Abstract
The rapid expansion of West Nile virus (WNV) throughout the New World has raised interest in understanding the population dynamics and patterns of dispersal of emerging infectious diseases by wildlife. WNV affects humans, although its main reservoirs are various species of birds. Here we analyse the prevalence of WNV-neutralizing antibodies in nearly full-grown chicks belonging to seven different species of colonial waterbirds at three localities in southern Spain. Chicks with neutralizing antibodies against WNV were detected in three species and at all three localities. However, the low antibody titres suggest the presence of antibodies is probably due to maternal transfer of antibody, presumably from exposure of the adult birds to WNV or a similar flavivirus at some stage of their lives. The analyses of the movements of tagged birds confirmed that all species with antibody visit regions that have had reports of WNV infection over the past decade.
Prévalence des anticorps neutralisants anti-virus West Nile chez des d'oiseaux aquatiques vivant en colonie dans le sud de l'Espagne.
L'expansion rapide du virus West Nile (WNV) dans le nouveau monde a soulevé un intérêt dans la compréhension des dynamiques de population et des caractéristiques de la dispersion des maladies infectieuses émergentes par la faune sauvage. Le WNV affecte l'homme, bien que ses principaux réservoirs soient les diverses espèces d'oiseaux. Ici nous analysons la prévalence des anticorps neutralisants anti-WNV chez de jeunes oiseaux presque adultes appartenant à sept espèces différentes d'oiseaux aquatiques vivant en colonies dans trois localités du sud de l'Espagne. De jeunes oiseaux avec des anticorps neutralisants anti-WNV ont été détectés chez trois espèces et dans les trois localités. Cependant, les titres faibles en anticorps suggèrent que la présence des anticorps est probablement due au transfert maternel des anticorps, sans doute à partir d'oiseaux adultes exposés au WNV ou à un flavivirus similaire à un moment de leur vie. Les analyses des mouvements des oiseaux bagués ont confirmé que toutes les espèces avec des anticorps visitent des régions où des infections à WNV ont été rapportées au court de la dernière décennie.
Vorkommen von Antikörpern gegen West-Nil-Virus bei koloniebildenden Wasservögeln in Südspanien
Die schnelle Ausbreitung des West-Nil-Virus (WNV) in der Neuen Welt hat das Interesse am Verstehen der Populationsdynamik und der Verbreitungsmuster von neu aufkommenden Infektionskrankheiten bei Wildtieren geweckt. Das WNV infiziert Menschen, aber sein Hauptreservoir sind verschiedene Vogelspezies. In dieser Studie untersuchten wir die Prävalenz von WNV neutralisierenden Antikörpern in nahezu ausgewachsenen Jungtieren von sieben verschiedenen Spezies von koloniebildenden Wasservögeln in drei Regionen in Südspanien. In allen drei Regionen wurden bei Jungtieren von drei Spezies neutralisierende Antikörper gegen WNV nachgewiesen. Die festgestellten Antikörpertiter waren jedoch so niedrig, dass ihre Anwesenheit wahrscheinlich auf die maternale Übertragung von Antikörpern, die vermutlich auf eine Exposition der adulten Tiere mit WNV oder eines ähnlichen Flavivirus zu irgendeinem Zeitpunkt in ihrem Leben beruhen. Die Analyse der Flugrouten markierter Vögel bestätigte, dass alle Spezies mit Antikörpern Regionen aufgesucht haben, in denen über das Vorkommen von WNV-Infektionen in den letzten zehn Jahren berichtet worden ist.
Prevalencia de anticuerpos neutralizantes frente al virus de West Nile en aves acuáticas residentes en el sur de España
La rápida expansión del virus de West Nile (WNV) en todo el Nuevo Mundo ha creado interés por entender la dinámica de poblaciones y los patrones de diseminación de enfermedades infecciosas emergentes a través de aves salvajes. WNV afecta a los humanos, a pesar de que sus principales reservorios son diversas especies aviares. En este estudio analizamos la prevalencia de anticuerpos neutralizantes frente a WNV en pollitos casi adultos pertenecientes a siete especies diferentes de aves acuáticas residentes en tres localidades del sur de España. Se detectaron pollitos con anticuerpos neutralizantes frente a WNV en tres especies y en las tres localidades. Sin embargo, los títulos bajos de anticuerpos sugieren que la presencia de anticuerpos es debida probablemente a la transferencia materna de anticuerpos, supuestamente por la exposición de aves adultas en algún momento de su vida a WNV o a flavivirus similares. Los estudios de los movimientos de las aves identificadas confirmaron que todas las especies con anticuerpos visitaban regiones en las que se había descrito infección por WNV en la última década.
Introduction
West Nile virus (WNV) is a flavivirus associated with wild animals and mainly occurs in birds, although occasional transmission to humans and horses has been documented (Zeller & Schuffenecker, Citation2004). The virus's usual cycle involves transmission between birds via a number of ornithophilic mosquito species (Work et al., Citation1955; Hayes et al., Citation2005). Other modes of infection may include oral, faecal and transovarian transmission (Banet-Noach et al., Citation2003; Komar et al., Citation2003; Hayes et al., Citation2005) and it has also been suggested that the virus can be transmitted to birds that feed on dead infected birds. West Nile disease was first described in a febrile woman in Uganda in 1937, while in Europe and the Mediterranean basin (Zeller & Schuffenecker, Citation2004) several outbreaks affecting humans and/or equines have occurred recently after an absence of 30 years in Algeria (1994), Morocco (1996, 2003), Romania (1996), Tunisia (1997), Czech Republic (1997), Italy (1998), Portugal (1998, 2004), Russia (1999 to 2001), Israel (1999 to 2000), and France (2000, 2003) (Hubalek et al., Citation2000; Del Giudice et al., Citation2004; Zeller & Schuffenecker, Citation2004; Esteves et al., Citation2005; Schuffenecker et al., Citation2005). In Spain, evidence of recent WNV circulation has been demonstrated by detection of the presence of WNV-specific antibodies in humans (Bofill et al., Citation2006). Unlike the situation in the New World, where WNV was first recorded in 1999 in New York (Ludwig et al., Citation2002) and then subsequently spread across all of North America and part of central America (Centers for Disease Control and Prevention, Citation2005), in Europe WNV has traditionally been considered a rare and minor arbovirosis (Zeller & Schuffenecker, Citation2004). In the New World, epidemic relapses occur every summer to autumn, killing thousands of birds and producing severe illness and even fatalities in a small portion of the human population exposed to the virus (Zeller & Schuffenecker, Citation2004).
In the work described here we examined sera from fledgling birds breeding in southern Spain for WNV-neutralizing antibodies. This area is within 100 km of recent WNV foci in Portugal and Morocco and previous results have shown a significant prevalence of antibodies in some species of wild birds in this area (Jiménez-Clavero et al., Citation2005). The objectives of this study were to determine the prevalence of WNV-neutralizing antibodies in nearly full-grown chicks of a number of different colonial species of waterbirds living in mosquito-rich habitats (wetlands, streams and marshes) and to then investigate the implications of these results in light of the migratory and dispersive movements of the bird species studied.
Materials and Methods
Nearly full-grown chicks of seven different species of bird were sampled at three different locations (Doñana, Odiel Marshes and Fuente de Piedra) (). Chicks were trapped by hand at or near the nest before they were old enough to fly. Blood samples were taken with syringes from the brachial or femoral vein and birds were released after manipulation. The volume of blood extracted depended on the size of the species and never exceeded 1% of the body mass (range 0.100 to 1 ml). Blood was collected in microfuge tubes, allowed to clot at ambient temperature and then placed in coolers until centrifuged (always on the same day).
Table 1. Species, locality, and date of blood samples
Both the WNV strain E101 and the E6 clone of Vero cells (Vero E6) used for virus propagation were obtained from Hervé Zeller (Institut Pasteur de Lyon). Viral titres were determined by end-point titration (Reed & Muench, Citation1938). Neutralizing antibody titres to WNV were determined using a micro virus-neutralization test (micro-VNT) in 96-well plates using an adaptation of a previously described method (Weintgartl et al., Citation2003). Serum samples were inactivated at 56°C for 30 min prior to analysis. Dilutions of test sera (25 µl) were mixed with a 25 µl volume containing 100 median tissue culture infectious dose of WNV strain E101 and incubated for 1 h at 37°C in Eagle's minimum essential medium (EMEM) supplemented with l-glutamine, 100 U penicillin/ml and 100 µg streptomycin/ml. A 50 µl volume of a suspension of Vero E6 cells (2×104 cells/ml) was added to the same medium with foetal calf serum to a final concentration of 5% v/v. The plates were incubated for 6 to 7 days at 37°C until a cytopathic effect was seen in control wells containing 10 median tissue culture infectious dose of virus. Serum samples were screened at dilutions of 1/10 and 1/20. Samples that neutralized the virus at one or both of the dilutions tested were tested again to confirm the result and then further titrated by testing serial serum dilutions from 1/10 to 1/640. The neutralization titre was reciprocal of the highest dilution completely preventing cytopathic effect.
All the available information on the migratory and/or dispersive movements of those species containing neutralizing antibodies was examined. For a number of years Greater Flamingos (Phoenicopterus ruber) have been the focus of intensive study using both sightings of individuals marked with polyvinylchloride (PVC) rings and satellite transmitters (Amat et al., Citation2005). Several hundred Yellow-legged Gulls (Larus cachinnans) and thousands of Glossy Ibis (Plegadis falcinellus) chicks from the colonies sampled in this study have been individually marked with PVC rings bearing a three-digit code that can be read with the aid of telescopes at a distance of over 100 m. The worldwide observations and recaptures of these marked birds have been used to characterize the approximate ranges of these species. Good information is also available on the movements of PVC-ringed White Storks (Ciconia ciconia), although none of the chicks of this species contained neutralizing antibodies and so data for this species is only presented for comparative purposes and because it is thought that C. ciconia introduced West Nile disease from central Europe into Israel in 1998 (Malkinson et al., Citation2001).
Results
The results are presented in . Neutralization at 10-fold or higher dilutions was detected in three of the seven species examined. The prevalence of neutralizing antibody in P. ruber was similar in Doñana and Fuente de Piedra (χ2=2.56, 1 degree of freedom, P=0.11). The highest prevalence (11.0% to 17.9%) was seen in this species. Neutralizing antibody was detected in birds at all three localities—in L. cachinnans in Odiel, P. falcinellus and P. ruber in Doñana and P.ruber in Fuente de Piedra. The titres were low in all three species, with maximum titres of 40.
Table 2. Summary of the analyses of antibody titres in nearly full-grown chicks of seven different waterbird species in southwest Spain
Discussion
There are three explanations for the presence of neutralizing antibodies in chicks. Firstly, these chicks may have been infected by mosquitoes, suggesting local circulation of WNV. However, if this was the case, at least some individuals would be expected to have high titres (80 or greater), as has been reported in experimentally (Komar et al., Citation2003) or naturally infected birds (i.e. individuals repeatedly captured and sero-converted in the field; authors’ unpublished data). In addition there would probably be differences in the prevalence between localities because the number of mosquitoes and prevalence of the virus would not necessarily be the same in each locality.
The second possibility is that the antibodies were passively transferred. Maternal antibodies transferred via egg yolk may survive for over 1 month (Komar, Citation2001; Gibbs et al., Citation2005). Greater Flamingos, the species in which we found the highest prevalence of antibodies, feed their chicks with a secretion that is produced by glands in the upper digestive tract and that is very rich in blood, protein, carbohydrate and fat (Lang, Citation1963). This is a similar mechanism to that used by pigeons to feed their chicks. Immunoglobulin A and immunoglobulin G are present in the crop milk produced by pigeons (Engberg et al., Citation1992) and consequently chicks may receive maternal and paternal antibodies, even after hatching. Parental transmission of antibodies, although not yet demonstrated in flamingos, would explain the similar prevalences of WNV-neutralizing antibodies in Fuente de Piedra and Doñana. The habitat in Doñana is only suitable for breeding by Greater Flamingos some years, and birds breeding there originate mainly from the Fuente de Piedra population and, to a lesser extent, La Camargue (Nager et al., Citation1997; Rendón-Martos et al., Citation2001). The transfer of antibodies by this secretion could also explain the higher prevalence of antibodies in this species as both males and females would be able to transfer antibodies to the chicks.
The third possibility is that the antibody was not induced by WNV infections, but by a cross-reacting virus antigenically related to WNV. Neutralizing antibody titres are often determined in parallel against different flaviviruses to establish the specificity of the immune response. Unfortunately, this analysis could not be carried out during our study due to the small volumes of sera available from most birds. However, WNV foci have been identified in two places within 100 km of the sampling areas, in Kenitra, Morocco (Schuffenecker et al., Citation2005) and in the Algarve, Portugal (Esteves et al., Citation2005), and immunoglobulin M specific for WNV has been detected recently in a human in southern Spain (Bofill et al., Citation2006), confirming WNV activity close to the areas under study. In addition, a previous survey of adult wild birds from these areas detected WNV neutralizing antibody titres in a number of bird species, and in all cases the titres against another related flavivirus (Usutu virus) were consistently lower than those against WNV (Figuerola et al., unpublished data). The specificity of the virus-neutralization test employed in our study has been analysed previously as part of an external quality assurance test (Niedrig et al., Citation2006) using a panel of sera with specificity for different flaviviruses (WNV, yellow fever virus, dengue virus and tick-borne encephalitis virus), and titres of 10 or higher were only detected in sera from WNV-infected individuals. We cannot rule out the possibility of a cross-reaction with a related flavivirus, particularly in birds with lower titres. Nevertheless, even if these low titres were discounted, the main conclusions of this study would remain unchanged.
Although we were unable to determine conclusively whether the antibodies had a parental origin or were produced by the chicks, the differences between the species and similarities between localities support the importance of parental transmission as an explanation for our results. Results from a previous study indicate that some birds breeding in Spain have been exposed to WNV and that the prevalence of antibodies is higher in migratory species (Figuerola et al., unpublished data). All the species included in our study were migratory. In recent years, the movements of P. ruber have been studied with satellite transmitters (Amat et al., Citation2005). During the breeding season flamingos very frequently move distances of up to 200 km between wetlands in southern Spain to collect food for their chicks. During the non-breeding season movements are less frequent but occur over greater distances (up to 1189 km) and individuals visit localities in northern and north-western Africa (Algeria, Tunisia, Morocco, Western Sahara, and Mauritania) that overlap with areas affected by recent outbreaks of WNV (see a).
Figure 1. Movements of (1a) Phoenicopterus ruber, marked with satellite trackers, redrawn from Amat et al. (Citation2005). (1b) Plegadis falcinellus, (1c) Larus cachinnans and (1d) Ciconia ciconia.
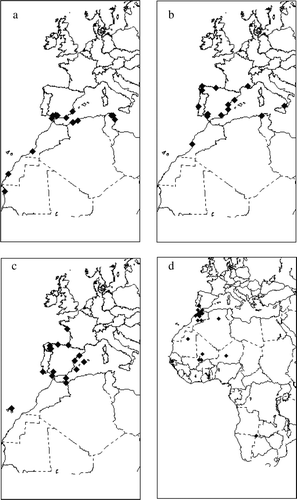
Many Glossy Ibises stay in the Doñana area until work ends in the rice fields and then move to North Africa or, as was the case during the extreme drought of the winter 2004 to 2005, to western Europe (b). Most sightings of Yellow-legged Gulls from the colonies studied have occurred in Spain, although some individuals have been observed along the Atlantic coast of France, Portugal and Morocco (see c).
No antibodies were found in the chicks of the other four species studied: the Slender-billed Gull (Larus genei), the Gull-billed Tern (Sterna nilotica), the Common Pochard (Aythya ferina) and the White Stork (C. ciconia). While L. genei and A. ferina remain in Europe or the Mediterranean Basin during the winter, S. nilotica winters farther south in West and East Africa (Delany & Scott, Citation2002). C. ciconia is a migratory species in Europe and many individuals migrate to Africa (see d). Spanish populations, however, have become all but sedentary due to their ability to exploit the permanent food resources provided by rubbish tips (Tortosa et al., Citation1995), and most sightings of marked C. ciconia were in Spain (5733 out of 5760). In previous analyses of adult birds we found that only one of 12 adult C. ciconia contained neutralizing antibodies (Figuerola et al., unpublished data). There are no data on the prevalence of antibodies in adults of the other species.
Our results are consistent with the hypothesis that adult birds have been exposed to WNV (or a highly related flavivirus). This may have occurred in Spain or in other areas visited during their migrations. During some of these movements, birds visit areas where WNV has been reported over the past decade. However, our results should be interpreted with caution. The presence of antibodies does not indicate that an individual will disperse the virus; rather, it only means that the individual has been exposed to the virus and has as a result developed immunity against it. For successful dispersal of the virus between migratory stopovers, birds would have to accumulate fat reserves, fly for a long time over large distances and then be bitten by a suitable vector during the viraemic phase of the infection, which usually lasts much less than 7 days (Komar et al., Citation2003) after arrival in a new area. Sub-clinical effects of virus infection on the activity of birds and their capacity to migrate would further reduce the likelihood of dispersal, and the importance of these processes in long-distance dispersal of pathogens merits further attention, given the growing interest in the role of wildlife in the epidemiology of emerging zoonotic diseases.
Acknowledgements
Our research was supported by the Spanish Ministry of Health cooperative research network EVITAR (G03/059) and by the Junta de Andalucía projects PAI RNM118, RNM157, and C03-059. Necessary permits were provided by the Junta de Andalucía, Parque Nacional de Doñana, and Paraje Natural Marismas de Odiel. Special thanks to J. A. Amat, M. A. Rendón, M. Rendón, O. González, M. Vázquez, and E. García for all their help during the development of the project. The Equipo de Seguimiento de Procesos Naturales (EBD-CSIC), Grupo de Anillamiento Zamaya, and Gosur assisted in the capture of the birds. P. Rodriguez and M. Adrian allowed us to work on their properties. J. L. Arroyo, F. Carro, J. J. Chans, L. García, F. Ibáñez, B. Jáñez, M. Mañez, R. Rodríguez, J. L. del Valle, B. Ramos, J. C. Rubio, C. Sánchez, J. M. Sayago, M. Vega, and volunteers from the Spanish Ornithological Society and the Junta de Andalucía volunteer programme helped in the collection of samples.
References
- Amat , J.A. , Rendón , M.A. , Rendón-Martos , M. , Garrido , A. and Ramírez , J.M. 2005 . Ranging behaviour of greater flamingos during the breeding and post-breeding periods: linking connectivity to biological processes . Biological Conservation , 125 : 183 – 192 .
- Bofill , D. , Domingo , C. , Cardeñoso , N. , Zaragoza , J. , de Ory , F. , Minguell , S. , Sánchez-Seco , M.P. , Dominguez , A. and Tenorio , A. 2006 . Human West Nile virus infection, Catalonia, Spain . Emerging Infectious Diseases , 12 : 1307
- Banet-Noach , C. , Simanov , L. and Malkinson , M. 2003 . Direct (non-vector) transmission of West Nile virus in geese . Avian Pathology , 32 : 489 – 494 .
- Centers for Disease Control and Prevention ( 2005 ). Maps of West Nile activity . Available online at: http://www.cdc.gov/ncidod/dvbid/westnile/birdspecies.htm (accessed 5 January 2005) .
- Del Giudice , P. , Schuffenecker , I. , Vandenbos , F. , Counillon , E. , Zeller , H. ( 2004 ). Human West Nile Virus, France . Emerging Infectious Diseases , 10 , 1885 – 1886 .
- Delany , S. and Scott , D. 2002 . Waterbird Population Estimates , 3rd edn , Wageningen : Wetlands International .
- Engberg , R.M. , Kaspers , B. , Shranner , I. , Kösters , J. and Lösch , U. 1992 . Quantification of the immunoglobulin classes IgG and IgA in the young and adult pigeon (Columba livia) . Avian Pathology , 21 : 409 – 420 .
- Esteves , A. , Almeida , A.P.G. , Galao , R.P. , Parreira , R. , Piedade , J. , Rodrigues , J.C. , Sousa , C.A. and Novo , M.T. 2005 . West Nile virus in southern Portugal, 2004 . Vector-Borne and Zoonotic Diseases , 5 : 410 – 413 .
- Gibbs , S.E.J. , Hoffman , D.M. , Stark , L.M. , Marlenee , N.L. , Blitvich , B.J. , Beaty , B.J. and Stallknecht , D.E. 2005 . Persistence of antibodies to West Nile Virus in naturally infected rock pigeons (Columba livia) . Clinical and Diagnostic Laboratory Immunology , 12 : 665 – 667 .
- Hayes , E.B. , Komar , N. , Montgomery , S.P. , O'Leary , D.R. and Campbell , G.L. 2005 . Epidemiology and transmission dynamics of West Nile Virus disease . Emerging Infectious Diseases , 11 : 1167 – 1173 .
- Hubalek , Z. , Savage , H.M. , Halouzka , J. , Juricova , Z. , Sanogo , Y.O. and Lusk , S. 2000 . West Nile virus investigations in South Moravia, Czechland . Viral Immunology , 13 : 427 – 433 .
- Jiménez-Clavero , M.A. , López , G. , Vidal , F. , Vázquez , A. , Figuerola , J. , Aranda , C. , Ruiz , S. , Escosa , R. , Masía , M. , Marqués , E. , Pujol , E. , Zaragoza , J. , Bofill , D. , de Ory , F. , Domingo , C. , Giovanni , C. , Kaptoul , D. , Sanbonmatsu , S. , Cardeñosa , N. , Gálvez , J.C. , Fernández , P. , Domínguez , A. , Gómez-Tejedor , C. , Soriguer , R. , Sánchez-Seco , M.P. and Tenorio , A. 2005 . Prevalence of neutralizing antibodies to West Nile virus in wild birds from wetland areas in Southern and Eastern Spain . Emirates Medical Journal , 23 : 25
- Komar , N. 2001 . West Nile virus surveillance using sentinel birds . Annals of New York Academy of Sciences , 951 : 58 – 73 .
- Komar , N. , Langevin , S. , Hinten , S. , Nemeth , N. , Edwards , E. , Hettler , D. , Davis , B. , Bowen , R. and Bunning , M. 2003 . Experimental infection of North American birds with the New York 1999 strain of West Nile virus . Emerging Infectious Diseases , 9 : 311 – 322 .
- Lang , E.M. 1963 . Flamingoes raise their young on a liquid containing blood . Experientia , 19 : 532 – 533 .
- Ludwig , G.V. , Calle , P.P. , Mangiafico , J.A. , Raphael , B.L. , Danner , D.K. , Hile , J.A. , Clippinger , T.L. , Smith , J.F. , Cook , R.A. and McNamara , T. 2002 . An outbreak of West Nile virus in a New York city captive wildlife population . American Journal of Tropical Medicine and Hygiene , 67 : 67 – 75 .
- Malkinson , M. , Banet , C. , Weisman , Y. , Pokamonski , S. , King , R. and Deubel , V. 2001 . Intercontinental transmission of West Nile virus by migrating White Storks . Emerging Infectious Diseases , 7 : 540
- Nager , R.G. , Johnson , A.R. , Boy , V. , Rendón-Martos , M. , Calderón , J. and Cézilly , F. 1997 . Temporal and spatial variation in dispersal in the greater flamingo (Phoenicopterus ruber roseus) . Oecologia , 107 : 204 – 211 .
- Niedrig , M. , Linke , S. , Zeller , H. and Drosten , C. 2006 . First International Proficiency Study on West Nile Virus Molecular Detection . Clinical Chemistry , 52 : 1851 – 1854 .
- Reed , L.J. and Muench , H. 1938 . A simple method of estimating fifty percent endpoints . American Journal of Hygiene , 27 : 493 – 497 .
- Rendón-Martos , M.A. , Garrido , A. , Ramírez , J.M. , Rendón-Martos , M. and Amat , J.A. 2001 . Despotic establishment of breeding colonies of greater flamingos, Phoenicopterus ruber, in southern Spain . Behavioral Ecology and Sociobiology , 50 : 55 – 60 .
- Schuffenecker , I. , Peyrefitte , C.N. , el Harrak , M. , Murri , S. , Leblond , A. and Zeller , H.G. 2005 . West Nile Virus in Morocco, 2003 . Emerging Infectious Diseases , 11 : 306 – 309 .
- Tortosa , F.S. , Máñez , M. and Barcell , M. 1995 . Wintering White Storks (Ciconia ciconia) in South West Spain in the years 1991 and 1992 . Vogelwarte , 38 : 41
- Weintgartl , H.M. , Drebot , M.A. , Hubalek , Z. , Halouzka , J. , Andonova , M. , Dibernardo , A. , Cottam-Birt , C. , Larence , J. and Marszal , P. 2003 . Comparison of assays for the detection of West Nile virus antibodies in chicken serum . Canadian Journal of Veterinary Research , 67 : 128 – 132 .
- Work , T.H. , Hurlbut , H.S. and Taylor , R.M. 1955 . Indigenous wild birds of the Nile delta as potential West Nile virus circulating reservoirs . American Journal of Tropical Medicine and Hygiene , 4 : 872 – 888 .
- Zeller , H.G. and Schuffenecker , I. 2004 . West Nile virus: an overview of its spread in Europe and the Mediterranean Basin in contrast to its spread in the Americas . European Journal of Clinical Microbiology and Infectious Diseases , 23 : 147 – 156 .