoptosis en tejidos de pollo, seis pollitos White Leghorn libres de patógenos específicos (SPF) de 3 semanas de edad se infectaron con ARV S1133. Los tejidos se marcaron doblemente para la detección simultánea de células apoptóticas y que contenían antígeno vírico. Se detectó fragmentación del DNA en tejidos de pollos infectados con ARV pero no en los controles negativos. El ensayo de marcaje doble reveló que la mayoría de las células que expresaban antígeno no eran apoptóticas. Sorprendentemente, algunas células apoptóticas pero que no expresaban antígeno estaban localizadas frecuentemente alrededor de las células que contenían antígeno. La formación de sincitios en los tejidos de pollos infectados con ARV que sufrían apoptosis era evidente, lo cual sugiere una correlación entre replicación vírica y apoptosis en tejidos de pollos.
Introduction
Cell death can be classified as either necrosis or apoptosis (Golstein et al., Citation1991). Apoptosis is genetically programmed death while necrosis is the name given to accidental death of cells and living tissue. Apoptosis or programmed cell death is a tightly controlled physiological process that can be triggered by various intracellular and extracellular stimuli (Kerr et al., Citation1972). Apoptosis plays a critical role in developmental modelling, immune repertoires, and homeostasis maintenance (Oppenheim, Citation1991). A number of viruses affect the viability of the host cell through either inhibiting or promoting host cell apoptosis (Shen & Shenk, Citation1995; O'brien, Citation1998; Labrada et al., Citation2002; Shih et al., Citation2004; Chang et al., Citation2005). Although apoptosis is a common mechanism of cell death for many viruses, little is known about the biochemical pathways that lead to this cellular response. Such knowledge is of critical importance to an understanding of viral disease mechanisms and has the potential to lead to the development of novel antiviral therapeutics capable of apoptosis blockade (Connolly et al., Citation2001).
Avian reovirus (ARV) is an important cause of diseases in poultry. In particular, conditions such as reovirus-induced arthritis, chronic respiratory diseases and malabsorption syndrome provoke considerable economic losses (Hieronymus et al., Citation1983; Kibenge & Wilcox, Citation1983; Robertson & Wilcox, Citation1986). ARV encodes at least 10 structural proteins and four non-structural proteins (Bodelon et al., Citation2001; Varela & Benavente, Citation1994). Previous investigations indicated that ARV infection causes cell damage in vivo in several organs, such as the liver, bursa, intestines, pancreas, thymus and spleen, characterized by lymphocyte depletion (Roessler & Rosenberger, Citation1989). Although association of ARV with various disease conditions in poultry has been demonstrated, the pathogenesis of these diseases still remains poorly understood. ARV protein σC, encoded by the third open reading frame of the S1 genome segment (Shapouri et al., Citation1995), is a cell attachment protein (Martinez-Costas et al., Citation1997) and apoptosis inducer (Shih et al., Citation2004). The present study is a continuation of our recent reports on σC-induced apoptosis in cultured cells (Shih et al., Citation2004), and activated a proapoptotic signal by linking Src to p53 (Lin et al., Citation2006) while p17 induced retarded cell growth through activation of a p53-dependent pathway (Liu et al., Citation2005). The results from this study provide the first demonstration that apoptosis induction by ARV in chicken tissues may be related to tissue injury.
Materials and Methods
Cell culture and virus preparation
BHK-21 cells were grown in minimal essential medium containing 5% foetal bovine serum, penicillin G50 (50 units/ml), streptomycin (50 µg/ml), and fungizone (1.25 µg/ml) at 37°C in a 5% CO2 incubator. Avian reovirus S1133 strain was propagated in Vero cells. Upon development of 80% cytopathic effect, the cells were harvested by centrifugation following three freeze–thaw cycles (Liu et al., Citation2003, Citation2004). The virus containing supernatant was collected and used in chicken inoculation.
Inoculation of chickens with ARV S1133
To compare tissue damage and apoptosis associated with ARV S1133, sections obtained from S1133-infected and mock-infected chicken tissues were immunostained with a monoclonal antibody against ARVσC (Hsu et al., Citation2006), terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick-end labelling (TUNEL) assayed, and cresyl violet stained. Six 3-week-old specific pathogen free White Leghorn chicks were used. The chicks were equally divided into two groups. Each group was inoculated by either ARV S1133 or PBS (mock infection). Each bird was inoculated in one nostril with 50 µl of a virus suspension containing 10 chicken infected doses per millilitre. The birds were housed in separate modified Horsfall–Bauer isolation units with filtered air. At 5 days postinfection, the chickens were sacrificed and formalin-fixed paraffin-embedded tissues were prepared for histological examination and dual-labelling assay.
DNA fragmentation analysis
Both mock-infected and ARV-infected tissues were harvested, washed, and lysed in lysis buffer (50 mM Tris, pH 7.5, 20 mM ethylenediamine tetraacetic acid, 1% Nonidet p-40). The supernatant was collected and incubated with RNase A at a final concentration of 500 µg/ml for 1 h at 37°C followed by proteinase K at 500 µg/ml for at least 2 h at 55°C. The DNA was extracted using phenol/chloroform and precipitated with ethanol in TE buffer (10 mM Tris, pH 8.0, 1 mM ethylenediamine tetraacetic acid), and run on a 1.5% agarose gel for DNA fragmentation analysis.
Nested polymerase chain reaction
Nucleic acids were extracted from mock-infected and ARV-infected chicken tissues (heart, liver, kidney, small intestine, bursa, and tendon). To detect the σC-encoding gene, nucleic acids extracted from the mock-infected and ARV-infected chicken tissues were subjected to reverse transcription-polymerase chain reaction (RT-PCR) and nested PCR. Two sets of primer pairs described previously were used to amplify the σC-encoding gene of ARV (Liu et al., Citation1999b). Primers sequences were as follows: S1M, 5′-CAACTAATGCAATTTCGCTGG-3′ (identical to nucleotides 586-606); and S1N, 5′-GAAAGTAATTGTGA AGACACG-3′ (complementary to nucleotides 915 to 895). The expected size of the PCR products was 330 base pairs in length. The nested primers (S1P and S1N) were as follows: S1P, 5′-CGCACTGATTATATGATGTC-3′ (identical to nucleotides 676 to 695). The expected size of the PCR products was 239 base pairs in length. The nucleic acids were denatured in boiling water for 10 min, chilled on ice for 5 min, and then used as a template. RT-PCR reactions were performed according to procedures provided by Roche Applied Sciences. RT was carried out at 50°C for 30 min. PCR reactions were subjected to 35 cycles consisting of denaturation for 1 min at 94°C, annealing for 1 min at 55°C and, extension for 90 sec at 72°C, and one final extension cycle at 72°C for 7 min. Five microlitres of the PCR products were run on a 1.5% agarose gel, containing 5 µg/ml ethidium bromide, and were subsequently visualized by ultraviolet transillumination.
Dual-labelling assay (immunofluorescence assay and TUNEL assay) of chicken tissues
ARV σC in ARV-infected chicken tissues was detected by immunofluorescence assay (IFA), and fragmented DNA was detected by TUNEL assay. TUNEL results in labelling of the available 3′ OH ends of DNA frequently generated during apoptosis by endonuclease-mediated fragmentation. The tissue sections, including heart, liver, kidney, small intestine, bursa, and tendon, were fixed in methanol and acetone then digested with proteinase K (20 µg/µl) at 37°C for 15 min. The sections were incubated with an anti-σC monoclonal antibody (Hsu et al., Citation2006) and TUNEL kit, respectively. The bound antibody was visualized by immunostaining with FITC-conjugated second antibody raised against mouse IgG (Amersham Pharmacia Biotech). The dual-labelling results were obtained by SPOT software version 4.5.
Results
Histological examination and tissue injury associated with apoptosis
Histological examination of the heart and gastrocnemius tendon from ARV-infected chickens showed apparent inflammation not seen in tissues from other organs and those from mock-infected chickens. The synovial membrane was mildly thickened and oedematous due to synovial cell hypertrophy and mild hyperplasia. Numerous lymphocytes and fewer plasma cells, heterophils, and macrophages infiltrated the synovial membrane and gastrocnemius tendon and sheath. Focal infiltration of numerous lymphocytes and small numbers of heterophils and macrophages were also observed in myocardium and epicardium.
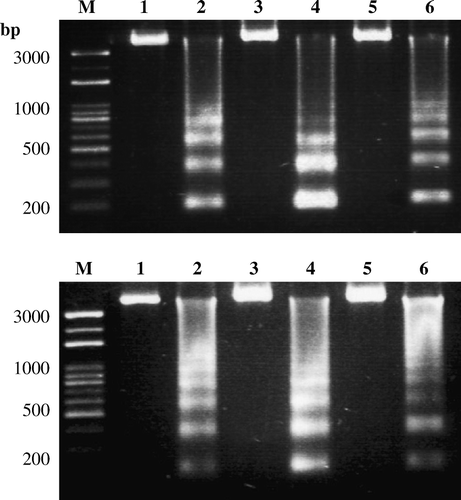
The ARV σC-encoding gene was detected by nested PCR in ARV-infected chicken tissues (). The presence of apoptosis could be confirmed by the presence of the characteristic intranucleosomal cleavage pattern of extracted DNAs. Upon agarose gel electrophoresis, DNA from infected tissues showed the typical pattern of DNA fragmentation (), suggesting that apoptosis occurred in ARV-infected tissues.
Figure 1. Detection of ARV σC-encoding gene in ARV-infected chicken tissues by nested PCR. The σC-encoding gene of ARV in ARV-infected chicken tissues, heart, kidney, bursa, liver, tendon, and intestine (upper and lower panels; lanes 1, 3, 5, 7, 9, and 11) was detected by nested PCR. The expected sizes of first PCR (upper panel) and second PCR (lower panel) products were 330 base pairs and 239 base pairs, respectively. Mock-infected chicken tissues, heart, kidney, bursa, liver, tendon, and intestine used as negative controls (upper and lower panels; lanes 2, 4, 6, 8, 10, and 12). Lane M, Bio 100 DNA ladder™ molecular weight marker.
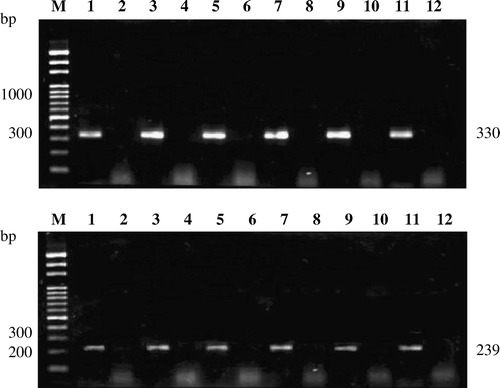
Figure 2. DNA fragmentation analysis in ARV-infected chicken tissues. Apoptosis induction by ARV S1133 in chicken tissues, heart, kidney, bursa (upper panel; lanes 2, 4, and 6), liver, tendon, and intestine (lower panel; lanes 2, 4, and 6) was detected by DNA fragmentation analysis. Mock-infected chicken tissues, heart, kidney, bursa (upper panel; lanes 1, 3, and 5), liver, tendon, and intestine (lower panel; lanes 1, 3, and 5) were used as negative controls. The chromosomal DNA was separated on a 1.5% agarose gel.
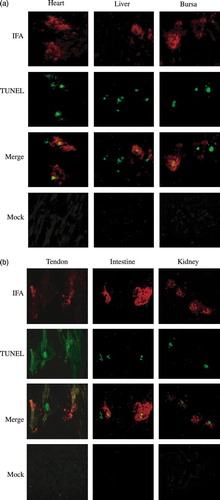
Extensive areas of apoptotic TUNEL-positive nuclei were noted in the ARV-infected heart and tendon not in the mock tissues. Large regions of avian reovirus antigen-positive tissues were noted in the ARV-infected heart and tendon in the same areas as tissue injury and TUNEL-positive cells (a,b). Taken together, these findings indicate that ARV-induced chicken tissue injury may be associated with apoptosis.
Figure 3. Dual-labelling assay for ARV antigens and apoptosis in ARV-infected tissue sections. Apoptosis induced by ARV in chicken tissues, heart, liver, and bursa (3a) and in tendon, intestine, and kidney (3b) was detected by dual-labelling assay. Extensive syncytium formation was observed in chicken tissues infected with ARV S1133. The dual-labelling (IFA; red) and (TUNEL; green) results (merge) were obtained by SPOT software version 4.5. Mock-infected chicken tissues (mock) were used as negative controls and showed no signals.
Apoptosis induced by ARV in chicken tissues
To determine whether apoptotic cells contained reovirus antigens, tissue sections from ARV S1133-infected chickens were assayed simultaneously for the presence of ARV σC by IFA and for apoptosis by TUNEL. In all infected regions, mixtures of doubly and singly labelled cells were observed (a,b). Some cells contained both ARV σC and fragmented DNA, but it was often difficult to distinguish co-labelling when single cells had appeared to shrink and break into apoptotic bodies. Many cells were labelled for either ARV σC or fragmented DNA but not for both. The results of the dual-labelling assay suggest that ARV induces apoptosis in both ARV antigen-positive and ARV antigen-negative cells. This provides evidence that apoptosis in ARV-infected regions of the chicken tissues occurs in both productively infected and uninfected cells.
Discussion
This study is an extension of our previous observations on the role of ARV in apoptosis induction (Shih et al., Citation2004; Lin et al., Citation2006) and tissue injury causation in vivo (Liu et al., Citation1999a). Apoptosis plays an important role in virus-induced tissue injury in vivo (Finkel et al., Citation1995; Tanimura & Sharma, Citation1998; Jungmann et al., Citation2001). It is generally accepted that virus-induced syncytium formation contributes to cytopathic effects. However, little is known how extensive syncytium formation is induced and its relationship to apoptosis. Syncytium formation has been reported on histopathological examination of tissues from ARV-infected chickens (Hieronymus et al., Citation1983; Kibenge et al., Citation1985; Kibenge & Dhillon, Citation1987). Here, we demonstrate for the first time that apoptosis is present within tissues with extensive syncytium after ARV infection, suggesting that there may be a correlation between virus replication and apoptosis in chicken tissues. Direct correlation between cytopathogenesis and apoptosis in vivo has been demonstrated for a subset of viruses (Finkel et al., Citation1995; Shen & Shenk, Citation1995). Our unpublished results indicated that ARV induced caspase-dependent apoptosis in BHK-21 cells.
It was noted that tenosynovitis and myocarditis were present in ARV-infected chickens whose tissues also showed apoptosis. Tenosynovitis still remains a serious problem for chickens, causing heavy economic losses to the poultry industry. The mechanisms that lead to arthritis are still not clearly understood. During apoptosis in vivo, the apoptotic bodies are phagocytosed by neighbouring cells in such a way that the contents are degraded intracellularly, without provoking inflammation. However, there are instances in tissue injury due to viral infection where apoptotic and necrotic features often coexist, resulting in the blurring of the long-held distinction between necrotic and apoptotic cell death. In fact, the mitochondrial permeability transition is a pathophysiological mechanism shared by both apoptosis and necrosis (Lemasters, Citation1999). Whereas apoptosis requires adenosine triphosphate, both in cell and cell-free systems, intracellular adenosine triphosphate is known to actually prevent onset of necrotic cell death (Leist et al., Citation1997). Our findings suggest that ARV-induced apoptosis and inflammation in the same region of chicken tissues may be due to a process called necrapoptosis leading to both forms of cell death (Lemasters, Citation1999).
Some apoptotic but non-antigen-expressing cells were observed in ARV-infected chicken tissues. The small number of apoptotic cells in the ARV antigen-negative cells could result from the apoptotic death of uninfected tissues that have lost trophic support from a connecting cell that has been eliminated by cell death due to ARV infection. Another possibility might be the release of an apoptosis-inducing factor(s) by cells expressing viral antigens. In contrast to the above findings, large numbers of TUNEL-negative, reovirus antigen-positive cells may represent cells that are infected but have not yet become apoptotic or reached the stage of DNA fragmentation. Many TUNEL-positive, ARV antigen-negative cells had undergone extensive cytoplasmic shrinkage, which may have precluded the detection of antigen. Additionally, proteolysis of cytoplasmic proteins, which occurs during apoptosis, may have altered or degraded antigenic sites beyond recognition. Since ARV replicates exclusively in the cytoplasm, these events could result in an underestimation of the number of ARV antigen-positive cells undergoing apoptosis.
Acknowledgement
This work was supported by the grant awarded to Dr Hung-Jen Liu by the National Science Council (NSC-95-2313-B-020-003), Taiwan.
References
- Bodelon , G. , Labrada , L. , Martinez-Costas , J. and Benavente , J. 2001 . The avian reovirus genome segment S1 is a functionally tricistronic gene that express one structural and two nonstructural proteins in infected cells . Virology , 290 : 181 – 191 .
- Chang , C.J. , Shih , W.L. , Yu , F.L. , Liao , M.H. and Liu , H.J. 2005 . Apoptosis induced by bovine ephemeral fever virus . Journal of Virological Methods , 122 : 165 – 170 .
- Connolly , J.L. , Barton , E.S. and Dermody , T.S. 2001 . Reovirus binding to cell surface sialic acid potentiates virus-induced apoptosis . Journal of Virology , 75 : 4029 – 4039 .
- Finkel , T.H. , Tudor-Williams , G. , Banda , N.K. , Cotton , M.F. , Curiel , T. , Monks , C. , Baba , T.W. , Ruprecht , R.M. and Kupfer , A. 1995 . Apoptosis occurs predominately in bystander cells and not in productively infected cells of HIV and SIV infected lymph nodes . Nature Medicine , 1 : 129 – 134 .
- Golstein , P. , Ojcius , D.M. and &.Young , J.D. 1991 . Cell death mechanisms and the immune system . Immunological Review , 121 : 29 – 65 .
- Hieronymus , D.R.K. , Villegas , P. and Kleven , S.H. 1983 . Identification and serological differentiation of several reovirus strains isolated from chickens with suspected malabsorption syndrome . Avian Diseases , 27 : 246 – 254 .
- Hsu , C.J. , Wang , C.Y. , Lee , L.H. , Shih , W.L. , Chang , C.I. , Cheng , H.L. , Chulu , J.L.C. , Ji , W.T. and Liu , H.J. 2006 . Characterization of monoclonal antibodies against avian reovirus S1133 σC protein produced in insect cells and their application in detection of ARV isolates . Avian Pathology , 35 ( 4 ) : 320 – 326 .
- Jungmann , A. , Nieper , H. and Muller , H. 2001 . Apoptosis is induced by infectious bursal disease virus replication in productively infected cells as well as in antigen-negative cells in their vicinity . Journal of General Virology , 82 : 1107 – 1115 .
- Kerr , J.F. , Wyllie , R.A.H. and Currie , A.R. 1972 . Apoptosis: a basic biological phenomenon with wide-ranging implication in tissue kinetics . British Journal of Cancer , 26 : 239 – 247 .
- Kibenge , F.S.B. and Dhillon , A.S. 1987 . A comparison of the pathogenicity of four avian reovirus in chickens . Avian Diseases , 31 : 39 – 42 .
- Kibenge , F.S.B. and Wilcox , G.E. 1983 . Tenosynovitis in chickens . Veterinary Bulletin , 53 : 431 – 443 .
- Kibenge , F.S.B. , Gwaze , G.E. , Jones , R.C. , Chapman , A.F. and Savage , C.E. 1985 . Experimental reovirus infection in chicken: observations on early viraemia and virus distribution in bone marrow, liver, and enteric tissues . Avian Pathology , 14 : 87 – 98 .
- Labrada , L. , Bodelon , G. , Vinuela , J. and Benavente , J. 2002 . Avian reoviruses cause apoptosis in cultured cells; viral uncoating but not viral gene expression is required for apoptosis induction . Journal of Virology , 76 : 7932 – 7941 .
- Leist , M. , Single , B. , Castoldi , A.F. , Kuhnle , S. and Nicotera , P. 1997 . Intracellular adenosine triphosphate (ATP) concentration: a switch in the decision between apoptosis and necrosis . Journal of Experimental Medicine , 185 : 1481 – 1486 .
- Lemasters , J.J. 1999 . Mechanisms of hepatic toxicity. V. Necrapoptosis and the mitochondrial permeability transition: shared pathways to necrosis and apoptosis . American Journal of Physiology , 276 : 1 – 6 .
- Lin , P.Y. , Liu , H.J. , Yu , F.L. , Hsu , H.Y. , Lee , J.W. and Shih , W.L. 2006 . Avian reovirus activates a novel proapoptotic signal by linking Src to p53 . Apoptosis , 11 : 2179 – 2193 .
- Liu , H.J. , Liao , M.H. , Chang , C.D. , Chen , J.H. , Lin , M.Y. and Tung , M.C. 1999a . Comparision of two techniques for the detection of avian reovirus in formalin-fixed paraffin-embedded chicken tissues . Journal of Virological Methods , 80 : 197 – 201 .
- Liu , H.J. , Chen , J.H. , Liao , M.H. , Lin , M.Y. and Chang , G.N. 1999b . Identification of the sigma C-encoded gene of avian reovirus by nested PCR and restriction endonuclease analysis . Journal of Virological Methods , 81 : 83 – 90 .
- Liu , H.J. , Lee , L.H. , Hsu , H.W. , Kuo , L.C. and Liao , M.H. 2003 . Molecular evolution of avian reovirus: evidence for genetic diversity and reassortment of the S-class genome segments and multiple cocirculating lineages . Virology , 314 : 336 – 349 .
- Liu , H.J. , Lee , L.H. , Shih , W.L. , Li , Y.J. and Su , H.Y. 2004 . Rapid characterization of avian reoviruses using polylogenetic analysis, reverse transcription-polymerase chain reaction and restriction enzyme fragment length polymorphism . Avian Pathology , 33 : 171 – 180 .
- Liu , H.J. , Lin , P.Y. , Lee , J.W. , Hsu , H.Y. and Shih , W.L. 2005 . Retardation of cell growth by avian reovirus p17 through the activation of p53 pathway . Biochemical and Biophysical Research Communication , 336 : 709 – 715 .
- Martinez-Costas , J. , Grande , A. , Varela , R. , Garcia-Martinez , C. and Benavente , J. 1997 . Protein architecture of avian reovirus S1133 and identification of the cell attachment protein . Journal of Virology , 71 : 59 – 64 .
- O'brien , V. 1998 . Viruses and apoptosis . Journal of General Virology , 79 : 1833 – 1845 .
- Oppenheim , R.W. 1991 . Cell death during development of the nervous system . Annual Review of Neuroscience , 14 : 453 – 501 .
- Roessler , D.E. and Rosenberger , J.K. 1989 . In vitro and in vivo characterization of avian reoviruses III. Host factors affecting virulence and persistence . Avian Diseases , 33 : 555 – 565 .
- Robertson , M.D. and &Wilcox , G.E. 1986 . Avian reovirus . Veterinary Bulletin , 56 : 154 – 174 .
- Shapouri , M.R.S. , Kane , M. , Letarte , M. , Bergeron , J. , Arella , M. and Silm , A. 1995 . Cloning, sequencing, and expression of the S1 gene of avian reovirus . Journal of General Virology , 76 : 1515 – 1520 .
- Shen , Y. and Shenk , T.E. 1995 . Viruses and apoptosis . Current Opinion in Genetic Development , 5 : 105 – 111 .
- Shih , W.L. , Hsu , W.H. , Liao , M.H. , Lee , L.H. and Liu , H.J. 2004 . Avian reovirus σC protein induces apoptosis in cultured cells . Virology , 321 : 65 – 74 .
- Tanimura , N. and Sharma , J. 1998 . In situ apoptosis in chicken infected with infectious bursal disease virus . Journal of Comparative Pathology , 118 : 15 – 27 .
- Varela , R. and Benavente , J. 1994 . Protein coding assignment of avian reovirus S1133 . Journal of Virology , 68 : 6775 – 6777 .