Abstract
In The Netherlands between January 2002 and December 2004, numerous psittaciformes died showing severe splenomegaly and hepatomegaly with multifocal acute necrosis. At the start of the outbreaks mostly parakeets were affected, but later larger parrots were also involved. Seventy-eight birds showed the same features and six were examined completely, including a virological examination. Tests for polyomavirus, Pacheco's disease (herpesvirus) and circovirus psittacine beak and feather disease (PBFD) viruses and Chlamydophila psittaci were carried out. All results were negative, except for two cases of circovirus infection. Many concurrent bacterial and parasitic infections were seen. Immunohistochemistry revealed reovirus antigen in intralesional mononuclear cells, and reovirus-like particles could be observed by negative contrast electron microscopy. A reovirus was grown and the isolates reacted with polyclonal reovirus antiserum but did not react with monoclonal antibodies against chicken reovirus. The virus was therefore considered a psittacine reovirus. Because reoviruses were seen consistently, they seemed to be the most probable cause of the outbreaks. Climate, the introduction of new birds and the transportation of birds might be other factors involved in the disease seen in The Netherlands. No regional influence could be seen; therefore, we suggested that the virus might be widespread and carriers could be a source of re-introduction.
Infections à réovirus associées à une mortalité élevée chez les psittaciformes aux Pays-Bas
Aux Pays-Bas entre janvier 2002 et décembre 2004 de nombreux psittaciformes sont morts présentant une sévère spléno et hépatomégalie avec des multiples foyers de nécrose aiguë. Au début de l'épisode, la plupart des perruches ont été affectées, mais plus tard des perroquets plus gros ont été touchés. Soixante-dix-huit oiseaux ont montré les mêmes caractéristiques et six ont été examinées complètement, en incluant un examen virologique. Les tests pour la détection du polyomavirus, du virus de la maladie de Pacheco (herpesvirus), du circovirus du PBFD et de Chlamydophila psittaci ont été réalisés. Tous les résultats ont été négatifs excepté pour deux cas d'infection à circovirus. Beaucoup d'infections bactériennes et parasitaires intercurrentes ont été observées.
L'immunohistochimie a révélé la présence de l'antigène réovirus dans les cellules mononuclées et par la coloration négative en microscopie électronique, il a été mis en évidence des particules ressemblant à des réovirus. Un réovirus a été cultivé et les isolats ont réagi avec un sérum polyclonal anti-réovirus mais n'a pas réagi avec les anticorps monoclonaux anti-réovirus du poulet. Par conséquent, le virus a été considéré comme étant un réovirus de psittacidés. Du fait que les réovirus ont été observés régulièrement, ils sont apparus être la cause la plus probable des cas. Le climat, l'introduction de nouveaux oiseaux et le transport des oiseaux peuvent être d'autres facteurs impliqués dans la maladie observée aux Pays-Bas. Aucune influence régionale n'a pu être mise en évidence, nous suggérons donc que le virus pourrait être disséminé et des porteurs pourrait être la source de la réintroduction.
Mit hoher Mortalität assozierte Reovirusinfektionen bei Psittaciformes in den Niederlanden
Zwischen Januar 2002 und Dezember 2004 starben in den Niederlanden zahlreiche Psittaciformes, die bei der Sektion hochgradige Spleno- und Hepatomegalie mit multifokalen akuten Nekrosen aufwiesen. Zu Beginn der Ausbrüche waren meistens Sittiche, später aber auch Großpapageien betroffen. 76 Vögel zeigten die gleichen Befunde und sechs von ihnen wurden einer vollständigen weitergehenden, einschließlich einer virologischen Untersuchung unterzogen. Es wurden Tests auf Polyomavirus, Herpesvirus (Pachecosche Krankheit) und Circovirus (Psittacine Beak and Feather Disease) sowie Chlamydophila psittaci durchgeführt. Alle Tests waren bis auf zwei Fälle von Circovirusinfektionen negativ. Außerdem wurden zahlreiche, gleichzeitige Infektionen mit Bakterien und Parasiten festgestellt. Immunhistochemisch wurden in den mononukleären Zellen im Bereich der Läsionen Reovirusantigen nachgewiesen und bei der elektronenmikroskopischen Untersuchung konnten Reovirus-ähnliche Partikel beobachtet werden. Reovirus wurde angezüchtet und das Isolat reagierte mit polyklonalem Reovirusantiserum, aber nicht mit monoklonalen Antikörpern gegen Hühnerreovirus. Aus diesem Grund wurde angenommen, dass es sich bei dem Virus um Psittazidenreovirus handelt. Da Reoviren immer wieder nachgewiesen wurden, waren sie höchstwahrscheinlich die Ursache für die Krankheitsausbrüche. Aber auch das Klima sowie Zukauf und Transport von Vögeln können an dieser in den Niederlanden aufgetretenen Krankheit beteiligte Faktoren gewesen sein. Ein regionaler Einfluss konnte nicht festgestellt werden, so dass wir annehmen, dass das Virus weit verbreitet ist und Virusträger eine Quelle für eine Wiedereinschleppung sein könnten.
Infecciones por reovirus en psitácidas asociadas a mortalidades altas en Países Bajos
Numerosas psitácidas murieron en Países Bajos entre Enero de 2002 y Diciembre de 2004, mostrando esplenomegalia y hepatomegalia graves con necrosis aguda multifocal. Al principio los brotes afectaron mayoritariamente periquitos, pero posteriormente también afectó a loros más grandes. Setenta y ocho aves mostraron las mismas características y seis se examinaron completamente, incluyendo estudios virológicos. Se llevaron a cabo pruebas frente a poliomavirus, enfermedad de Pacheco (herpesvirus), circovirus (PBFD) y Chlamydophila psittaci. Todos los resultados fueron negativos, excepto en dos casos de infección por circovirus. Se observaron muchas infecciones bacterianas y parasitarias concurrentes.
La inmunocitoquímica mostró la presencia de antígeno de reovirus intracelularmente en células mononucleares y se observaron partículas compatibles con reovirus mediante tinción negativa de contraste en microscopía electrónica. Se cultivó un reovirus y los aislados reaccionaron con antisueros policlonales frente a reovirus pero no reaccionaron con anticuerpos monoclonales frente al reovirus del pollo. Por lo tanto, se consideró que el virus era un reovirus de las psitácidas. Teniendo en cuenta que los reovirus se observaron de manera consistente, pareceron ser la causa más probable de los brotes. El clima, la introducción de nuevas aves y el transporte de aves podrían ser otros factores implicados en la enfermedad observada en los Países bajos. No se observaron influencias regionales, y por lo tanto se sugerimos que el virus podría estar distribuido y los portadores podrían ser una fuente de reintroducción.
Introduction
Avian reoviruses have a worldwide distribution in different species of birds and they are associated with viral arthritis/tenosynovitis, malabsorption syndrome, stunting/runting syndromes, enteric disease, immunosuppression and respiratory disease in poultry (Jones, Citation2000). Reoviruses are non-enveloped double-stranded RNA viruses with a segmented genome and vary between 75 and 80 nm in diameter. They are not always pathogenic and have been found on routine examination in apparently healthy poultry (Robertson et al., Citation1984; Jones, Citation2000). Avian reoviruses may cause serious disease in birds; especially in poultry, they can cause important losses (Tang et al., Citation1987). Outbreaks of reovirus infection in psittacines have previously been reported and have increased in frequency over the years (Senne et al., Citation1983; Ashton et al., Citation1984; Wilson et al., Citation1985; Graham, Citation1987; Conzo et al., Citation2001; Sanchez-Cordon et al., Citation2002). They often involved imported consignments of African grey parrots (Psittacus e. erithacus) and also other species, such as Australian king parrots (Alisterus scapularis). In disease caused by reovirus, pathological examination, as reported in the above publications, revealed splenomegaly, hepatic congestion and enteritis. Histopathology shows hepatic coagulation necrosis and less prominent necrosis in spleen and bone marrow. Senne et al. (Citation1983) described reovirus infection in asymptomatic psittacines in quarantine. In Amazon parrots, chronic respiratory signs were reported. In cockatoos, non-specific clinical signs such as incoordination, emaciation and diarrhoea were seen (Wilson et al., Citation1985; Conzo et al., Citation2001). Sudden death and hepatomegaly are reported to be suggestive of reoviruses in lories, lorikeets (Trichoglossus sp.), and in a less severe form in Senegal parrots (Poicephalus senegalus) and Jardine's parrots (Poicephalus gulielmi) (Gaskin, Citation1987; Pennycott, Citation2004). Reoviruses were previously thought to be mildly pathogenic for budgerigars (Melopsittacus undulates), but since October 2002 in Scotland and other parts of the UK high mortality was observed in different flocks of adult breeding budgerigars (Manvell et al., Citation2004; Pennycott, Citation2004). The birds showed splenomegaly and hepatomegaly with multifocal acute fibrinoid necrosis. Reoviruses were isolated in typically affected birds. Reoviral infection in New World (South America) psittaciformes is suggested to be rare. These species are thought to be more resistant than the Old World parrots (those of Australian, Asian and African origin) and could recover more easily when given appropriate supportive care (Ritchie & Carter, Citation1995; Conzo et al., Citation2001; Smidt et al., Citation2003; Manvell et al., Citation2004).
From January 2002, in The Netherlands a disease was observed among different species of psittacine birds exhibiting a very high mortality and associated with reovirus infection. During the following years this disease reappeared every few months. The disease usually occurred in larger flocks. Only occasionally was a single bird of a private owner affected. This paper describes the pathological changes in different species of psittaciformes associated with reovirus infection during outbreaks in The Netherlands and the isolation and characterization of a psittacine reovirus.
Materials and Methods
Case history
Starting in January 2002 many psittacine birds with more or less the same history were offered for autopsy at the Department of Pathobiology, Section of Diseases of Exotic Animals and Wildlife, Utrecht University, The Netherlands. Up to December 2004, hundreds of birds died. Eighty-four cases were presented for necropsy, of which six cases are described below.
| • | Case 1: A budgerigar (M. undulates) died and about 30 additional parakeets of different species died within a few weeks. Three weeks before the mortality started, new birds had been introduced into the flight. The birds showed ruffled feathers, became apathic and died. | ||||
| • | Case 2: A red-rumped parrot (Psephotus haematonotus) that was housed with other psittacines, including king parrots (Alisterius amboinensis) and pennant's rosellas (Platycercus elegans), died. Many birds died within several days. | ||||
| • | Case 3: A Bourkes parakeet (Neophema bourkii) was sent in with two other birds of the same species. All three birds came out of a large flock of mostly neophemas, located next to other flocks of both Australian and South-American parakeets. Four weeks before the problems started, a large group of neophemas was bought and put into the flock. Clinically, the birds sat on the floor, slept a lot, became anorexic and died after 3 days. In total, 17 out of 49 birds died; both young and adult animals. | ||||
| • | Cases 4, 5 and 6: An eclectus parrot (Eclectus roratus), a yellow-faced Amazon (Amazona xanthops) and a ring-necked parakeet (Psittacula krameri) came from one owner as a sample from many deaths recorded, including different species such as yellow-crowned Amazons (Amazona ochrocephala) and Cuban Amazons (Amazona leucocephala). The problems had started 3 weeks earlier, after visiting a bird market, when many birds showed conjunctivitis, diarrhoea and death several days after the onset of the disease. | ||||
An additional 78 birds presented for necropsy came from the same or from different owners. Often they were birds of different species out of a larger flock where many birds seemed to be affected and most, or even all, birds died within a few weeks. Administration of different medications had no effect. Initially the birds were mostly parakeets, but larger psittacines were also later affected. The history frequently showed the introduction of new birds into the collections. The problems started 2 to 5 days later, with a few birds (both new and present birds) showing ruffled feathers, listless behaviour and anorexia. Some were showing emaciation and diarrhoea before they finally died. The birds were of different ages; however, the very young birds (nestlings) seemed to be less affected.
Within 1 to 3 weeks, many birds in each flock died in varying numbers, from about 70 to 100%. The greatest number of cases was seen during wintertime.
Histology
At necropsy, tissue samples from the lung, heart, liver, spleen, kidney, intestine and brain were fixed in 4% phosphate-buffered formalin, embedded in paraffin, sections cut at 4 µm and stained with haematoxylin and eosin.
Cytology
Impression smears of the liver, spleen, lung and rectum were examined microscopically after staining with a quick stain (Hemacolor®; Merck, Darmstadt, Germany). STAMP staining (Machiavello's technique) and direct immunofluorescence assays—using Imagen™ Chlamydia with monoclonal antibody (mAb) K6106 (routinely provided by DakoCytomation, Ely, UK)—of impression smears were performed to identify Chlamydophila psittaci.
Bacteriology
Aerobic bacterial culture of the liver and rectum was performed in all 84 cases using standard techniques. Additional organs were cultured when gross pathological changes were suggestive for bacterial or mycotic infections and bacteria were seen in impression smears.
On tissues of four birds a quantitative polymerase chain reaction (PCR) was performed by Dr Van Haeringen (Laboratorium b.v., Wageningen, The Netherlands), and real time (RT)-PCR was performed on two birds by Dr Gendika (Veendam, The Netherlands) for detection of C. psittaci.
Virus detection
Immunohistochemistry was performed on paraffin sections of the liver, spleen, lung and intestine of four birds using the avidin–biotin–peroxidase complex (ABC) methods, following standard procedures, for polyomavirus (3G10G5 antibody against VP1 of avian polyomavirus; produced by the Institute for Virology, Leipzig, Germany) and Pacheco's Disease (routinely provided by Animal Sciences Group, Wageningen University, The Netherlands).
For detection of reovirus, 4 µm thick paraffin sections were treated with 1% hydrogen peroxide in methanol for 30 min in order to remove endogenous peroxidase activity. After incubation with normal rabbit serum for 30 min, the chicken anti-reovirus polyclonal serum, diluted to 1:200, was incubated at 4°C overnight. Anti-reovirus polyclonal serum (supplied by Dr Jaspers, Intervet International BV, Boxmeer, The Netherlands) was produced against strain 1733/2408 of avian reovirus. A rabbit anti-chicken peroxidase-labelled antibody was used as a secondary antibody. The reaction was visualized with 3,3′-diaminobenzidine tetrahydrochloride. Phosphate-buffered saline (PBS) was used in place of specific primary antibody as negative control. Rinsing steps were preformed with PBS/Tween 20.
On the spleen, liver and kidney tissues of four birds, a quantitative PCR using TaqMan® was performed by Dr Van Haeringen, and RT-PCR was performed by Dr Gendika on the same tissues of two birds for detection of polyomavirus and psittacine beak and feather disease (PBFD).
For virus isolation, the liver, intestine and intestinal contents of Cases 1 to 6 were frozen at −70°C. Thawed tissues were homogenized and a 20% suspension with an antibiotic solution (penicillin, streptomycin, mycostatin and gentamycin) was prepared. The supernatant from the suspension was inoculated on confluent monolayers of baby hamster kidney (BHK-21) cells (Simoni et al., Citation1999; Manvell et al., Citation2004) in 96-well polystyrene microtitre plates and was examined daily for cytopathic effect (CPE) by light microscopy. The supernatant of the tissue homogenates from the different birds, and from different reovirus strains as control, were inoculated onto confluent BHK-21 cells grown in micro-titre plates. Uninfected cells served as controls. After 24 h incubation at 37°C with 5% CO2, inoculated monolayers were fixed with cold 96% alcohol. The plates were washed with wash buffer and 100 µl different reovirus mAb hybridoma cell supernatants (Int-13-6, Int-15-1, Int-14-11 diluted 1:50 and Int-14-67 diluted 1:200; van Loon et al., Citation2001) diluted in PBS, or polyclonal anti-reovirus chicken serum (directed against reovirus strain 1733/2408) diluted 1:600 in PBS, was added to different wells of the same isolate. The plates were incubated for 60 min at 37°C, washed three times with wash buffer and reacted with 1:100 PBS-diluted FITC conjugate rabbit-anti-mouse or goat-anti-chicken antibody. Finally the plates were washed and fixed with a glycerol/PBS solution (1:1). The presence of fluorescence was observed microscopically. Cultures showing widespread CPE were freeze-thawed, and culture fluid concentrated by ultra-centrifugation and examined by negative contrast electron microscopy.
Results
Pathology
Case 1. Based on the observation of atrophic muscles and lack of fat tissue at necropsy, the budgerigar was considered to be in a poor condition. The liver and spleen were enlarged and pale.
Case 2
This bird, a red-rumped parrot, was also in a poor condition. The liver was slightly enlarged with multiple white small foci. The spleen was severely enlarged, showing a pale and dull cut surface and firm consistency. The kidneys were swollen and pale with a few small foci. The lungs were dark red, suggesting pneumonia.
Case 3
The cloaca and feet of the Bourkes parakeet were covered with thin faeces and it was in a poor condition. The liver and spleen were enlarged with a mottled appearance and pale, dull foci. The intestines had a hyperaemic wall and were filled with dark-coloured, watery thin faeces.
Cases 4, 5 and 6
These three birds (the eclectus parrot, the yellow-faced Amazon and the ring-necked parakeet) showed the same pathology. They were in a moderate to poor condition. They had severely enlarged livers and spleens with multiple white small foci with a dull appearance. The kidneys were pale and showed urates within the tubules. The intestines were partially filled with thin dark coloured faeces.
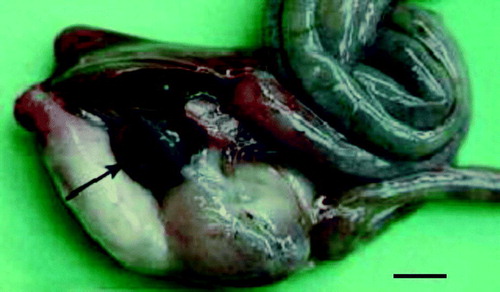
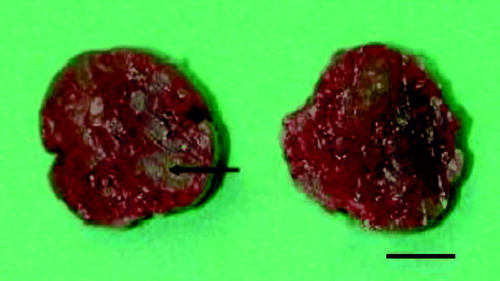
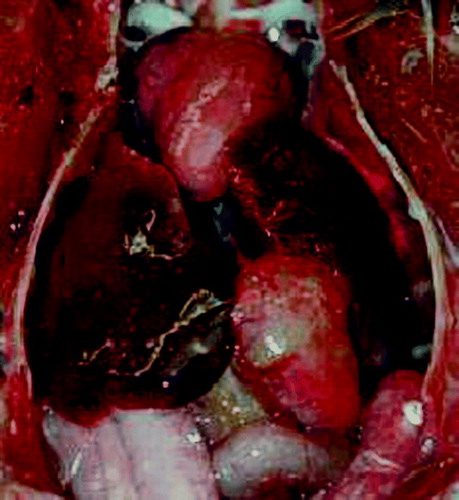
At necropsy the other 78 birds were in a moderate condition, but each had a large spleen with a mottled dull appearance and very often an enlarged, but not swollen, liver, both with multiple foci; pale, dull and 1 to 3 mm in size (Figures ). The lungs were hyperaemic and often haemorrhagic. The kidneys were hyperaemic with congestion of urates within the tubules in many cases. A few birds had intestines filled with haemorrhagic contents suggestive of haemorrhagic diathesis. Two birds had mites (Dubininia sp.) and two birds were infected with Ascaridia platyceri.
Figure 1. In-situ view of an Amazon parrot carcass (A. xanthops) showing mild hepatomegaly with multiple small necrotic foci.
Histology
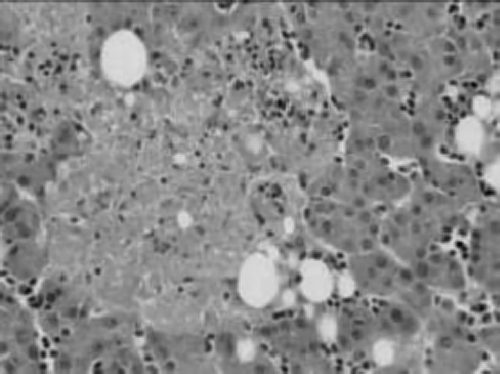
The histology of all of the cases was similar with little variations in the severity of the pathological lesions. Microscopically, the spleens showed multifocal, severe acute necrosis with plasmalymphocytic infiltrates, proliferation of macrophages (often filled with hemosiderin) and occasionally multifocal suppurative inflammation and fibrin deposits (). The liver showed multifocal necrotic lesions scattered through the parenchyma with varying numbers of plasma cells, lymphocytes and macrophages (). The hepatocytes were vacuolated; fibrosis and bile duct proliferation was found in the portal areas with infiltration of few plasma cells and lymphocytes. In the kidney, acute tubular degeneration and necrosis was seen, with some calcium deposits and perivascular plasmacellular infiltrates. Plasmacellular infiltrates were also found in the pancreas and surrounding feather follicles, and occasional necrotic foci could be seen in a few other organs (pancreas, lungs and testis). The heart showed mild degeneration of the myocardium. The bursa of Fabricius had very few active lymphoid components and some necrotic cells within the medulla. The lung of Case 2 showed a severe multifocal granulomatous mycotic pneumonia with numerous fungal hyphae up to 18 µm in diameter, multifocally many macrophages and several multinucleated giant cells.
Cytology
Cytology of rectum smears showed Macrorhabdus ornithogaster in seven of the 84 birds. The STAMP and immunofluorescence test (IFT) were negative for C. psittaci in all cases.
Bacteriology
Aerobic and anaerobic bacterial culture of the intestinal contents, liver and lung grew numerous colonies of different bacteria as follows: intestine (n=84): Enterobacter (two), Lactobacillus (nine), Streptococcus (nine), Pseudomonas (four), Enterococcus durans (two), Escherichia coli (five), Staphylococcus (two), and Clostridium (one); liver (n=84): Staphylococcus (two), Enterococcus durans (two), Bacillus sp. (one), E. coli (one), Streptococcus (one), Acinetobacter (one), and Pseudomonas (one); lung (n=2): Staphylococcus (one); air sac (n=1): Corynebacterium falsenii (one); and spleen (n=3): Lactobacillus (one). Yeasts (mostly Candida albicans) were found in the intestines (four), and fungi (three) were cultured from the lungs (n=3) including Case 2.
The RT-PCRs for C. psittaci were negative.
Virus detection
The ABCs for polyomavirus and Pacheco's disease were negative.
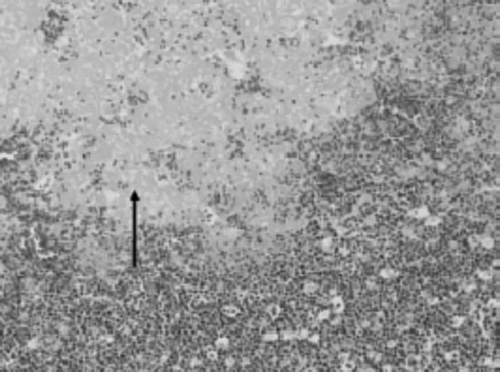
Immunohistochemical techniques used to detect avian reovirus revealed viral antigen in large mononuclear cells (possibly macrophages) in affected areas in the liver, spleen, lung, kidney, bursa and intestine ().
Figure 6. Immunohistochemical detection of avian reovirus. Viral antigen was detected in mononuclear cells of the liver in areas with necrosis. ABC method, ×200.

The quantitative PCR and RT-PCR for polyomavirus were negative. Two of the selected birds were positive for PBFD in the PCR.
Attempted virus isolation carried out on BHK-21 cell cultures from Cases 1 to 6 showed varying degrees of CPE when observed after less than 24 h incubation at 37°C. Concentrated cell culture fluids from all six cases showing widespread CPE revealed reovirus-like particles when examined by negative contrast electron microscopy. Spherical–polyhedral virions were seen, measuring 77 to 81 nm in diameter. The electron-dense virion nucleus was surrounded by a moderately electron-dense outer ring.
The different psittacine reovirus isolates (Cases 3 to 6) and chicken reovirus strains were characterized using the different mAbs (). Data for reovirus chicken strains that have been described in the literature are also shown. The chicken reovirus isolates showed a distinct reaction pattern with the mAbs. However, the psittacine reovirus reacted only with the polyclonal reovirus-antiserum, not with any of the mAbs. The reaction pattern of the reovirus isolates found in budgerigars in the UK was identical to the reaction pattern of the reovirus isolates from different psittacine species in The Netherlands.
Table 1. Identification by means of immunofluorescence of different recognized chicken reovirus strains compared with Cases 3 to 6 described in this study, as well as with reovirus isolates from budgerigars in the UK
Discussion
The outbreak of disease in psittaciformes in The Netherlands showed a high mortality in all age groups, both from large flocks and from individual pet birds. Therefore an infectious disease agent was suggested, such as C. psittaci or a virus, possibly Pacheco's disease, polyomavirus, circovirus or paramyxovirus. The histology, IFT, ABC and PCR were all negative for these agents, except for two birds that were positive for PBFD. The course of this disease was suggestive of a highly pathogenic virus. The multifocal necrosis and variable mononuclear infiltrates in the liver and spleen were consistent with reovirus infections. Immunohistochemistry detected reovirus antigen in mononuclear cells within affected areas, whereas electron microscopy on infected BHK cell cultures showed viral particles consistent with reovirus.
At the start of this outbreak, the disease seemed to affect only parakeets, but later larger psittacines such as Eclectus parrots and Amazons were also affected. Because reoviruses were seen consistently, they seemed to be the most probable cause of the outbreak. Although reovirus infections are found in different psittacine species, it is said that Old World species such as the African grey parrot (P. e. erithacus), are more sensitive, while New World species are less affected and show more resistance (Conzo et al., Citation2001; Smidt et al., Citation2003; Manvell et al., Citation2004). In this report, both New World and Old World species showed high mortality, while Old World species were affected twice as often. Within aviaries both New World and Old World species became affected, suggestive of a disease that affects all psittaciformes regardless of the assumed higher resistance of certain species. It is possible that the virus changed over the period of time (antigenic variation) so that it can infect other species as well (Vasserman et al., Citation2004). There is a range of strains of avian reovirus with different antigenicity that cause different disorders in poultry (Hsu et al., Citation2005). Also one cannot exclude the possibility that the simultaneous presence of more than one reovirus strain could show different levels of pathogenicity for different species (Conzo et al., Citation2001). Characterization of psittacine reovirus isolates with a panel of mAbs revealed that these strains showed a distinct pattern compared with reovirus strains commonly isolated from chickens. Interestingly, the pattern of the reovirus isolated from a case of high mortality in budgerigars in UK in 2003 (unpublished data) was identical to that of reoviruses from the Dutch outbreaks in psittacine cases during 2002 to 2004.
The exact route of transmission of reovirus in psittaciformes is still unclear. In chickens the transmission is primarily by the horizontal routes, through direct or indirect contact with contaminated faeces (Jones et al., Citation1989) and less by vertical transmission (Mendenez et al., Citation1975). Outbreaks of reovirus infection are reported to have been complicated by the presence of a variety of concurrent infections, including salmonellosis, aspergillosis and avian paramyxovirus 3 (Clubb et al., Citation1985; Ritchie & Carter, Citation1995; Smidt et al., Citation2003). Reoviruses are suggested to have an immunosuppressive activity, which is most probably caused by lymphodepletion mediated by factors released by macrophages (Sharma et al., Citation1994; Pertile et al., Citation1996; Sanchez-Cordon et al., Citation2002). This immunosuppression could facilitate the growth of bacteria and parasites and allow other viruses to co-infect the bird. The concurrent infections as seen in the birds were bacterial, parasitic, yeast-like and mycotic infections. The M. ornithogaster found in the cytology is often seen in our department in birds with immunosuppression.
The two birds that were positive by PCR for circovirus showed no clinical signs of PBFD, indicating that this infection was unlikely to be the cause of the lesions found. Few birds showed haemorrhagic contents in the gastrointestinal tract suggestive for haemorrhagic diathesis due to anorexia for a longer period of time.
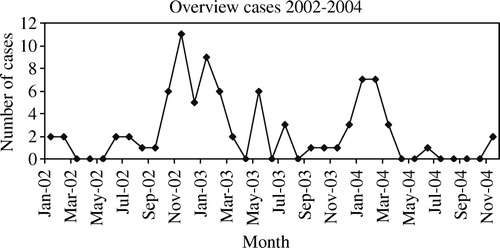
Viral infections as well as stress factors are thought to be an important cause of mortality in birds in quarantine (Meulemans et al., Citation1983; Graham, Citation1987). Almost one-half (39 out of 84) of the birds examined in this study had a known history of introduction of new birds. It is very difficult to determine the influence of stress factors, which could include attending bird shows or environmental changes within the flocks. The occurrence of the high mortality was more often seen during the colder months (October until March) and less in late spring, summer and autumn (). Since many of these birds were kept outside, an explanation could be that keeping these exotic and tropical birds in outside aviaries in the temperate climate of The Netherlands could induce stress. Temperature stress is known to increase the susceptibility to infectious diseases (Beard & Mitchell, Citation1987). In a few of the presented cases, translocation of birds to other cages is suggested to be a factor that could cause stress, and therefore induce an immunosuppression.
Figure 7. The number of cases received for necropsy at the Pathology Department of Utrecht University per month in 2002 to 2004, showing a higher frequency between October and March.
The fact that reovirus was found in cases from all over The Netherlands and that comparable reovirus infections in budgerigars occurred in the UK suggests that the virus may even be present in more countries. There seems to be no geographical preference, but the disease could be spread by migrating wild birds, attending bird-shows and visiting bird markets, and by the introduction of new birds into existing collections. Clinically affected birds that recovered from the disease may be carriers and thus could be a source of further outbreaks (Senne et al., Citation1983; Jones, Citation2000).
In poultry, serology for detection of reovirus is of little value because of the prevalence and the frequency of subclinical infections, as well as the antigenic variation of different virus strains (Vasserman et al., Citation2004). In psittacine birds it is expected that reovirus infections are less common, and a diagnosis may be possible in live birds by demonstrating precipitating or virus-neutralizing antibodies in animals with suggestive signs. Enzyme-linked immunosorbent assays are commonly used for this purpose in chickens and it would be useful to develop a tool to diagnose reovirus infections clinically in psittaciformes (Liu et al., Citation2002).
Reovirus vaccines that are used in chickens were found to be of little value for the Psittaciformes in earlier infections because the virus strains commonly found in these species are antigenically unrelated to those found in poultry (Clubb et al., Citation1985; Gaskin, Citation1989). However, reduction in morbidity and mortality has been seen in African grey parrots and cockatoos using an experimental inactivated vaccine produced from a psittacines reovirus isolate (Clubb et al., Citation1985). Vasserman et al. (Citation2004) suggest that vaccines in poultry should be adjusted to the changes in the field strains of reovirus, and that the main field strains of that area should be determined using RT-PCR and sequencing. In psittacine birds, such adjustments could be made to produce a correct vaccine.
This study reports the results of an extensive examination of numerous psittaciformes that died showing severe splenomegaly and hepatomegaly with multifocal acute necrosis. In all cases the presence of a reovirus was revealed, indicating that this virus could be the cause of the high mortality observed. Moreover, this virus was shown to be specific for psittacines, as it did not react with mAbs against chicken reovirus.
The assumption that such specific psittacine reovirus is responsible for high mortality is highly plausible and could perhaps be demonstrated by experimental infection of a limited number of small psittacines. Also, much is still unclear regarding the epidemiology and pathogenicity of reovirus infections in psittacines. Therefore, specific PCRs need to be developed to screen psittacines flocks (healthy flocks and known infected flocks) for the presence of psittacines reovirus. Because psittacines, specified pathogen free for reovirus, are not available, the developing a reliable screening method for the detection of antibodies against reovirus is essential to perform a good infection model.
Acknowledgements
The authors wish to thank Ruby Wagensveld for collecting the data, the technical staff of the Department of Pathobiology for their assistance with the immunohistochemistry, and Dr Jaspers from Intervet for providing the antibody.
References
- Ashton , W.L.G. , Randall , C.J. , Dagless , M.D. and Eaton , T.M. 1984 . Suspected reovirus-associated hepatitis in parrots . Veterinary Record , 114 : 476 – 477 .
- Beard , C.W. and Mitchell , B.W. 1987 . Influence of environmental temperatures on the serological responses of broiler chickens to inactivated and viable Newcastle disease vaccines . Avian Diseases , 31 : 321 – 326 .
- Clubb , S.L. , Gaskin , J.M. & Jacobson E.R. ( 1985 ). Psittacine reoviruses. An update including a clinical description and vaccination . Proceedings of the Association of Avian Veterinarians 1985 Annual Conference (pp. 83 – 90 ).
- Conzo , G. , Magnino , S. , Sironi , G. , Lavazza , A. , Vigo , P.G. , Fioretti , A. and Kaleta , E.F. 2001 . Reovirus infection in two species of Psittaciformes recently imported into Italy . Avian Pathology , 30 : 43 – 47 .
- Gaskin , J.M. ( 1987 ). Considerations in the diagnosis and control of psittacine viral infections . Proceedings of the Association of Avian Veterinarians 1987 Annual Conference (pp. 1 – 14 ).
- Gaskin , J.M. 1989 . Psittacine viral disease: a perspective . Journal of Zoo and Wildlife Medicine , 20 : 249 – 264 .
- Graham , D.L. 1987 . Characterization of a reo-like virus and its isolation from and pathogenicity for parrots . Avian Diseases , 31 : 411 – 419 .
- Hsu , H.W. , Su , H.Y. , Huang , P.H. , Lee , L.H. and Liu , H.J. 2005 . Sequence and phylogenetic analysis of P10- and P17-encoding genes of avian reovirus . Avian Diseases , 49 : 36 – 42 .
- Jones , R.C. 2000 . Avian reovirus infections . Review Scientifique et technique—Office Internationales Epizootics , 19 : 614 – 625 .
- Jones , R.C. , Islam , M.R. and Kelly , D.F. 1989 . Early pathogenesis of experimental reovirus infection in chickens . Avian Pathology , 18 : 239 – 253 .
- Liu , H.J. , Kuo , L.C. , Liao , M.H. and Lien , Y.Y. 2002 . Development of an ELISA for detection of antibodies to avian reovirus in chickens . Journal of Virological Methods , 102 : 129 – 138 .
- Manvell , R. , Gough , D. , Major , N. and Fouchier , R.A.M. 2004 . Mortality in budgerigars associated with a reovirus-like agent . Veterinary Record , 154 : 539 – 540 .
- Mendenez , N.A. , Calnek , B.W. and Cowen , B.S. 1975 . Experimental egg-transmission of avian reovirus . Avian Diseases , 19 : 104 – 111 .
- Meulemans , G. , Dekegel , D. , Charlier , G. , Froyman , R. , Van Tilburg , J. and Halen , P. 1983 . Isolation of orthoreoviruses from psittacines birds . Journal of Comparative Pathology , 93 : 127 – 134 .
- Pennycott , T. 2004 . Mortality on budgerigars in Scotland: pathological findings . Veterinary Record , 154 : 538 – 539 .
- Pertile , T.L. , Karaca , K. , Walser , M.M. and Sharma , J.M. 1996 . Suppressor macrophages mediate depressed lymphoproliferation in chickens infected with avian reovirus . Veterinary Immunology and Immunopathology , 53 : 129 – 145 .
- Ritchie , B.W. and Carter , K. 1995 . “ Reoviridae ” . In Avian Viruses: Function and Control , Edited by: Ritchie , B.W. 335 – 350 . Lake Worth , FL : Wingers Publishing Inc .
- Robertson , M.D. , Wilcox , G.E. and Kibenge , F.S.B. 1984 . Prevalence of reoviruses in commercial chickens . Australian Veterinary Journal , 61 : 319 – 322 .
- Sanchez-Cordon , P.J. , Hervas , J. , Chacon de Lara , F. , Jahn , J. , Salguero , F.J. and Gomez-Villamandos , J.C. 2002 . Reovirus infection in Psittacine birds (Psittacus erithacus): morphologic and immunohistochemical study . Avian Diseases , 46 : 485 – 492 .
- Senne , D.A. , Pearson , J.E. , Miller , L.D. and Gustafson , G.A. 1983 . Virus isolations from pet birds submitted for importation into the United States . Avian Diseases , 27 : 731 – 744 .
- Sharma , J.M. , Karaca , K. and Pertile . 1994 . Virus-induced immunosuppression in chickens . Poultry Science , 73 : 1082 – 1086 .
- Simoni , I.C. , Bittencourt Fernandes , M.J. , Custodio , R.M. , Backx Noronha Madeira , A.M. and Weis Arns , C. 1999 . Susceptibility of cell lines to avian viruses . Revista de Microbiologia , 30 : 373 – 376 .
- Smidt , R.E. , Reavill , D.R. and Phalen , D.N. 2003 . Pathology of pet and aviary birds , 1st edn , 74 Ames : Iowa State Press .
- Tang , K.N. , Fletcher , O.J. and Villegas , P. 1987 . Comparative study of the pathogenicity of avian reoviruses . Avian Diseases , 31 : 577 – 583 .
- van de Zande , S. and Kuhn , E.-M. 2007 . Central nervous system signs in chickens caused by a new avian reovirus strain: a pathogenesis study . Veterinary Microbiology , 120 : 42 – 49 .
- van Loon , A.A.W.M. , Koopman , H.C. , Kosman , W. , Mumczur , J. , Szeleszcuk , O. , Karpinska , E. , Kosowska , G. and Lütticken , D. 2001 . Isolation of a new serotype of avian reovirus associated with malabsorption syndrome in chickens . Veterinary Quarterly , 23 : 129 – 133 .
- Vasserman , Y. , Eliahoo , D. , Hemsani , E. , Kass , N. , Ayali , G. , Pokamunski , S. and Pitcovski , J. 2004 . The influence of reovirus Sigma C protein diversity on vaccination efficiency . Avian Diseases , 48 : 271 – 278 .
- Wilson , R.B. , Holscher , M. , Hodges , J.R. and Thomas , S. 1985 . Necrotizing hepatitis associated with a reo-like virus infection in a parrot . Avian Diseases , 29 : 568 – 571 .