Abstract
Experimental aspergillosis was induced in 1-day-old turkeys by intra-air-sac inoculation of a spore suspension of a 3-day-old Aspergillus fumigatus culture (CBS 144.89) containing 107 spores. Ten additional poults were used as controls. Infected and non-infected animals were closely observed at least twice a day for the appearance of clinical signs and were sequentially sacrificed at days 1, 2, 3, 5 and 7 post-inoculation. In the infected group, most lung tissues and air sac swabs were culture positive from day 1 to day 5. At 1 day post-inoculation, air sac membranes were multifocally and moderately to severely thickened by an oedema and covered by an exudate. A small number of germinating conidia were present in the superficial exudate, already giving rise to small radiating hyphae. Lung lesions were mild, dominated by a diffuse congestion and a mild heterophilic infiltration. From 2 to 3 days post-inoculation, air sac membranes were more severely affected and several granulomas were observed. Both granulomas and exudates were rich in germinated conidia and hyphae. Pulmonary lesions consisted in a diffuse pneumonia. Five days post-inoculation, air sac membrane lesions progressed to a severe, multifocal, heterophilic and granulomatous inflammation. Seven days post-inoculation, a reduction of the severity of the diffuse pneumonia was detected. Concomitantly, the fungal elements were mainly observed as fragmented tubules in the cytoplasm of multinucleate giant cells. The present study demonstrated that healthy turkey poults might be able to withstand exposure to 107 A. fumigatus spores.
Résultats clinique, mycologique et pathologique chez des dindes infectées expérimentalement par Aspergillus fumigatus
L'aspergillose expérimentale a été induite chez des dindonneaux âgés d'un jour, par inoculation dans les sacs aériens d'une culture de 3 jours d'Aspergillus fumigatus (CBS 144.89) contenant 107 spores en suspension. Dix dindonneaux supplémentcaires ont servi de témoins. Les animaux infectés et non infectés ont été observés, au moins deux fois par jour, pour noter les symptômes et ont été sacrifiés à 1, 2, 3, 5, et 7 jours après l'inoculation (pi). Dans le groupe infecté, la plupart des cultures de tissu pulmonaire et d'écouvillons de sac aérien ont été positives du 1er jour au 5ème jour. Au 1er jour pi les membranes des sacs aériens ont présenté, en de multiples foyers, un épaississement modéré à important sous forme d'œdème couvert par un exsudat. Un petit nombre de conidies germant était présent dans l'exsudat superficiel donnant déjà croissance à de petits hyphes rayonnants. Les lésions des poumons étaient peu importes, dominées par une congestion diffuse et une infiltration d'hétérophiles peu importante. Du 2ème au 3ème jour pi, les membranes des sacs aériens étaient plus sévèrement affectées et plusieurs granulomes ont été observés. Les granulomes et les exsudats étaient riches en conidies germinant et en hyphes. Les lésions pulmonaires comprenaient une pneumonie diffuse. Au 5ème jour pi, les lésions des membranes des sacs aériens ont progressé en de multiples et d'importants foyers d'inflammation d'hétérophiles. Au 7ème pi une réduction de la sévérité de la pneumonie diffuse a été détectée. Concomitamment, les éléments fongiques principalement observés étaient des tubules fragmentés dans le cytoplasme de cellules géantes multinuclées. La présente étude a démontré que des dindonneaux en bonne santé pouvaient être capables de résister à une exposition de 107 spores d'A. fumigatus.
Klinische, mykologische und pathologische Befunde in experimentell mit Aspergillus fumigatus infizierten Puten
In eintägigen Putenküken wurde experimentell durch Inokulation von einer Sporensuspension aus einer drei Tage alten Aspergillus fumigatus-Kultur (CBS 144.89) mit 107 Sporen eine Aspergillose induziert. Zehn weitere Putenküken dienten als Kontrolle. Die infizierten und die nicht infizierten Küken wurden wenigstens zweimal täglich genau auf das Auftreten von klinischen Symptomen untersucht und einige von ihnen wurden jeweils am 1., 2., 3., 5. und 7. Tag post inoculationem (pi) seziert. In der infizierten Gruppe ließ sich der Erreger vom ersten bis zum fünften Tag pi aus fast allen Lungen und Luftsackabstrichen reisolieren. Am ersten Tag pi waren die Luftsackmembranen multifokal gering- bis hochgradig ödematös verdickt und mit einem Exsudat bedeckt. Eine geringe Anzahl von keimenden Konidien mit ersten kleinen ausstrahlenden Hyphen waren in dem oberflächlichen Exsudat zu finden. Die milden Lungenläsionen waren gekennzeichnet von einer diffusen Kongestion und einer geringgradigen heterophilen Infiltration. Vom zweiten bis zum dritten Tag pi waren die Luftsäcke hochgradig verändert und es wurden etliche Granulome beobachtet. Sowohl die Granulome als auch die Exsudate enthielten reichlich keimende Konidien und Hyphen. Die Lungenläsionen bestanden in einer diffusen Pneumonie. Fünf Tage pi hatten sich die Luftsackveränderungen zu einer schweren, multifokalen, heterophilen und granulomatösen Entzündung entwickelt. Am 7. Tag pi nahm der Schweregrad der diffusen Pneumonie wieder ab. Begleitend wurden die fungalen Elemente hauptsächlich als fragmentierte Hyphen im Zytoplasma von multinukleären Riesenzellen festgestellt. Die vorliegende Studie hat gezeigt, dass gesunde Putenküken einer Infektion mit 107 A. fumigatus-Sporen wiederstehen können.
Hallazgos clínicos, micológicos y patológicos en pavos infectados experimentalmente con Aspergillus fumigatus
Se indujo experimentalmente aspergilosis en pavos de un día de vida mediante inoculación en los sacos aéreos de una suspensión de esporas procedente de un cultivo de Aspergillus fumigatus de 3 días (CBS 144.89) que contenía 107 esporas. Se usaron otros 10 pavipollos como controles. Se evaluaron de forma detallada al menos dos veces al día los signos clínicos en los animales infectados y no infectados y se realizaron sacrificios secuenciales a los 1, 2, 3, 5 y 7 días post-inoculación (pi). En el grupo infectado, la mayoría de muestras de pulmón e hisopos de sacos aéreos fueron positivos entre los días 1 y 5. A 1 día pi se observaba de moderado a intenso engrosamiento multifocal en las membranas de los sacos aéreos debido al edema y presencia de exudado en su superficie. En el exudado superficial había un número bajo de conidias germinales, que ya daban lugar a hifas radiales pequeñas. Las lesiones en los pulmones fueron leves, básicamente congestión difusa y leve infiltrado heterofílico. De los 2 a 3 días pi la afectación de las membranas de los sacos aéreos fue más grave y se observaron diversos granulomas. Tanto los granulomas como los exudados contenían gran cantidad de conidias germinales e hifas. Las lesiones pulmonares consistían en neumonía difusa. A los cinco días pi las lesiones en las membranas de sacos aéreos progresaron hasta inflamación heterofílica y granulomatosa multifocal. A los siete días pi se observó una reducción en la gravedad de la neumonía difusa asociada a la presencia de elementos fúngicos como túbulos fragmentados principalmente en el citoplasma de las células gigantes. Este estudio demuestra que pavipollos sanos serían capaces de resistir la exposición a 107 esporas de A. fumigatus.
Introduction
In the early 1800s, moulds, probably belonging to the genus Aspergillus, were described in wild birds in Europe. Since then aspergillosis has been described worldwide in a very large number of avian species, and probably all birds are susceptible to infection (Richard, Citation1997; Kearns, Citation2003; Tell, Citation2005). Turkey poults in large confinement houses, quail, marine birds that are brought into rehabilitation, captive raptors, and penguins being maintained in zoological parks commonly die from aspergillosis (Redig, Citation1993). Young birds appear to be much more susceptible than adults. In turkey poults, aspergillosis leads to consequential economic losses related to low productivity, mortality and carcass condemnations at slaughter inspection (Morris & Fletcher, Citation1988; Richard, Citation1997). Two forms of the disease are regularly reported in turkey poults. The first form is an acute aspergillosis leading to severe outbreaks in very young birds. Clinical signs usually include dyspnoea, gasping and inappetence. The chronic form of aspergillosis most commonly occurs in 13-week-old to 18-week-old turkeys, late in the growing cycle.
Aspergillus spp. are opportunistic pathogens, causing disease in immunocompromised birds or in birds exposed to overwhelming numbers of fungal spores. In most cases, the primary site of development is the respiratory tract (air sacs and lungs) but blood dissemination frequently occurs, leading to macroscopic lesions in a wide range of organs or tissues. In spontaneous cases, lesions range from miliary to larger granulomatous foci (Olson, Citation1969; Reece et al., Citation1986; Perelman & Kuttin, Citation1992; Singh et al., Citation1994), which are white in colour and protrusive to the surface of the internal organ (Reece et al., Citation1986; Perelman & Kuttin, Citation1992; Richard, Citation1997). Thickening of the walls of the air sacs is frequently reported. Microscopic examination reveals the presence of granulomatous foci and necrosis with a surrounding region of proliferation including giant cells, macrophages, heterophils and lymphocytes and an outer capsule of connective tissue. Branching and septate fungal hyphae are systematically observed within the lesions (Reece et al., Citation1986; Perelman & Kuttin, Citation1992; Singh et al., Citation1994). Despite advances in the study of diseases related to Aspergillus spp., the physiopathology of avian aspergillosis has not yet been fully elucidated. In previous investigations, experimental aspergillosis was induced in chickens (O'Meara & Chute, Citation1959; Taylor & Burroughs, Citation1973; Van Cutsem, Citation1983; Fadl Elmula et al. Citation1984), turkeys (Richard et al., Citation1981; Richard & Thurston, Citation1983; Redig et al., Citation1986; Richard et al., Citation1991; Kunkle & Rimler, Citation1996; Kunkle & Sacco, Citation1998; Kunkle et al., Citation1999), starlings (Atasaver & Gümüssoy, Citation2004), ducks (Graczyk et al., Citation1998), Japanese quail (Olson, Citation1969; Chaudhary & Sadana, Citation1988; Gümüssoy et al. Citation2004), pigeons (Van Cutsem et al., Citation1989) and ostriches (Walker, Citation1915).
The aim of the present study was to evaluate clinical, mycological and pathological findings in turkey poults experimentally infected by Aspergillus fumigatus. Experimentally infected poults were killed from day 1 to day 7 post-inoculation (p.i.) and a subsequent histological analysis was performed in order to describe the first steps of Aspergillus development and concomitant immune response in tissues.
Materials and methods
Animals
Thirty-one turkey poults of the British United Turkeys 9 strain were selected for the present study. These animals originated from a conventional breeding unit in France. Throughout the experiment, the poults were housed in cages (cages E1CCBAC010; Charles River Laboratories, France) with filtrating covers (E4FVC04910). They were fed a commercial poult mash and water ad libitum. The feed was sterilized by ionization. The water was sterilized by heat.
Experimental inoculum
The strain CBS 144.89, initially isolated from a human patient with invasive aspergillosis in France and obtained from the Centraalbureau voor Schimmelculture, Utrecht, The Netherlands, was used. The strain was routinely maintained on Sabouraud dextrose agar plates supplemented with chloramphenicol (0.5 g/l). To obtain asexual spores (conidia), cultures were grown on YM agar (0.3% yeast extract, 0.3% malt extract, 0.5% peptone, and 0.5% agar) at 37°C. After 3 days growth, a large number of conidia was produced from specific cells (phialids) that radiate from a vesicle at the top of a conidiophorous hypha. The conidia were harvested by flooding the plates with sterile distilled water. They were then pelleted by centrifugation, washed in phosphate-buffered saline (0.15 M) and quantified using a Malassez cell.
Experimental design
Six 1-day-old poults were killed at the beginning of the experiment (pre-inoculation controls). Their lungs were removed and contamination by A. fumigatus was checked for by application of lung sections onto Sabouraud dextrose agar. The plates were incubated at 41°C and the presence of A. fumigatus colonies was checked for every day for 1 week. The remaining 1-day-old poults were randomly divided into two groups. Each bird in the infected group (n=15) was anaesthetized by intramuscular injection of 15 µl ketamine and 10 µl diazepam (5 mg/kg), 15 µl imalgene 1000 mg/10 ml + 10 µl valium, 5 mg/ml; this birds were then inoculated by transcutaneous injection into the right caudal thoracic air sac with 100 µl spore suspension of a 3-day-old A. fumigatus culture containing 107 spores. The birds in the control group (n=10) were anaesthetized and similarly given 20 µl sterile saline solution. Birds from the two groups were placed in separate cages. The poults were closely observed at least twice a day for the appearance of clinical signs. Three randomly selected poults from the infected group and two from the control group were killed 1, 2, 3, 5 and 7 days p.i. A postmortem examination was performed and the following organs were removed: lungs, thoracic air sac, liver and brain.
Mycological and histological analyses
Sections of the lung, liver and brain were applied on Sabouraud dextrose agar with 0.5% chloramphenicol in Petri dishes. Sampling of the thoracic air sac was made with a sterile cotton-swab. The plates were incubated for 4 days at 37°C. When fungal colonies developed, species identification was done by microscopic examination of conidiophores and conidia, in addition to the observation of colony morphology. A. fumigatus is characterized by green echinulate conidia, 2 to 3 µm in diameter, produced in chains from greenish phialids, 6 to 8 µm by 2 to 3 µm in size (de Hoog et al., Citation2000). All the organs and tissues were further fixed in 10% formaldehyde. Paraffin wax-embedded specimens were sectioned at 4 µm and stained with haematoxylin–eosin–safran (HES), methenamine silver stain (MS) and periodic acid–Schiff. Immuno-histochemical staining was performed with a Ventana NexES automated immunostainer on 4 µm sections using the avidin–biotin–peroxidase complex method with diaminobenzidine as a substrate and haematoxylin as counterstaining (Ventana iView DAB detection kit). After deparaffinization, unmasking of the antigens by heat (microwave 750 W, 10 min) and inhibition of endogenous peroxidase activity (by a specific buffer included in the Ventana iView DAB detection kit), some sections were incubated with a rabbit polyclonal antibody specific for A. fumigatus conidia (Sturtevant & Latgé, Citation1992) diluted 1:50, for 30 min at 37°C.
Results
Clinical signs
In the infected group no clinical signs were noticed until day 6 p.i., when one poult (out of six) presented respiratory distress and diarrhoea. The birds in the control group remained apparently normal throughout the study.
Mycological cultures
A. fumigatus was not recovered from the six poults that were killed at the beginning of the study. In the infected group, a large number of A. fumigatus colonies could be isolated from thoracic air sac and lung tissue from days 1 to 5 p.i. (). Mycological cultures were negative at 7 days p.i. A small number of A. fumigatus colonies were isolated from liver tissue in two experimentally infected animals at days 3 and 5. A. fumigatus was not isolated from brain tissue. In the control group, mycological cultures were all negative.
Table 1. Recovery of A. fumigatus and evidence of macroscopic lesions in the lung tissue and thoracic air sac of experimentally infected turkey poults
Macroscopic lesions
Gross lesions were detected at necropsy in nine infected birds (). Lesions consisted of small (1 to 3 mm) white nodules protrusive to the surface of the lungs. A thickening of the walls of the thoracic air sacs (along with small plaques) was also noticed in some animals. No macroscopic lesions were detected in the liver or the brain of experimentally infected poults, or in the control group.
Histological observations
The development of the lesions was followed from 1 to 7 days p.i. on air sac membranes and lung parenchyma. The character and severity of the lesions were generally comparable between the different animals of the same group, in spite of a slight individual variability. No lesions or fungal elements were seen in the liver or the brain. At 1 day p.i., air sac membranes were multifocally and moderately to severely thickened by a clear oedema containing dispersed, non-degenerate, heterophils; mononuclear inflammatory cells were rare. Multifocally, the epithelium was ulcerated and replaced by a loosely arranged eosinophilic exudate, containing blood cells (mainly red blood cells) and degenerate heterophils (a). In other places, the epithelium was intact or slightly hyperplasic. No granuloma could be observed at this stage. A small number of swollen and germinating conidia was present in the superficial exudate, giving already rise to small radiating hyphae (b,c). No fungal element was observed in intact structures or in the interstitial exudative changes. Lung lesions were mild, dominated by a diffuse congestion and a mild hererophilic infiltration. A few, more severe, inflammatory lesions were focally present on the pleura and the underlying pulmonary lobules (a). Those focal pleural lesions were characterized by a severe thickening due to a clear oedema and a moderate infiltration by heterophils, lymphocytes and plasma cells. Lesions of the underlying pulmonary alveoli were severe and characterized by a parenchymal infiltration with similar numbers of heterophils and macrophages; rare, small, multinucleated giant cells began to appear (b). A moderate perivascular cuffing by lymphocytes and macrophages was inconstantly present. The epithelium of parabronchi was necrotic and their lumen was filled with a slightly eosinophilic exudate containing few red blood cells and numerous heterophils. A small number of germinating conidia was present in the pulmonary lesions, confined to the lobular parenchyma, leaving free the parabronchi and the overlying pleura (c).
Figure 1. Air sac 1-day p.i. (1a) Oedema of the air sac membrane (asterisk) and heterophil-rich exudate collected in the lumen (frame). HES. Bar = 50 µm. (1b) Details of the exudate, showing numerous small radiating hyphae strongly stained in black by MS. Bar = 10 µm. (1c) Same sample stained with periodic acid–Schiff, allowing the observation of septae within the hyphae (arrows); swollen conidia (arrowheads) are present and characterized by a larger diameter than the hyphae. Bar = 10 µm.
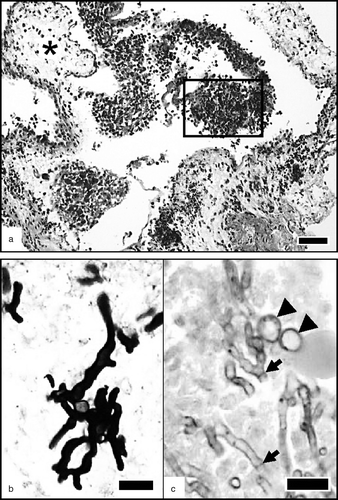
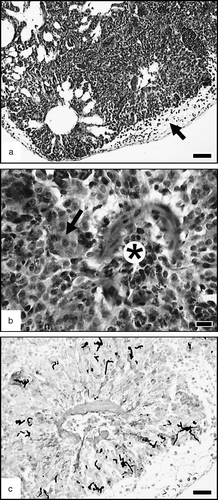
Figure 2. Lung, 1 day p.i. (2a) Pleural oedema (arrow); diffuse densification of the parenchyma by a congestion and by an inflammatory cellular infiltration. HES. Bar = 50 µm. (2b) Details showing a parabronchus filled by a heterophil-rich exudate (asterisk) and a parenchymal infiltration by heterophils and mononuclear inflammatory cells (presumptive macrophages); a multinucleate giant cell is already present (arrow). HES. Bar = 10 µm. (2c) Small hyphae radiating in a pulmonary lobule. MS. Bar = 25 µm.
Two days p.i., air sac membranes were more severely affected, being diffusely thickened, either by a clear oedema containing few cells or focally by a protein-rich exudate containing numerous heterophils and macrophages. Several granulomas were observed, composed of a necrotic, amorphous, eosinophilic centre, surrounded by degenerate and intact heterophils, and by a thin, discontinuous rim of macrophages. The superficial exudate was condensed in highly eosinophilic deposits, containing numerous necrotic and degenerate heterophils. Both types of lesions were rich in germinated conidia and hyphae. Multinucleate giant cells were present but remained rare. Pulmonary lesions were also more severe, consisting in a diffuse pneumonia, with a marked mixed cellular infiltrate associating heterophils, macrophages and lymphocytes. These lesions were especially severe in superficial lobules, with a strong macrophagic infiltration, rich in multinucleate giant cells (a). Germinating conidia were numerous in giant cell-rich areas (b).
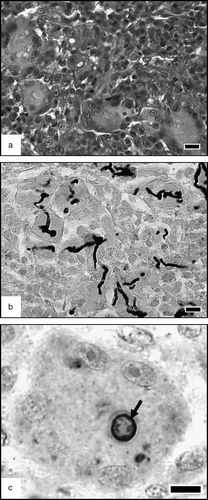
Figure 3. Early evolution of the inflammatory cell population. (3a) Pulmonary parenchyma, 3 days p.i. Mononuclear cells are more numerous than at 1 day p.i. and heterophils are fewer. HES. Bar = 10 µm. (3b) Same sample showing hyphae in black within the multinucleated giant cells (cytoplasm stained in green). MS. Bar = 10 µm. (3c) A swollen conidium (arrow), phagocytized by a multinuclear giant cell. Anti-Aspergillus immunolabelling, showing that the fungi stained by MS belong to the species A. fumigatus . Bar = 5 µm.
By 3 days p.i., pleural lesions were characterized by a less severe oedema than in previous stages and by a stronger infiltration with heterophils, macrophages and lymphocytes. Large areas of the lung presented a severe pneumonia dominated by macrophages, epithelioid cells and multinucleate giant cells; the infiltration by heterophils was diffuse and moderate; small foci of lymphoid cells were present and inconstantly organized in periarteriolar sheets. Several granulomas were present in the lobular parenchyma, with a central core of necrotic heterophils and a large rim of multinucleate giant cells. Fungal elements were numerous and obvious, either with HES or with MS staining. Immunohistochemical techniques, using a rabbit polyclonal antibody specific for A. fumigatus conidia (Sturtevant & Latgé, Citation1992), confirmed that the fungal elements observed in the lesions belonged to A. fumigatus (c).
At 5 days p.i., air sac membrane lesions progressed to a severe, multifocal, heterophilic and granulomatous inflammation, with large accumulations of necrotic heterophils, surrounded by a continuous rim of epithelioid and multinucleate giant cells. Fungal elements were mainly confined to the centre of the granulomas. Pleural and pulmonary lesions were similar to those observed at day 3, with persistence of well-organized granulomas where fungi were restricted.
At 7 days p.i., a reduction in the severity of the diffuse pneumonia was detected. Concomitantly, destruction of the fungal elements occurred. These elements were mainly observed in the cytoplasm of multinucleate giant cells, either in the air sac membranes or in the alveolar parenchyma, as irregular and fragmented tubules () or dust-like debris, attesting their destruction by the inflammatory cells.
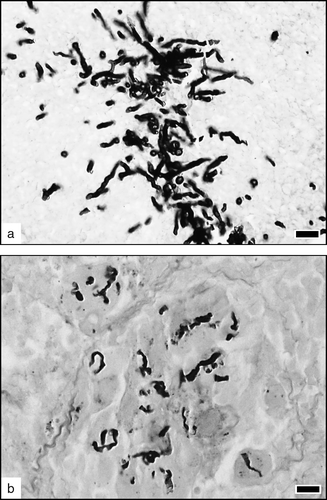
Figure 4. Morphological evolution of fungi. (4a) Numerous, well-stained and well-delineated conidia and hyphae in an acute exudative lesion, 2 days p.i. MS. Bar = 1 µm. (4b) Intra-granulomatous fungi, phagocytized by multinucleate giant cells, 7 days p.i. Hyphae are badly delineated and fragmented, attesting their destruction by the inflammatory cells. MS. Bar = 10 µm.
Discussion
Aspergillosis is considered to be a common and life-threatening infection in many avian species. Predisposition of birds to aspergillosis may be attributed to some anatomical peculiarities that preclude the mechanisms of ejection of inhaled fungal spores. The absence of resident macrophages within airway lumens and the dependence on heterophils (that use cationic proteins, hydrolase and lysosyme rather than catalase and myeloperoxidase) may also be responsible for the increased sensitivity of birds to aspergillosis (Klika et al., Citation1996; Harmon, Citation1998). As a consequence, information provided by models using common laboratory animals (rodents and rabbit) may not be valuable for birds. Therefore, the development of specific avian models is critical to our current understanding of the pathogenesis of avian aspergillosis and to the advancement of prevention and therapy. Previous models have used a variety of avian species (chickens, turkeys, quail, starling, pigeon and ostriches), with ages ranging from hatchlings to adult birds. Different routes of inoculation and challenge dosage have been tested. Inhalation chambers have been used to obtain experimental aspergillosis in chickens (O'Meara & Chute, Citation1959; Taylor & Burroughs, Citation1973) and turkeys (Richard et al., Citation1981, Citation1991; Richard & Thurston, Citation1983). Intra-tracheal challenge has been performed in ducks (Graczyk et al., Citation1998), quail (Chaudhary & Sadana, Citation1988) and ostriches (Walker, Citation1915). Intra-air sac inoculation was tested in turkeys (Kunkle & Rimler, Citation1996; Kunkle & Sacco, Citation1998; Kunkle et al., Citation1999). Exposure to aerosolized spores probably represents the model that most closely mimics natural conditions. However, this model requires specific equipment (inhalation chambers) and the standardization of challenge dose may be difficult.
In the present study, intra-air sac inoculation was selected with a controlled inoculum of 107 spores/animal. Experimental infection was performed in 1-day old turkeys because aspergillosis is regularly reported in this species and because young turkeys are believed to be more susceptible than adults. In the present study, sequential observations from 1 to 3 days p.i. did not differ significantly from previous ones, especially from those of Kunkle & Rimler (Citation1996) performed using 9-week-old and 19-week-old turkeys. Our observations confirmed that lesions are confined to air sac membranes and lungs, and that the formation of granulomas occurs very soon after experimental inoculation. However, Kunkle & Rimler (Citation1996) detected granulomas (50 to 200 µm in diameter) as soon as 1 day p.i., whereas in the present study similar lesions were first observed 2 days p.i. Kunkle & Rimler examined experimentally infected turkeys only at days 1, 2, 3 and 4 p.i., whereas in the present study the lesions were described for a longer period (1 week) and this allowed us to demonstrate that fungal elements begin to be destroyed by the multinucleate giant cells at day 7 p.i. This result suggests that healthy turkey poults can withstand considerable exposure to A. fumigatus spores. Chaudhary & Sadana (Citation1988) made similar observations in quail. Clinical signs were consistently observed for 7 to 10 days following intra-tracheal inoculation, but thereafter the surviving birds appeared normal. O'Meara & Chute (Citation1959) reported that hatching poults were easily infected with A. fumigatus spores, but poults older than 3 days were resistant to the infection. In the study by Taylor & Burroughs (Citation1973), histological evidence of Aspergillus infection was noted in lung tissue at about the third day post aerosol exposure of chickens, but generally disappeared during the subsequent 7 to 10 days. On the contrary, lesions persisted in air sacs, for 3 weeks or more in some birds.
Acute inflammatory reactions in birds frequently include multinucleate giant cells that are characteristic of more chronic reactions in mammals (Kunkle & Rimler, Citation1996). These elements, together with the findings from the present study, suggest that the involvement of macrophages and multinucleate giant cells in the early development of aspergillosis in turkeys is a non-specific process. Effective destruction of A. fumigatus was confirmed in the present study by negative cultures from lungs and air sacs at day 7 p.i. Further experiments have to be conducted with older animals. Of course, a single injection of a large amount of Aspergillus conidia within an air sac does not represent the model that most closely mimics natural conditions. In animal facilities, birds may be exposed to a small number of airborne Aspergillus conidia for a long time (several days). In such circumstances, we can imagine that pathogenesis of avian aspergillosis may be different from that described after a single inoculation. Pathogenesis of avian aspergillosis may also be related to the virulence of A. fumigatus isolates. Using the analysis of microsatellite markers polymorphism, a recent investigation demonstrated that the same genotype was detected in both healthy and infected birds, suggesting the absence of particular virulent genotypes for turkeys (Lair-Fulleringer et al., Citation2003). However, Peden & Rhoades (Citation1992) reported that A. fumigatus isolates from different sources showed a range of virulence and lethal infection in intra-air sac inoculated turkeys. The single environmental isolate produced no mortality. The strain used in the present study (CBS 144.89) was initially isolated from a human with invasive aspergillosis. In further experiments, turkeys should be challenged with A. fumigatus of avian origin.
Acknowledgements
The authors express their gratitude to Agnès Champeix, Patricia Wattier and Sophie Chateau-Joubert (Ecole Nationale Vétérinaire d'Alfort) for the histological technique.
References
- Atasever , A. and Gümüssoy , K.S. 2004 . Clinical and mycological findings in experimental aspergillosis infections of starlings . Journal of Veterinary Medicine Series A , 51 : 19 – 22 .
- Chaudhary , S.K. and Sadana , J.R. 1988 . Experimental aspergillosis in Japanese quails (Coturnix coturnix japonica), clinical signs and haematological changes . Mycopathologia , 102 : 179 – 184 .
- de Hoog , G.S. , Gene , J. & Figueras , M.J. ( 2000 ). Atlas of Clinical Fungi 2nd edm . Utrecht , , The Netherlands : Centraalbureau voor Schimmelcultures .
- Fadl Elmula , A. , Abu Elgasim , A.I. and El Mubarak , A.K. 1984 . Experimental aspergillosis in young chicks . Revue Elevage et Médecine Vétérinaire Pays tropicaux , 37 : 437 – 441 .
- Graczyk , T.K. , Cranfield , M.R. and Klein , P.N. 1998 . Value of antigen and antibody detection, and blood evaluation parameters in diagnosis of avian aspergillosis . Mycopathologia , 140 : 121 – 127 .
- Gümüssoy , K.S. , Uyanik , F. , Atasever , A. and Çam , Y. 2004 . Experimental Aspergillus fumigatus infection in quails and results of treatment with itraconazole . Journal of Veterinary Medicine Series B , 51 : 34 – 38 .
- Harmon , B. 1998 . Avian heterophils in inflammation and disease resistance . Poultry Sciences , 77 : 972 – 977 .
- Kearns , K.L. 2003 . “ Avian aspergillosis ” . In Recent Advances in Avian Infectious Diseases , Edited by: Kearns , K.S. and Loudis , B. Ithaca , NY : International Information Service .
- Klika , E. , Scheuermann , D.W. , De Groodt-Lasseel , M.H.A. , Bazantova , I. and Switka , A. 1996 . Pulmonary macrophages in birds (barn owl, Tyto tyto alba), domestic fowl (Gallus gallus domestica), quail (Coturnix coturnix) and pigeon (Columbia livia) . Anatomy Record , 246 : 87 – 97 .
- Kunkle , R.A. and Rimler , R.B. 1996 . Pathology of acute aspergillosis in turkeys . Avian Diseases , 40 : 875 – 886 .
- Kunkle , R.A. and Sacco , R.E. 1998 . Susceptibility of convalescent turkeys to pulmonary aspergillosis . Avian Diseases , 42 : 787 – 790 .
- Kunkle , R.A. , Rimler , R.B. and Steadham , E.M. 1999 . Absence of protection against challenge with Aspergillus fumigatus by adoptive transfer of splenocytes from convalescent turkeys . Avian Diseases , 43 : 678 – 684 .
- Lair-Fulleringer , S. , Guillot , J. , Desterque , C. , Seguin , D. , Warin , S. , Chermette , R. and Bretagne , S. 2003 . Differentiation of Aspergillus fumigatus isolates from breeding turkeys and their environment by genotyping with microsatellite markers . Journal of Clinical Microbiology , 41 : 1798 – 1800 .
- Morris , M.P. and Fletcher , O.J. 1988 . Disease prevalence in Georgia turkey flocks in 1986 . Avian Diseases , 32 : 404 – 406 .
- O'Meara , D.C. and Chute , H.L. 1959 . Aspergillosis experimentally produced in hatching chicks . Avian Diseases , 3 : 404 – 406 .
- Olson , L. 1969 . Aspergillosis in japanese quail . Avian Diseases , 13 : 225 – 227 .
- Peden , W.M. and Rhoades , K.R. 1992 . Pathogenicity differences of multiple isolates of Aspergillus fumigatus in turkeys . Avian Diseases , 36 : 537 – 542 .
- Perelman , B. and Kuttin , E.S. 1992 . Aspergillosis in ostriches . Avian Pathology , 21 : 159 – 163 .
- Redig , P.T. 1993 . “ Avian aspergillosis ” . In Zoo and Wild Animals Medicine , Edited by: Fowler , M.E. 178 – 181 . Philadelphia , PA : WB. Saunders Company .
- Redig , P.T. , Post , G.S. , Concannon , T.M. & Dunette , J. ( 1986 ). Development of an ELISA for the detection of aspergillosis in avian species . Proceedings of the Association Avian Veterinarians Niami (pp. 165 – 178 ).
- Reece , R.L. , Taylor , T.K. and Dickson , D.B. 1986 . Mycosis of commercial Japanese quail, ducks and turkeys . Australian Veterinary Journal , 63 : 196 – 197 .
- Richard , J.L. 1997 . “ Aspergillosis ” . In Diseases of Poultry , Edited by: Calnek , B.W. 351 – 365 . London : Mosby-Wolfe .
- Richard , J.L. and Thurston , J.R. 1983 . Rapid hematogenous dissemination of Aspergillus fumigatus and A. flavus spores in turkey poults following aerosol exposure . Avian Diseases , 27 : 1025 – 1033 .
- Richard , J.L. , Cutlip , R.C. , Thurston , J.R. and Songer , J. 1981 . Response of turkey poults to aerosolized spores of Aspergillus fumigatus and aflatoxinogenic and non aflatoxinogenic strains of Aspergillus flavus . Avian Diseases , 25 : 53 – 67 .
- Richard , J.L. , Peden , W.M. and Sacks , J.M. 1991 . Effects of adjuvant-augmented germling vaccines in turkey poults challenged with Aspergillus fumigatus . Avian Diseases , 35 : 93 – 99 .
- Singh , H. , Grewal , G.S. and Singh , N. 1994 . Mycotic salpingitis in a Japanese quail (Coturnix coturnix japonica) . Avian Diseases , 38 : 910 – 913 .
- Sturtevant , J. and Latgé , J.P. 1992 . Interactions between the spore of Aspergillus fumigatus and human complement component C3 . Infection and Immunity , 60 : 1913 – 1918 .
- Taylor , J.J. and Burroughs , E.J. 1973 . Experimental avian aspergillosis . Mycopathologia Mycologia Applicata , 51 : 131 – 141 .
- Tell , L.A. 2005 . Aspergillosis in mammals and birds: impact in veterinary medicine . Medical Mycology , 43 : S71 – S73 .
- Van Cutsem , J. 1983 . Antifungal activity of enilconazole on experimental aspergillosis in chickens . Avian Diseases , 27 : 36 – 42 .
- Van Cutsem , J. , Van Gerven , F. and Janssen , P.A. 1989 . Oral and parenteral therapy with saperconazole (R 66905) of invasive aspergillosis in normal and immunocompromised animals . Antimicrobial Agents and Chemotherapy , 33 : 2063 – 2068 .
- Walker , J. 1915 . Aspergillosis in the ostrich chick . Union South Africa Department Agriculture Annual Report , 3–4 : 535 – 574 .