Abstract
Histomonas meleagridis is a protozoan parasite causing histomoniasis (histomonosis), a disease of gallinaceous fowl. In order to determine whether the lesser mealworm, Alphitobius diaperinus, is capable of harbouring H. meleagridis, the presence of the parasite was tested by polymerase chain reaction in lesser mealworms collected in the field. Parasite DNA was detected in two larvae from two farms undergoing an outbreak of histomoniasis. Insects were also artificially infected, killed after incubation and analysed by polymerase chain reaction for the presence of parasite DNA. After 4 days, two larvae (out of 20) remained positive. In another experimental infection to investigate the viability of histomonads in the larvae, living parasites were detected in five of 20 larvae 4 days after infection. These results indicate that although A. diaperinus can become infected with H. meleagridis it appears to have a low susceptibility to infection and would probably not be a major route of contamination between flocks.
Etude préliminaire de l'infection naturelle et expérimentale du petit ténébrion, Alphitobius diaperinus (Coleoptera: Tenebionidae) par Histomonas meleagridis (Protozoa: Sarcomastigophora)
Histomonas meleagridis est un protozoaire parasite responsable de l'histomonose, une maladie affectant les galliformes. Afin de déterminer si Alphitobius diaperinus est capable de porter H. meleagridis, la présence du parasite a été recherchée par la réaction de polymérisation en chaîne (PCR) chez des petits ténébrions collectés sur le terrain. De l'ADN parasitaire a été détecté dans deux larves provenant d’élevages subissant des épisodes d'histomonose. Des insectes ont également été infectés artificiellement, sacrifiés après une période d'incubation puis analysés par PCR pour détecter la présence d'ADN parasitaire. Après 4 jours, deux larves (sur 20) étaient encore positives. Une autre expérience d'infection expérimentale a été menée dans le but de vérifier la viabilité d’H. meleagridis chez la larve. Des parasites vivants ont été détectés dans 5 larves sur 20, quatre jours après infection. Ces résultats montrent que bien qu’A. diaperinus puisse porter H. meleagridis, il semble qu'il ait une faible capacité à s'infecter et ne constituera pas une voie majeure de contamination entre bandes.
Vorläufige Studie zur natürlichen und experimentellen Infektion des glänzendschwarzen Getreideschimmelkäfers, Alphitobius diaperinus, (Coleoptera: Tenebrionidae) mit Histomonas meleagridis (Protozoa: Sarcomastigophora)
Histomonas meleagridis ist ein protozoärer Parasit, der die Histomonadose, eine Krankheit bei Hühnervögeln, verursacht. Um zu ermitteln, ob der glänzendschwarze Getreideschimmelkäfer, Alphitobius diaperinus, Histomonas meleagridis beherbergen kann, wurden mittels Polymerasekettenreaktion (PCR) im Feld gesammelte glänzendschwarze Getreideschimmelkäfer auf das Vorhandensein des Parasiten getestet. In zwei Larven aus zwei Farmen mit Histomonadose-Ausbrüchen wurde die parasitäre DNS nachgewiesen. Außerdem wurden Käfer künstlich infiziert, nach Inkubation getötet und mit PCR auf das Vorhandensein von parasitärer DNS getestet. Nach 4 Tagen waren noch zwei von 20 Larven positiv. In einem anderen Experiment zur Untersuchung der Lebensfähigkeit von Histomonaden in den Larven wurden lebende Parasiten 4 Tage nach der Infektion in fünf von 20 Larven entdeckt. Diese Ergebnisse weisen darauf hin, dass, obwohl A. diaperinus sich mit H. meleagridis infizieren kann, die Empfänglichkeit nicht sehr hoch zu sein scheint und er wahrscheinlich nicht die Hauptursache für eine Übertragung zwischen Herden ist.
Estudio preliminar de la infección natural y experimental de larvas de Alphitobius diaperinus (Coleóptera: Tenebrionidae) con Histomonas meleagridis (Protozoa: Sarcomastigophora)
Histomonas meleagridis es un parásito protozoario causante de la histomoniasis, una enfermedad de aves gallináceas. Con el objetivo de determinar si la larva, Alphitobius diaperinus, es capaz de transmitir H. meleagridis, se evaluó la presencia del parásito mediante la reacción en cadena de la polimerasa (PCR) en larvas obtenidas en el campo. El DNA del parásito se detectó en dos larvas de dos granjas que sufrían un brote de histomoniasis. Los insectos también se infectaron experimentalmente, se sacrificaron tras la incubación y se analizaron mediante PCR para determinar la presencia del DNA del parásito. Tras 4 días, dos larvas (de 20) seguían siendo positivas. En otra infección experimental para investigar la viabilidad de las histomonas en las larvas, se detectaron parásitos vivos en 5 de 20 larvas a los 4 días post infección. Estos resultados indican que aunque A. diaperinus puede infectarse con H. meleagridis parece tener una menor susceptibilidad a la infección y probablemente no se trata de una de las principales fuentes de contaminación entre lotes.
Introduction
Histomonas meleagridis is a protozoan parasite causing histomoniasis (histomonosis), a disease of poultry leading to high mortality especially in turkeys. Although the infection results mainly from ingestion of eggs of caecal worms (Heterakis gallinarum), it has been shown that H. meleagridis was present in some farms in which its presence was dissociated from that of the nematode (Chossat, Citation2002), suggesting an alternative source of contamination. Lateral transmission was demonstrated in turkeys by Hu & McDougald (Citation2003) in the absence of worms and arthropods, and the authors speculated that ‘The only opportunity for the birds to become infected was from usual litter pecking and coprophagy and possibly from contamination of the feed by infected pen mates’. However, alternative means of contamination should also be explored.
The lesser mealworm (also called the darkling beetle), Alphitobus diaperinus (Panzer), is often present in very large numbers in poultry units. It readily feeds on diverse sources of nutrients ranging from chicken feed to poultry faeces and dead chickens (Kumar, Citation1986). During this feeding, the beetle may become contaminated with several pathogenic agents. It has been reported to harbour viruses (McAllister et al., Citation1995; De Las Casas et al., Citation1976), bacteria (De Las Casas et al., Citation1972; McAllister et al., Citation1996), fungi (De Las Casas et al., Citation1972) and also parasite tapeworms, in which a true host relationship is established (Elowni & Elbihari, Citation1979; Espaine & Jurasek, Citation1971). It has been shown that it could play a role in the transmission of protozoa; that is, Eimeria spp. (Reyna et al., Citation1983; Goodwin & Waltman, Citation1996).
In order to determine whether A. diaperinus is able to harbour H. meleagridis, adults and larvae collected in the field were examined for the presence of the parasite. Insects were also artificially infected and analysed by the polymerase chain reaction (PCR) for the presence of parasite DNA. Another experimental infection was performed to investigate the viability of histomonads in the insects.
Materials and Methods
DNA amplification
An individual insect was crushed in 200 µl extraction buffer (2% cethyl trimethyl ammonium bromide, 1.4 M NaCl, 20 mM ethylenediamine tetraacetic acid, 100 mM Tris–HCl, pH 8.0, 0.2% 2β-mercaptoethanol) and incubated at 65°C for 1 h. Proteins were removed with one volume of chloroform. DNA was precipitated with one volume of isopropanol, the pellet was washed with ethanol (70% v/v) and resuspended in 50 µl distilled water. The PCR amplified a DNA target of 209 base pairs (bp) of the small subunit ribosomal DNA sequence and was performed as described by Huber et al. (Citation2005a). Two microlitres of DNA were used as the template in the PCR, which had a final volume of 12.5 µl and contained 1x buffer (Invitrogen, Carlsbad, California, USA), 1.5 mM MgCl2, 60 µM each dNTP (Amersham Biosciences, Buckinghamshire, UK), 5 pmol each primer (HIS5F, 5′-CCTTTAGATGCTCTGGGCTG-3′; and HIS5R, 5′-CAGGGACGTATTCAACGTG-3′) and 0.25 U Taq polymerase (Invitrogen). They were performed in a TGradient thermocycler (Biometra, Goettingen, Germany). The reaction conditions were 5 min at 96°C, 40 cycles each of 1 min at 95°C, 1 min at 59°C and 1 min at 72°C, with a final extension period of 5 min at 72°C. DNA fragments were separated on 3% (w/v) agarose gels and visualized by ultraviolet illumination of ethidium bromide stained DNA.
The sensitivity of the PCR for adult and larval beetles was assessed using artificially infected materials. A known number of H. meleagridis obtained by serial dilutions in order to have concentrations ranging from 1×103 to 1×10–1 parasites per beetle were added to single insects (adults and larvae) coming from an uninfected farm. These samples were subjected to DNA extraction and PCR as described above.
Field samples
Adults and larvae were collected from 14 farms from France between years 2003 and 2005 () representing three types of infection status: (1) four farms free of histomoniasis; (2) five farms having undergone an outbreak of histomoniasis in previous flocks; (3) five farms undergoing an outbreak of histomoniasis. Samples were stored at −20°C.
Table 1. PCR analysis of A. diaperinus collected from poultry farms between January 2003 and May 2005
Experimental infections
The suspension of H. meleagridis used to prepare the inocula was obtained from the HmP-12 stain (Callait et al., Citation2002). Lesser mealworms were obtained from a laboratory colony started in 2004 from individuals originating on a farm that was free of histomoniasis. They were maintained in plastic boxes at room temperature and fed with commercial chicken feed (Agri Sud Est, Vienne, France). Water-saturated cotton was placed into the boxes to provide humidity and water.
Six groups of 10 adults and six groups of 10 larvae ranging in length from 3 to 12 mm from the seventh and eighth generation were placed in Petri dishes. The beetles were starved for 24 h, after which time absorbent cotton soaked with 7 ml cultured H. meleagridis (6×105 parasites per ml, HmP-12 strain) was placed in the dishes of four groups of adults and four groups of larvae. The cotton for the remaining larvae and adult groups was soaked with Stepkowski media (Stepkowski & Klimont, Citation1979) as a negative control. After 24 h, two infected groups and one control group of adults and two infected groups and one control group of larvae exposed to H. meleagridis were removed and frozen at –20°C. The remaining lesser mealworms were removed from the Petri dishes and placed in other sterile dishes that contained sterile chicken feed and cotton soaked with sterile deionized water. Four days after the infection, they too were frozen at −20°C.
One group of larvae and one group of adults frozen after 24 h and after 4 days were individually placed in 1 ml paraformaldehyde 4%, agitated and incubated for 7 min. This step was repeated. Then they were rinsed in 1 ml distilled water. All samples were subjected to DNA extraction and PCR as described above.
In order to test the parasite viability in the larvae, six groups of 10 larvae were infected as described previously. After 24 h, two infected groups and one control group of larvae exposed to H. meleagridis were removed and treated. Individual larvae were crushed aseptically in 50 µl Stepkowski media (Stepkowski & Klimont, Citation1979) at 39°C and then transferred to a flat-bottom glass tube containing 500 µl Stepkowski media. Tubes were vortexed and then incubated at 39°C. Samples of 15 µl were taken daily for 8 days from the bottom of the tube, and examined microscopically (magnification x400) to detect live histomonads. Every day, 300 µl supernatant were removed and replaced by fresh media and then the tubes were vortexed.
The remaining larvae were removed from the Petri dishes and placed in sterile dishes that contained sterile chicken feed and cotton soaked with sterile deionized water. Four days after the infection, they were treated as described above.
One group of larvae treated at 24 h and one group treated at 4 days were washed with paraformaldehyde as described above.
Results and Discussion
The sensitivity of the PCR reaction evaluated was found to be one parasite per adult beetle and 10 per larvae (). Field samples from farms free of histomoniasis or having undergone an outbreak of histomoniasis in previous flocks were all negative. Adult beetles coming from farms actually undergoing an outbreak of histomoniasis were also all negative, but parasite DNA was detected in two larvae from two of these farms ().
Figure 1. Sensitivity of the PCR for the detection of H. meleagridis in A. diaperinus samples. Adult (1a) and larvae (1b) were artificially infected to obtain concentrations of 103 H. meleagridis per beetle (lane 1), 102 parasites per beetle (lane 2), 10 parasites per beetle (lane 3), 1 parasite per beetle (lane 4), 10−1 parasites per beetle (lane 5), positive control for H. meleagridis (lane 6), negative control (lane 7) and a 100 bp DNA ladder (lane 8).
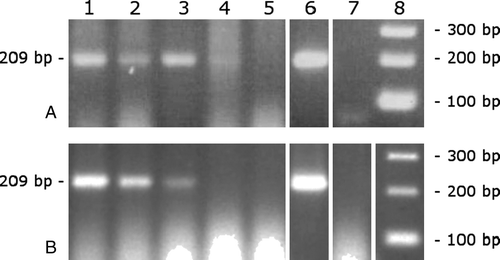
In order to examine the ability of the A. diaperinus to become infected, an experimental infection was performed (). All the control groups were negative and the parasite DNA was never detected in the adults. However, 24 h after infection the parasite DNA was detected in five larvae (out of 20), one from the unwashed group and four from the washed group. After 4 days two larvae remained positive (out of 20), one in the unwashed group and one in the washed group.
Table 2. Number of positive A. diaperinus (adults and larvae) detected by PCR and number of A. diaperinus larvae carrying viable histomonads, 24 h and 4 days after experimental infection with H. meleagridis
To investigate the viability of histomonads in the larvae, another experimental infection was performed (). Twenty-four hours after infection, living parasites were detected in four larvae (out of 20), two from the unwashed group and two from the washed group. After 4 days five larvae remained positive (out of 20), four in the unwashed group and one in the washed group.
These results show that A. diaperinus has a low susceptibility to infection. The histomonas DNA was not detected in adult beetles whereas it was detected in larvae. Adult and larvae have different feeding habits. Adults are essentially mycophagous but larvae tend to feed more frequently on dead chicken and poultry faeces (Huber et al., Citation2005b). In H. meleagridis infection of turkeys, parasites are excreted in caecal droppings during the acute phase of the disease (Huber et al., Citation2006), allowing the larvae to become contaminated. However, since histomonas DNA was not detected in adult beetles, the chance of this insect spreading the parasite within and among farms is probably low under normal circumstances.
Living parasites were observed in larvae 4 days after infection, demonstrating the ability of this insect to maintain the contamination for several days. It is necessary to determine exactly how long the larvae can remain contaminated in order to assess the relative importance of A. diaperinus as a biological source of contamination.
A. diaperinus may be only a mechanical vector of the parasite. Mechanical transmission of a pathogen in insects may occur in two forms. The agent may be transported on the cuticle of the insect, or may be ingested and retained in a viable state as it passes through the insect gut. After the treatment of the external teguments of A. diaperinus, the PCR and the culture of some individuals remained positive, indicating that the parasite is not on the tegument but inside the larvae—but the next step will be to verify whether contaminated lesser mealworms are able to infect turkeys.
This study has shown that A. diaperinus is unlikely to be an important vector for H. meleagridis but that it could provide an accidental means of residual environmental contamination. An increase in A. diaperinus density could also increase the chance of contact between pathogen and host so a proper poultry management system to control beetle infestation is essential to reduce the risk of accidental transmission.
Acknowledgements
The authors wish to thank Lise Roy and Arnaud Aymonin-Glorieux for their technical assistance, Loïc Balaine, Joël Bertin, Xavier Chatenet, Laurent Deffreix, Julien Flori, Maud Guimiot, Philippe Marie, Isabelle Petetin, Jacques Roberton and Benoit Thuillier for providing insect samples, Claude Chauve for useful suggestions on the manuscript, and Richard Sullivan for English-language corrections.
References
- Callait , M.-P. , Granier , C. , Chauve , C. and Zenner , L. 2002 . In vitro activity of therapeutic drugs against Histomonas meleagridis (Smith, 1895) . Poultry Science , 81 : 1122 – 1127 .
- Chossat , L. ( 2002 ). L'histomonose en production AOC ‘Dinde Fermière de Bresse’. Essai de prevention par phytothérapie ( 96 pp ). Veterinary Thesis, Université Claude Bernard, Lyon.
- De Las Casas , E. , Harien , P.K. and Pomeroy , B.S. 1972 . Bacteria and fungi within the lesser mealworm collected from poultry brooder houses . Entomops , 1 : 27 – 30 .
- De Las Casas , E. , Harein , P.K. , DeshMuck , D.R. and Pomeroy , B.S. 1976 . Relationship between the lesser mealworm, fowl pox and Newcastle disease in poultry . Journal of Economic Entomology , 69 : 775 – 779 .
- Elowni , E.E. and Elbihari , S. 1979 . Natural and experimental infection of the beetle Alphitobius diaperinus (Coleoptera: Tenebrionidae) with Choanotaenia infundibulum and other chicken tapeworms . Veterinary Science Communications , 3 : 71 – 173 .
- Espaine , L. and Jurasek , V. 1971 . Primer hallazgo de los cysticercoides de Raillietina cesticillus (Molin, 1858) en los coleopteros Carcinops troglodytes, en las condiciones naturales de la crianza de gallinas . Revista Cubana de Ciencias Veterinarias , 2 : 217 – 222 .
- Goodwin , M.A. and Waltman , W.D. 1996 . Transmission of Eimeria, viruses, and bacteria to chicks: darkling beetles (Alphitobius diaperinus) as vectors of pathogens . Journal of Applied Poultry Research , 5 : 51 – 55 .
- Hu , J. and McDougald , L.R. 2003 . Direct lateral transmission of Histomonas meleagridis in turkeys . Avian Diseases , 47 : 489 – 492 .
- Huber , K. , Chauve , C. and Zenner , L. 2005a . Detection of Histomonas meleagridis in turkeys caecal droppings by PCR amplification of the small subunit ribosomal DNA sequence . Veterinary Parasitology , 131 : 311 – 316 .
- Huber , K. , Gouilloud , L. and Zenner , L. 2005b . L'implication du petit ténébrion (Alphitobius diaperinus) dans la transmission d'agents pathogènes . Bulletin des GTV , 31 : 17 – 21 .
- Huber , K. , Reynaud , M.-C. , Callait , M.-P. and Zenner , L. 2006 . Histomonas meleagridis in turkeys: dissemination kinetics in the host tissues after cloacal infection . Poultry Science , 85 : 1008 – 1014 .
- Kumar , P. 1986 . Flesh eating behaviour of Alphitobius diaperinus Panz. (Tenebrionidae: Coleoptera) . Indian Journal of Entomology , 48 : 113 – 115 .
- McAllister , J.C. , Steelman , C.D. , Newberry , L.A. and Skeeles , J.K. 1995 . Isolation of infectious bursal disease virus from the lesser mealworm, Alphitobius diaperinus (Panzer) . Poultry Science , 74 : 45 – 49 .
- McAllister , J.C. , Steelman , C.D. , Skeeles , J.K. , Newberry , L.A. and Gbur , E.E. 1996 . Reservoir competence of Alphitobius diaperinus (Coleoptera: Tenebrionidae) for Escherichia coli (Eubacteriales: Enterobacteriaceae) . Journal of Medical Entomology , 33 : 983 – 987 .
- Reyna , P.S. , McDougald , L.R. and Mathis , G.F. 1983 . Survival of coccidia in poultry litter and reservoirs of infection . Avian Diseases , 27 : 464 – 473 .
- Stepkowski , S. and Klimont , S. 1979 . Obserwacje nad hodowla in vitro Histomonas meleagridis (Smith, 1895) . Medycyna Weterynaryjna , 35 : 502 – 505 .