Abstract
The poultry red mite (Dermanyssus gallinae) is the most important and common ectoparasite of laying hens in Europe. This haematophagous mite has been experimentally demonstrated to be a vector of Salmonella Enteritidis by acquiring bacteria through the blood meal or cuticular contact. We have evaluated another route of infection by orally inoculating chicks with mites previously infected by S. Enteritidis. Two methods of infecting the mites were tested: mites contaminated by cuticular contact or during the blood meal. After the washing of mites with paraformaldehyde, groups of 10 Salmonella-contaminated mites were inoculated individually into 1-day-old chicks. The titre of the inoculum suspension was evaluated by crushing mites and followed by bacteriological counting. It was 3×104 colony-forming units/chick and 2.7×106 colony-forming units/chick, respectively, for cuticular contact and orally mediated contamination of mites. Each bird was found to be positive 12 days post-inoculation. Salmonella colonized the intestinal tracts and invaded the livers and spleens. The caecal content concentration reached a mean level of S. Enteritidis of 8.5×104 most probable number (MPN) Salmonella/g. This experiment demonstrated the ability of mites to orally infect 1-day-old chicks with subsequent colonization and multiplication of Salmonella. Consequently, mites infected by S. Enteritidis constitute potential reservoir hosts of this bacterium, allowing it to persist in the poultry house as a source of infection for newly introduced animals. If contaminated mites are found in poultry facilities, effective red mite control should be performed before new batches are introduced into the facility.
Colonisation et invasion de poussins après inoculation expérimentale par des Dermanyssus gallinae contaminés par Salmonella Enteritidis
Les poux rouges, Dermanyssus gallinae, sont les plus représentés des ectoparasites chez les poules pondeuses en Europe. Cet arthropode hématophage s'est révélé porteur de Salmonella Enteritidis après l'ingestion de la bactérie ou par contamination trans-cuticulaire. Dans notre étude, la capacité de ces Dermanyssus contaminés de transmettre Salmonella Enteritidis à des poussins a été testée. Les deux modes de contamination des parasites ont été employés: par contact cuticulaire ou après ingestion de sang contaminé. Après décontamination des Salmonella en surface par un lavage à la paraformaldehyde, des inoculums de 10 Dermanyssus contaminés par salmonelle ont été administrés par voie orale à des poussins de 1 jour. L'inoculum, après broyage et homogénéisation contenait 3,0.104 UFC et 2,7.106 UFC/poussin respectivement pour les voies trans-cuticulaire et ingestion. Tous les poussins se sont révélés contaminés par S. Enteritidis au moment de l'autopsie réalisée 12 jours post inoculation. S Enteritidis colonisait le tractus digestif et les organes (foies et rates) de tous les oiseaux. La contamination caecale a été relevée à des niveaux de 8,5.10−4 Salmonella /g de contenu (évaluation par une technique NPP). Cette étude démontre la capacité de Dermanyssus contaminés par Salmonella de transmettre la bactérie à des poussins de 1 jour, et de permettre grâce à une multiplication dans les oiseaux, la colonisation par Salmonella. En conséquence ces arthropodes infectés par S. Enteritidis constitueraient un réservoir potentiel de cette bactérie, lui permettant de persister dans un bâtiment et de contaminer la bande suivante. Si la présence de Dermanyssus contaminés par Salmonella est confirmée en élevage de poules pondeuses, une maîtrise de ces parasites doit être atteinte avant le rechargement d'un bâtiment par un nouveau lot de poulette.
Kolonisation und Organinvasion bei Hühnern nach experimenteller Infektion mit Salmonella Enteritidis kontaminierten Dermannyssus gallinae
Die rote Vogelmilbe, Dermanyssus gallinae ist der bedeutendste und häufigste Ektoparasit bei Legehennen in Europa. Experimentell konnte nachgewiesen werden, dass diese blutsaugende Milbe ein Vektor für Salmonella Enteritidis ist, wobei die Bakterien mittels Blutmahlzeit oder Kutikulakontakt übertragen werden. Wir haben eine anderen Infektionsweg untersucht, bei dem Hühnerküken oral mit S. Enteritidis infizierten Milben inokuliert wurden. Dabei wurden zwei Infektionsmethoden für die Milben getestet: Kontamination mittels Kutikulakontakt oder während der Blutmahlzeit. Nach Waschung der Milben mit Paraformaldehyd wurden Gruppen von 10 Salmonellen-kontaminierten Milben individuell in Eintagsküken inokuliert. Der Titer der Inokulationssuspensionen wurde nach Zerreiben der Milben durch Bakterienzählung ermittelt. Sie enthielten nach Kontamination durch Kutikulakontakt 3×104 bzw. nach Blutmahlzeit 2,7×106 Kolonie bildende Einheiten (CFU). Alle inokulierten Küken waren bis 12 Tage nach der Inokulation positiv. Die Salmonellen besiedelten den Darmtrakt und drangen auch in Leber und Milz ein. Die zäkale S. Enteritidis-Konzentration erreichte im Mittel eine Anzahl von 8,5×104 MPN Salmonellen g-1. Dieses Experiment belegt die Fähigkeit von Milben, nach oraler Aufnahme durch Hühnerküken Salmonellen mit nachfolgender Kolonisation und Vermehrung zu übertragen. Dies bedeutet, dass mit S. Enteritidis infizierte Milben ein potenzielles Erregerreservoir für dieses Bakterium darstellen, was ihm erlaubt, im Geflügelstall als Infektionsquelle für neu eingestallte Tiere zu persistieren. Beim Nachweis von kontaminierten Milben in Geflügelhaltungen sollte vor der Neubelegung eine effektive Milbenbekämpfung durchgeführt werden.
Colonización e invasión de órganos en pollos infectados experimentalmente con Dermanyssus gallinae contaminado por Salmonella Enteritidis
El ácaro rojo de las aves, Dermanyssus gallinae es el ectoparásito más importante y común de las gallinas de puesta en Europa. Se ha demostrado experimentalmente que este ácaro hematófago es un vector de Salmonella Enteritidis adquiriendo la bacteria a través de la ingestión de sangre o del contacto cuticular. Hemos evaluado otra ruta de infección mediante la inoculación oral de pollos con ácaros previamente infectados con S. Enteritidis. Se evaluaron dos métodos para la infección de los ácaros: ácaros contaminados por contacto cuticular o durante la ingestión de sangre. Tras el lavado de los ácaros con Paraformaldehído, grupos de 10 ácaros contaminados con Samonella se inocularon individualmente en pollitos de 1 día de vida. Se evaluó el título del inóculo en suspensión mediante recuento bacteriano de los ácaros machacados. Éste fue de 3×104 unidades formadoras de colonias (CFU) y de 2.7×106 CFU/pollo para la contaminación mediante contacto cuticular u oral de los ácaros respectivamente. Todas las aves fueron positivas a los 12 días postinoculación. Salmonella colonizó los tractos intestinales e invadió el hígado y el bazo. La concentración de S. enteritidis en contenido cecal llegó a una media de 8.5×104 MPN Salmonella g−1. Este estudio demostró la capacidad de los ácaros de infectar oralmente pollitos de un día de vida con la posterior colonización y replicación de Salmonella. Por lo tanto, los ácaros infectados con S. enteritidis constituyen un reservorio potencial de esta bacteria, que le permite persistir en las naves como fuente de infección para los nuevos animales introducidos. Si se encuentran ácaros contaminados en las instalaciones de aves, se debe realizar un control efectivo de los ácaros rojos antes de la introducción de nuevos lotes en estas instalaciones.
Introduction
Salmonella is associated with many of the most important outbreaks of food-borne diseases worldwide (Lacey, Citation1993). Eggs or broiler meat contaminated with Salmonella enterica have been described as sources of infection for consumers. As poultry is one of the most important reservoirs of Salmonella, its transmission to humans through the food chain is a major public health concern. Since the 1980s, the serovar most often responsible for Salmonella intoxication in humans is Salmonella Enteritidis, strongly associated with egg production. Salmonella typhimurium human cases are more commonly associated with consumption of contaminated meat (poultry, pig and bovine) (European Food Safety Authority, Citation2005). Poultry facilities have identified risk factors associated with primary production contamination. Birds can be infected from contaminated food, water, litter and rodents, and the persistence properties of Salmonella in the birds’ environment still represents an important characteristic of Salmonella epidemiology in poultry (Davies & Breslin, Citation2003). Many investigations have emphasized the role of wildlife species such as litter beetles, nematodes or houseflies in the transmission or the persistence of S. Enteritidis in poultry (Noreen & Greenberg, Citation1980; Olsen & Hammack, Citation2000; Rose et al., Citation2000; Chadfield et al., Citation2001; Skov et al., Citation2004). The role of Dermanyssus gallinae as a causative agent in the transmission of Salmonella between laying hens in successive flocks is still under question.
The poultry red mite (D. gallinae) is a common haematophagous arthropod found in laying hen facilities where it can cause bird anaemia, decreased egg production and, in extreme cases, death of its bird hosts (Kirkwood, Citation1967). This mite parasites its host only during blood meals and the rest of time it hides in the darkness to digest and/or reproduce. It can also bite humans and is recognized as a real problem for people working on poultry farms (Chauve, Citation1998). Moreover, its population can rapidly reach great number of individuals since its lifecycle is particularly short (about 10 days), and, most of the current methods of control having low efficiency, it has become a very difficult pest to eradicate (Beugnet et al., Citation1997). D. gallinae is also suspected to be a possible vector of various pathogens (Valiente Moro et al., Citation2005) and has been demonstrated to be an experimental vector of S. Enteritidis (Valiente Moro et al., 2006). It has been shown that the mite can be infected by S. Enteritidis after both cuticular contact and during a contaminated blood meal, that the bacteria can multiply and can be transmitted to the next generation, and that transtadial transmission from protonymphs to deutonymphs is also possible. Finally, it has been shown that previously infected mites are also able to recontaminate the blood in an in vitro feeding device. Since birds commonly peck and even ingest red mites in breeding hen facilities, the ingestion of contaminated mites by chickens as a potential route for poultry infection is of particular interest and merits further investigation.
Therefore, we have orally contaminated chicks with mites previously infected by S. Enteritidis. The ability of Salmonella to colonize the intestinal tract of the birds and invade their internal organs after inoculation of mites was then analysed.
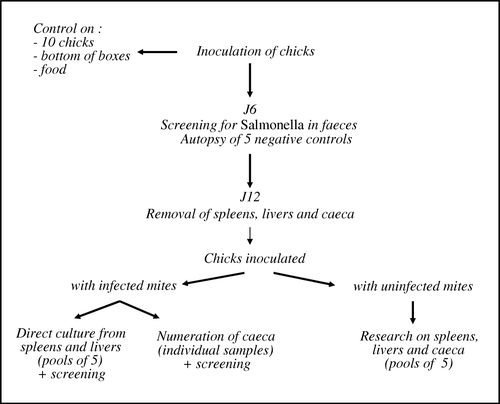
Materials and Methods
Mites and bacterial strain
Mites were collected from broiler chickens in the Rhône-Alpes region (Bresse, France) and were manipulated with a paint brush and a suction pump connected to a collector. The absence of Salmonella in the mites was confirmed on 20 groups of 10 mites by culture of crushed mites onto Salmonella medium identification (SM ID). The S. Enteritidis strain was provided by the “Laboratoire Centrale de Recherche Avicole et Porcine” (AFSSA, Ploufragan, France), which had previously isolated it from a poultry hatchery sample. It was maintained by regular plating on SM ID (Biomérieux, Craponne, France).
Chicks
One-day-old Isabrown chicks were used for the experiments. Chicks came from a layer hatchery free of Salmonella. Prior to the test, 10 chicks were confirmed to be S. Enteritidis-free by analysing their intestinal tract. The bottoms of the boxes in which the chicks had been transported as well as any droppings in the boxes were also checked for Salmonella contamination with negative results. All experimental birds received a commercial broiler finisher diet, which was confirmed S. Enteritidis free. All experimental procedures were performed according to French law concerning animal experimentation (decree 87-848 modified by 2001-464 of 29 May 2001).
Infection of mites
As described in our experimental model, two methods of infecting the mites with Salmonella were used (Valiente Moro et al., 2006). The infection during the blood meal consisted of in an in vitro feeding device using chicken skin over a pipette tip used as a blood reservoir. Adult chickens (Gallus gallus) were killed, their blood defibrinized by vigorous shaking and their skin carefully dissected from the corpses and scraped free of subcutaneous tissue. The absence of Salmonella in the blood used was previously checked by culture on specific medium SM ID. Blood was infected with S. Enteritidis at the rate of 108 colony-forming units (CFU/ml). Using a pipette the mites were then placed in contact with the chicken skin overlying the infected blood reservoir and allowed to feed for 4 h in an incubator under standardized conditions (temperature, 27±1°C; relative humidity, 75%; total darkness). This resulted in an average contamination of 2×104 Salmonella/mite. Cuticular infection of the mites was achieved by leaving them on a dry Salmonella coating. Briefly, 500 µl S. Enteritidis concentrated solution in Trypton Salt (TS) (BioMérieux) was streaked onto SM ID agar and incubated at 37°C for 18 to 24 h. Sterile Whatman paper was placed in contact with the bacterial coating and then transferred to a sterile petri dish. This allowed the mites to walk on the paper without suffering from the humidity produced by the agar. They remained in contact with Salmonella for 48 h at room temperature and were finally collected and stored in sterile plastic tubes until inoculation of chicks.
Inoculum preparation
Forty pools of 10 mites were selected by randomly taking engorged mites that had been artificially infected during bloodmeals 6 to 13 days before. The same sampling was performed for cuticle-infected mites that had been infected 6 to 10 days earlier. To ensure that any bacteria subsequently detected were those located inside the mites, we used the external decontamination and washing protocol previously described by Zeman et al. (Citation1982); each set of 10 mites was washed twice in 100 µl of 4% paraformaldehyde for 7 min, once in 100 µl sterile water, followed by a final washing in 100 µl TS. The final washing solutions were pooled and analysed for the presence of Salmonella to check the efficiency of the washing protocol. Each group of mites was finally suspended in 100 µl TS and stored at 4°C. These mite suspensions were used as the inoculum for the chicks. Control mites, namely non-contaminated mites, were also washed to check that the paraformaldehyde solution was not toxic for chicks. Six pools of 10 mites were kept for each route of infection to assess the titre of the feeding suspension. Mites were crushed in 200 µl TS and a 10-fold serial dilution was made. Then, 100 µl each dilution were streaked onto Rambach agar and incubated at 37°C for 18 to 24 h. When bacterial levels were undetectable, the presence of Salmonella in the remaining 80 µl initial solution was checked by pre-enrichment in buffered peptone water (BPW) (BioMérieux) and enrichment on modified semi-solid Rappaport Vassiliadis (MSRV) agar.
Experimental infection of chicks
Ninety-eight 1-day-old chicks were inoculated orally: 34 received mites contaminated during the blood meal, 34 were infected with mites contaminated by the cuticular route, and 30 chicks, the negative control, were inoculated with uncontaminated mites. Using a 200 µl pipette, each chick received orally 10 mites suspended in 100 µl TS. Each group of birds was housed in separate isolator units and kept for 12 days after inoculation.
Faecal samples
At 6 days post-inoculation (), faecal samples were randomly collected from each incubator (about 25 g per incubator). These samples were transferred to 225 ml BPW (BioMérieux) and mixed by stomaching for 30 sec. After pre-enrichment for 16 to 20 h at 37°C, an MSRV agar plate was spot-inoculated with three drops of the pre-enriched medium and incubated at 41.5°C for 24 h and if necessary for 48 h. On MSRV agar, Salmonella strains migrated from the inoculation spot. The margin of this migratory zone was streaked onto a Rambach agar plate. If no migration was noted after 48 h of incubation, the MSRV result was considered negative. After 24 h incubation of the Rambach agar plate, typical colonies were biochemically confirmed using API20E gallery (BioMérieux).
Internal organ samples
At 12 days after inoculation in each trial (), all chicks were sacrificed to allow bacteriological culture of their internal organs. For chicks inoculated with uncontaminated mites, livers, spleens and caeca were aseptically removed, pooled in groups of five, transferred to BPW (1/10 dilution w/w) and homogenized for 30 sec in a Stomacher Model. For chicks inoculated with contaminated mites, their livers and spleens were pooled in groups of five and mixed by stomaching for 30 sec in BPW. Salmonella detection was performed individually on their caeca. All samples were incubated for 16 to 20 h at 37°C, and a MSRV agar plate was spot-inoculated and incubated for 24 and 48 h at 41.5°C. Finally, a loopful was streaked onto Rambach and xylose lysine desoxycholate (XLD) agar and incubated for 18 to 24 h at 37°C. The enumeration of Salmonella was also performed by direct plating of 100 µl from the liver and spleen pool suspensions onto Rambach agar and cultured overnight at 37°C. For caeca individually isolated from contaminated chicks, the Salmonella count was performed using a most probable number (MPN) approach based on miniaturization of MSRV enrichment. The method consisted of three repeat successive dilutions using the mini MSRV method (Fravalo et al., Citation2003). Any presumed colonies of S. Enteritidis were confirmed biochemically and serotyped.
Results
Determination of the feeding suspension titres
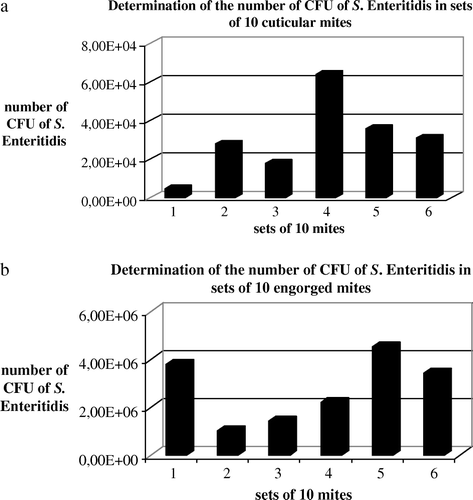
The level of Salmonella associated with the pool of 10 Dermanyssus inoculated per bird was determined by crushing mites, homogenizing the suspension and subsequent plating of convenient 10-fold serial dilutions in TS onto Rambach agar. The mean infectious titres were, respectively, 3.0×104 CFU/chick and 2.7×106 CFU/chick for birds inoculated with cuticular contaminated mites and those contaminated by blood-engorged mites (a,b). Before the chicks were inoculated, the final washing solutions of the mites were also controlled. The residual contamination measured for the 4 ml pools of the final washing solutions from the 340 cuticle-contaminated mites was 10 CFU/100 µl, and was 7.5×104 CFU/ 100 µl for the blood-contaminated mites.
Figure 2. Determination of the feeding suspension titres calculated from cuticular contaminated mites (a) and from blood-meal contaminated mites (b). The level of salmonella associated with the pool of 10 Dermanyssus inoculated per bird was determined by crushing mites, homogenizing the suspension and plating convenient 10-fold serial dilutions in TS onto Rambach agar. The experiment was repeated six times.
Isolation of S. Enteritidis from faecal samples and tissue
At 6 days after inoculation, faecal samples from both sets of infected chicks were positive for Salmonella while the control corresponding to chicks inoculated with uncontaminated mites remained negative for Salmonella. On day 12 post-inoculation, S. Enteritidis was isolated from the caeca of all birds that had received contaminated mites with an average number of S. Enteritidis above 8.5×104 MPN Salmonella/g (). Using direct plating, S. Enteritidis was counted from the spleen and the liver of all birds. Chi-square analysis did not show any significant difference (P<0.5) between the infection routes.
Table 1. Isolation and enumeration of S. Enteritidis in the spleen, liver and caecum on day 12 post-inoculation
Discussion
It has recently been demonstrated that D. gallinae is an experimental vector of S. Enteritidis (Valiente Moro et al., 2006). It has been shown that the mite can be infected through the blood meal or by cuticular contact. This infection is followed by bacterial survival for up to 14 days inside the mite as well as multiplication, trans-ovarial and transtadial transmission and contamination of host blood after a blood meal. However, the contamination of hens through eating previously infected mites has not yet been demonstrated. Therefore, in the present study, we inoculated chicks with mites experimentally infected by S. Enteritidis in order to assess the potential of D. gallinae to transmit Salmonella in this way.
The mean infectious titre of the inoculum given to chicks was equal to 3×104 CFU and 2.7×106 CFU for chicks respectively inoculated with cuticle-infected and engorged mites. Other experimental studies performed with young chicks at 1-day-old or 1-week-old showed that low dosages of S. Enteritidis ranging from less than 10 organisms to 5×104 CFU could induce infection in chicks (Cooper et al., Citation1994; Duchet-Suchaux et al., Citation1995; Van Immerseel et al., Citation2004). It is important to note that this dose is dependent on the age of the host, the number of bacteria in the organs and the serotype of Salmonella used. For example, it is about 1012 CFU for older chickens infected with S. typhimurium considering the fact that they can trigger their host's defence mechanisms after the colonization of the gastrointestinal tract (Muir et al., Citation1998). In our experiment, mites used as the infecting inoculum contained sufficient infectious levels to cause infection in 1-day-old chicks.
After the chicks had been orally inoculated with contaminated mites, excretion of S. Enteritidis was observed from 6 days post-inoculation in both infected groups. When the birds were sacrificed on day 12, all those that had received contaminated mites showed increased Salmonella levels in their livers, spleens and caeca (higher than 8.5×104 MPN Salmonella/g tissue), indicating colonization and multiplication of the bacteria. The results of our tests on the final washing solutions were, respectively, 10 CFU/100 µl and 7.5×104 CFU/100 µl for cuticle-infected and engorged mites. It suggests that the washing procedure was not performed adequately. However, it would be expected that the mites that were infected by walking on a Salmonella coating would have more Salmonella on the outside that the mites that have been infected by a blood meal. But the results show that the washing solution of the engorged infected mites is more contaminated than the other group. So, another explanation for this contamination could be the accidental crushing of a mite during the washing process. This hypothesis is supported by the fact that the level of contamination in the solution could be compatible with the concentration found in a single infected mite 7 or 14 days after the engorgement considering the fact that bacterial multiplication has been observed in some mites (Valiente Moro et al., 2006).
Previous studies on S. Enteritidis infections in poultry have been primarily carried out by inoculating chickens with a suspension of bacteria to demonstrate the invasive character of these bacteria when administered orally to chickens (Desmidt et al., Citation1997). In a model using 1-week-old chicks, CFU levels were highest during the first week(s) after oral inoculation with 5×104 S. Enteritidis and then decreased progressively (Duchet-Suchaux et al., Citation1995). Another study involving S. Enteritidis as an inoculum of 2×102 CFU administrated orally to 1-day-old chicks showed that the clearance of Salmonella was fast, with 60% of caecal samples found to be S. Enteritidis positive at 18 h post-inoculation (Asheg et al., Citation2001). Hinton et al. (Citation1989) further demonstrated that birds may readily become infected by eating feed contaminated with S. Enteritidis. This latter experiment is closer to our study because Salmonella transmitted via feed given to birds is similar to Salmonella transmitted via mites ingested by birds.
In spite of the Salmonella-positive results in the washing solutions, the level of infection obtained in both the infection models tested shows that previously inside-infected and outside-infected mites, when eaten by 1-day-old chicks, could represent a new source of Salmonella infection. The level of infection obtained in both the infection models tested shows that previously infected mites, when eaten by 1-day-old chicks, could represent a source of Salmonella infection. The invasion of organs such as the liver and spleen is an indication of systemic infection. So, the reproductive organs could be also contaminated and it could introduce a risk for humans when eggs are consumed. However, we cannot say how many mites are ingested each day by hens and it is difficult to assess the real impact of the mite in breeding facilities. However, we can state that this mode of infection is possible in 1-day-old chicks Moreover, the challenge model we have used has been performed with chicks, so, in future investigations, it would be interesting to confirm whether such infection takes place in older birds. To give a ruling on the possibility of this type of contamination in the field, it would also necessary to study the levels of Salmonella in facilities that have already had problems with Salmonella contamination or acarian infestation both before and after the arrival of new flocks. Finally, carriage of Salmonella by arthropods has already been reported in previous studies. Most recorded examples refer to litter beetles (Alphitobius diaperinus) or cockroaches, but their role in the transmission of infection remains unproven (McAllister et al., Citation1994; Davies & Wray, Citation1995; Gray et al., Citation1999). Ash & Greenberg (Citation1980) showed that the German cockroach (Blatella germanica) is an effective mechanical transmitter of S. typhimurium via faeces, although the bacteria were recoverable from its gut for about 10 days longer than from the faeces. In addition, the role of litter beetles as a potential reservoir for Salmonella enterica between broiler flocks has also been observed (Skov et al., Citation2004). Davies & Wray (Citation1993) demonstrated that the feeding of contaminated Lucilia sericata larvae to chickens is a potent means of establishing S. Enteritidis infection.
In conclusion, this study has demonstrated an experimental model of S. Enteritidis infection that could occur in poultry farms—natural consumption of contaminated mites leading to a lasting infection. It is the first time that Salmonella infection has been induced via the ingestion of an arthropod vector. Infected D. gallinae could represent a new source of contamination when ingested by chickens.
Acknowledgements
The authors gratefully acknowledge the technical assistance of the service personnel of “Elevage et Expérimentation en Pathologie Aviaire” (Agence Française pour la Sécurité Sanitaire des Aliments, Ploufragan) as well as Wanda Lipsky and Richard Sullivan for English-language correction.
References
- Ash , N. and Greenberg , B. 1980 . Vector potential of the German cockroach (dictyoptera: blatellidae) in dissemination of Salmonella enteritidis serotype typhimurium . Journal of Medical Entomology , 17 : 417 – 423 .
- Asheg , A.A. , Fedorova , V. , Pistl , J. , Levkut , M. , Revajova , V. , Kolodzieyski , L. , Sevcikova , Z. and Pilipcinec , E. 2001 . Effect of low and high doses of Salmonella Enteritidis PT4 on experimentally infected chicks . Folia Microbiologica (Praha) , 46 : 459 – 462 .
- Beugnet , F. , Chauve , C. , Gauthey , M. and Beert , L. 1997 . Resistance of the red poultry mite to pyrethroïd in France . Veterinary Record , 140 : 577 – 579 .
- Chadfield , M. , Permin , A. , Nansen , P. and Bisgaard , M. 2001 . Investigation of the parasitic nematode Ascaridia galli (Shrank 1788) as a potential vector for Salmonella enterica dissemination in poultry . Parasitology Research , 87 : 317 – 325 .
- Chauve , C. 1998 . The poultry red mite Dermanyssus gallinae (DeGeer, 1778): current situation and future prospects for control . Veterinary Parasitology , 79 : 239 – 245 .
- Cooper , G.L. , Venables , L.M. , Woodward , M.J. and Hormaeche , C.E. 1994 . Invasiveness and persistence of Salmonella Enteritidis, Salmonella Typhimurium, and a genetically defined S. Enteritidis aroA strain in young chickens . Infection and Immunity , 62 : 4739 – 4746 .
- Davies , R.H. and Breslin , M. 2003 . Persistence of Salmonella enteritidis phage type 4 in the environment and arthropod vectors on an empty free-range chicken farm . Environmental Microbiology , 5 : 79 – 84 .
- Davies , R.H. & Wray , C. ( 1993 ). Use of larvae of Lucilia serricata in colonization and invasion studies of Salmonella enteritidis in poultry . In Proceedings of the Flair Workshop No. 6 , 10–13 December 1992 (pp. 10 – 13 ). Southampton , , UK .
- Davies , R.H. and Wray , C. 1995 . The role of the lesser mealworm beetle (Alphitobius diaperinus) in carriage of Salmonella Enteritidis . Veterinary Record , 137 : 407 – 408 .
- Desmidt , M. , Ducatelle , R. and Haesebrouck , F. 1997 . Pathogenesis of Salmonella enteritdis phage type four after experimental infection of young chickens . Veterinary Microbiology , 5 : 99 – 109 .
- Duchet-Suchaux , M. , Léchopier , P. , Marly , J. , Bernardet , P. , Delaunay , R. and Pardon , P. 1995 . Quantification of experimental Salmonella enteritidis carrier state in B13 Leghorn chicks . Avian Diseases , 39 : 796 – 803 .
- European Food Safety Authority ( 2005 ). The Community Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents and Antimicrobial Resistance in the European Union in 2004 , 25 December .
- Fravalo , P. , Hascoet , Y. , Le Fellic , M. , Queguiner , S. , Petton , J. and Salvat , G. 2003 . Convenient method for rapid and quantitative assessment of Salmonella enterica contamination: the mini-MSRV technique . Journal of Rapid Methods and Automation in Microbiology , 11 : 81 – 88 .
- Gray , J.P , Maddox , C.W. , Tobin , P.C. , Gummo , J.D. and Pitts , C.W. 1999 . Reservoir competence of Carcinops pumilio for Salmonella Enteritidis . Journal of Medical Entomology , 36 : 888 – 891 .
- Hinton , M. , Pearson , G.R. , Threlfall , E.J. , Rowe , B. , Woodward , M. and Wray , C. 1989 . Experimental Salmonella enteritidis infection in chicks . Veterinary Record , 124 : 223
- Kirkwood , A.C. 1967 . Anaemia in poultry infested with the red mite Dermanyssus gallinae . Veterinary Record , 80 : 514 – 516 .
- Lacey , R.W. 1993 . Food-borne bacterial infections . Parasitology , 107 : S75 – S93 .
- McAllister , J.C. , Stellman , C.D. and Skeeles , J.K. 1994 . Reservoir competence of the lesser mealworm (Coleopta: tenebrionidae) for Salmonella typhimurium (Eubacterials: Enterobacteriacae) . Journal of Medical Entomology , 31 : 369 – 372 .
- Muir , W.I. , Bryden , W.L. and Husband , A.J. 1998 . Comparison of Salmonella typhimurium challenge models in chickens . Avian Diseases , 42 : 257 – 264 .
- Noreen , A. and Greenberg , B. 1980 . Vector potential of the german cockroach (dictyoptera:blatellidae) in dissemination of Salmonella enteritidis serotype typhimurium . Journal of Medical Entomology , 17 : 417 – 423 .
- Olsen , A.R. and Hammack , T. 2000 . Isolation of Salmonella spp. from the housefly, Musca domestica, and the dumpfly (Hydrotaea aenescens)(Wiedmann) (Diptera:Muscidae) at caged-layer houses . Journal of Food Protection , 63 : 958 – 960 .
- Rose , N. , Beaudeau , F. , Drouin , P. , Toux , J.X. , Rose , V. and Colin , P. 2000 . Risk factors for Salmonella persistence after cleansing and disinfection in French broiler-chicken houses . Preventive Veterinary Medicine , 44 : 9 – 20 .
- Skov , M.N. , Spencer , A.G. , Hald , B. , Petersen , L. , Nauerby , B. , Carstensen , B. and Madsen , M. 2004 . The role of litter beetles as potential reservoir for Salmonella enterica and thermophilic Campylobacter spp. between broiler flocks . Avian Diseases , 48 : 9 – 18 .
- Valiente Moro , C. , Chauve , C. and Zenner , L. 2005 . Vectorial role of some Dermanyssoid mites (Acari, Mesostigmata, Dermanyssoidea) . Parasite , 12 : 99 – 109 .
- Valiente Moro , C. , Chauve , C. and Zenner , L. 2007 . Experimental Salmonella Enteritidis infection by the poultry red mite . Dermanyssus gallinae, Veterinary Parasitology , 146 : 329 – 336 .
- Van Immerseel , F. , De Buck , J. , Pasmans , F. , Bohez , L. , Boyen , F. , Haesebrouck , F. and Ducatelle , R. 2004 . Intermittent long-term shedding and induction of carrier birds after infection of chickens early posthatch with a low or high dose of Salmonella Enteritidis . Poultry Science , 83 : 1911 – 1916 .
- Zeman , P. , Stika , V. , Shalka , B. , Bartik , M. , Dusbabeck , F. and Lavickova , M. 1982 . Potential role of Dermanyssus gallinae (DeGeer, 1778) in the circulation of the agent of pullurosis-typhus in hens . Folia Parasitologica , 29 : 371 – 374 .