Abstract
The acquisition of immunity by chickens infected 18 h post-hatch with 100 oocysts of Eimeria maxima and reared in floor-pens in contact with their droppings was investigated. In the first experiment, birds were placed on new litter and immunity was measured at 2, 3, 4, and 5 weeks by calculation of weight gain from days 0 to 7 following challenge with 100000 oocysts or by oocyst production in the faeces from days 5 to 8 following challenge with 500 oocysts. In the second experiment, birds were placed on new litter or reused litter from the first experiment (1 week after birds from the first experiment had been removed when 6 weeks of age), and were challenged at 1, 2, and 3 weeks of age. In the first experiment, immunity had developed in birds challenged at 3, 4, and 5 weeks, judged by weight gain and oocyst production, but immunity was not complete at 2 weeks. In the second experiment, immunity had developed in birds challenged at 1, 2, and 3 weeks measured by either criterion. In both experiments, birds produced small numbers of oocysts in their faeces following challenge. Judged by the weight gain following challenge, no significant difference in the acquisition of immunity was observed whether birds were reared on new or reused litter.
Mise en place de l'immunité vis-à-vis d'Eimeria maxima chez des poulets élevés sur litière neuve ou réutilisée après éclosion
La mise en place de l'immunité chez des poulets infectés 18 heures après l'éclosion avec 100 oocystes d'Eimeria maxima et élevés en parquets au contact de leurs déjections a été étudiée. Dans la première expérimentation les oiseaux ont été mis sur une litière neuve et l'immunité a été testée au bout de 2, 3, 4 et 5 semaines en calculant le gain de poids de 0 à 7 jours après l'épreuve réalisée avec 100 000 oocystes ou avec les oocystes produits dans les fientes du 5ème au 8ème jour après une épreuve avec 500 oocystes. Dans la deuxième expérimentation, les oiseaux ont été placés sur une litière neuve ou réutilisée provenant de la première expérimentation (une semaine après que les animaux ont été enlevés quand ils étaient âgés de 6 semaines) et éprouvés à 1, 2 ou 3 semaines. Dans la première expérimentation, en se basant sur le gain de poids et la production d'oocystes, l'immunité s'est développée chez les animaux éprouvés à 3, 4 et 5 semaines, mais à 2 semaines l'immunité n'était pas complète. Dans la seconde expérimentation, en se basant sur l'un ou l'autre des critères, l'immunité s'est développée chez les oiseaux éprouvés à 1, 2, et 3 semaines. Dans les deux expérimentations les oiseaux ont produit un petit nombre d'oocystes dans leurs fientes après l'épreuve. Aucune différence significative dans l'immunité acquise n'a été observée en se basant sur le gain de poids après épreuve, que les oiseaux aient été élevés sur litière neuve ou réutilisée.
Immunitätsbildung gegen Eimeria maxima in frisch geschlüpften Küken nach Aufzucht auf frischer oder wiederverwendeter Einstreu
Es wurde die Immunitätsbildung bei Hühnerküken untersucht, die 18 Stunden nach dem Schlupf mit 100 Eimeria maxima-Oozysten infiziert worden waren und die in Bodenhaltung in Kontakt mit ihrem Kot aufgezogen wurden. Im ersten Versuch waren die Küken auf neuer Einstreu eingestallt. Im Alter von 2, 3, 4 und 5 Wochen wurde die Immunität entweder durch Bestimmung der Gewichtszunahmen an den Tagen 0–7 nach Belastungsinfektion mit 100 000 Oozysten oder mittels Oozystenzählung im Fäzes vom 5.–8. Tag nach einer Belastungsinfektion mit 500 Oozysten überprüft. Im zweiten Versuch wurden die Küken entweder auf frische Einstreu oder auf die aus dem ersten Versuch stammende Einstreu (eine Woche nachdem die Küken aus dem ersten Versuch im Alter von 6 Wochen ausgestallt worden waren) gesetzt und im Alter von 1, 2 und 3 Wochen belastungsinfiziert. Im ersten Versuch hatten die Tiere, die im Alter von 3, 4 und 5 Wochen einer Belastungsinfektion ausgesetzt wurden, gemessen an den Gewichtszunahmen und der Oozystenproduktion eine Immunität ausgebildet, während die Immunität nach zwei Wochen noch nicht vollständig war. In zweiten Versuch waren die Küken aufgrund der Ergebnisse aus beiden Kriterien bereits nach Belastungsinfektion im Alter von 1, 2 und 3 Wochen immun. In beiden Versuchen schieden die Hühnerküken nach der Belastungsinfektion nur eine geringe Anzahl von Oozysten mit dem Fäzes aus. Gemessen an den Gewichtszunahmen nach den Belastungsinfektionen konnte kein signifikanter Unterschied in der Immunitätsbildung der auf frischer oder wiederverwendeter Einstreu gehaltenen Küken festgestellt werden.
Inmunización de pollitos recién nacidos criados en yacija nueva o reutilizada frente a Eimeria maxima
Se estudió cómo conseguir inmunidad en pollos infectados a las 18 horas post-nacimiento con 100 ooquistes de Eimeria Maxima y alojados en corralinas en contacto con sus heces. En el primer ensayo, las aves se alojaron en yacija nueva y su inmunidad se midió a las 2, 3, 4 y 5 semanas mediante el cálculo de la ganancia de peso entre los días 0–7 tras el desafío con 100 000 ooquistes o la producción de ooquistes en las heces entre los días 5–8 tras el desafío con 500 ooquistes. En el segundo ensayo, las aves se alojaron en yacija nueva o en la reutilizada del primer ensayo (1 semana después de eliminar las aves del primer ensayo a las 6 semanas de vida), y se desafiaron a 1, 2 y 3 semanas de vida. En el primer ensayo, la inmunidad se había desarrollado en las aves desafiadas a las 3, 4 y 5 semanas según la ganancia diaria y la producción de ooquistes, pero a las 2 semanas no estaba completamente desarrollada. En el segundo ensayo, la inmunidad se desarrolló en las aves desafiadas a 1, 2 y 3 semanas según cualquiera de los criterios utilizados. En ambos experimentos las aves produjeron un número bajo de ooquistes en las heces tras el desafío. Según la ganancia de peso tras el desafío, no se observaron diferencias significativas en el desarrollo de la inmunidad entre la crianza con yacija nueva o reutilizada.
Introduction
Eimeria maxima is a widespread protozoan parasite of poultry capable of stimulating a strong immune response in the fowl (Rose, Citation1974). The species is included in all commercial coccidiosis vaccines, some of which are administered to chicks in the hatchery before they are placed in the field (Chapman, Citation2000; Williams, Citation2002). Most studies of immunity have involved giving older birds high doses of Eimeria (for example, Long, Citation1962; Rose & Long, Citation1962). However, in a previous study, an inoculum of 100 oocysts of E. maxima, given to chicks 18 h after hatch, was shown to result in the development of protective immunity by 4 weeks of age (Chapman et al., Citation2005). Little information is available on how rapidly immunity is acquired when 1-day-old chicks are given doses of this magnitude. Coccidiosis is thought to occur most frequently when birds are 3 to 6 weeks of age (McDougald, Citation2003); however, it could occur earlier and therefore information on the rate of acquisition of immunity is desirable. The experiments reported here were designed to investigate the acquisition of immunity at different times in birds given 100 oocysts of E. maxima at 1 day of age. In some countries, the reuse of litter is widely practiced; the development of immunity in birds reared on new or reused litter was therefore compared.
Materials and Methods
Animals and husbandry
Broiler chickens (Cobb/Cobb strain) were obtained from a local hatchery and transferred to separate rooms in an isolation facility that had been thoroughly cleaned and disinfected before use. Each room was set up as a self-contained floor-pen with wood shavings for litter, and was serviced separately to avoid accidental exposure to Eimeria infection. Initial stocking densities and management of the birds followed guidelines for the care of animals in agricultural research (Anonymous, Citation1999).
Parasite
The strain of E. maxima used in the experiments has been maintained in the laboratory since 1994 when it was first isolated from a litter sample obtained from a broiler farm in northwest Arkansas. The strain was originally propagated from a single oocyst. This strain was used to infect chicks post-hatch and to challenge birds at various time intervals thereafter (homologous challenge).
Criteria for evaluating immunity
Weight gain and oocyst production following challenge were used as criteria to measure immunity. Previous experiments have shown that a dose of 100 000 oocysts of this strain of E. maxima causes a depression of weight gain without mortality (Chapman et al., Citation2005). Oocyst production was measured following challenge with 500 oocysts; high doses can give spurious results due to the “crowding effect” that can result in fewer oocysts being produced if large doses are given (Williams, Citation2001). Oocysts were counted as described by Long et al. (Citation1976).
Experiment 1
In the first experiment, 700 chicks were ordered and 233 allocated to each of three pens at an initial stocking density of 0.043 m2/bird. Birds in pen 1 were inoculated with 100 oocysts upon arrival from the hatchery (approximately 18 h post-hatch) and served as infected birds to be challenged (IC). Birds placed in pens 2 and 3 were not infected and served as uninfected challenged control birds (UCC) and uninfected unchallenged control birds (UUC).
Weight gain
Thirty birds from each pen were randomly allocated to wire-floored grower cages (five replicates of six birds/cage) at 12, 19, 26, and 33 days of age. They were given 2 days to acclimatize to the cages, and 2 days later (2, 3, 4, and 5 weeks of age) those from pens 1 and 2 were challenged with 100 000 oocysts (IC and UCC). Birds from pen 3 were not challenged (UUC). All birds were weighed when challenged and 7 days later, and the gain in weight was calculated.
Oocyst production
A further 12 birds from each pen were randomly allocated to cages (four replicates of three birds/cage) at 12, 19, 26, and 33 days of age. Two days later, those from pens 1 and 2 were challenged with 500 oocysts (IC and UCC). Birds from pen 3 were not challenged (UUC). Faeces were collected from cages from days 5 to 8 after infection and the total number of oocysts present counted.
Preparation for experiment 2
At 6 weeks of age, birds remaining in each of the three pens were removed and euthanased. Litter in pen 1 was left in place for 7 days prior to commencement of the second experiment in order to simulate a 1-week period during which birds were not present, such as might be employed in commercial practice. Pens 2 and 3 were thoroughly cleaned and new litter provided.
Experiment 2
Seven weeks after commencement of the first experiment, 600 chicks were ordered; 150 birds were placed in pens 1 and 2 and 300 in pen 3 (initial stocking densities of 0.067 and 0.134 m2/bird, respectively). Chicks in pens 1 and 2 were inoculated with 100 oocysts upon arrival from the hatchery and served as infected birds from reused (IRC) or new (INC) litter to be challenged respectively. Chicks in pen 3 were not infected and served as UCC and UUC.
Weight gain
Thirty birds from pens 1, 2, and 3 were randomly allocated to wire-floored grower cages (three replicates of 10 birds/cage) at 5, 12, and 19 days of age, and 2 days later (1, 2, and 3 weeks of age) were challenged with 100 000 oocysts (IRC, INC, and UCC). A further 30 birds from pen 3 were not challenged (UUC). Birds were weighed when challenged and 7 days later, and the gain in weight was calculated.
Oocyst production
Twelve birds from pens 1, 2, and 3 were transferred to cages (three replicates of four birds/cage) at 5, 12, and 19 days of age, and 2 days later were challenged with 500 oocysts (IRC, INC, UUC). A further 12 birds from pen 3 were not challenged (UUC). Faeces were collected from days 5 to 8 after challenge and the number of oocysts counted.
Oocysts in the litter
The course of infection in Experiment 1 was determined by counting the number of oocysts per gram present in the litter of the pens at 7-day intervals throughout the experiment. The number of oocysts in the litter of pens in Experiment 2 was determined when the birds were 1, 2, and 3 weeks old. A litter sample was collected from each pen and the number of oocysts present determined as described elsewhere (Chapman, Citation1992). Further litter samples were collected from the IC pen at 2 and 5 weeks (Experiment 1), weighed when collected, dried in an oven, and the percentage moisture content determined.
Analysis
In the first experiment, five replicates of six birds/cage were used for measurement of weight gain and four replicates of three birds/cage for measurement of oocyst production; in the second experiment, three replicates of 10 birds/cage were used to measure weight gain and three replicates of four birds/cage for measurement of oocyst production. Cage means served as the experimental unit for statistical analysis. Weight gains and oocyst numbers were analysed by one-way analysis of variance using the PROC analysis of variance procedure of SAS software (SAS Institute, Citation1991). Oocyst data were transformed before analysis using log(x+1); actual means and standard errors are presented in the tables. Means were separated and compared using Duncan's multiple-range test.
Results
Experiment 1
The weight gain and oocyst production, following challenge with 100 000 or 500 oocysts, respectively, are presented in . UCC birds gained significantly less weight than UUC birds when challenged at 2, 3, 4, and 5 weeks, indicating that birds reared in the absence of infection were susceptible to the challenge dose. At 2, 3, 4, and 5 weeks, the weight gain of IC birds was significantly greater than the UCC birds. At 2 weeks the weight gain of IC birds was significantly less than the UUC birds, but at 3, 4, and 5 weeks no significant difference was apparent. It is concluded that, judged by weight gain, IC birds had acquired immunity to the challenge infection at 3, 4, and 5 weeks, but immunity was not complete at 2 weeks.
Table 1. Weight gain and oocyst production of birds reared in floor-pens that were infected 18 h after hatch with 100 oocysts of E. maxima and challenged with 100 000 or 500 oocysts at 2, 3, 4, and 5 weeks of age
UCC and IC birds produced variable numbers of oocysts at 2, 3, 4, and 5 weeks following infection with 500 oocysts. IC birds produced significantly fewer oocysts than UCC birds at 2, 3, 4, and 5 weeks, indicating that, judged by oocyst production, birds had acquired immunity to the challenge infection. At 3 and 5 weeks, IC birds produced very few oocysts.
Experiment 2
The weight gain and oocyst production, following challenge with 100 000 or 500 oocysts, respectively, are presented in . UCC birds gained significantly less weight than UUC birds when challenged at 1, 2, and 3 weeks, indicating that birds reared in the absence of infection were susceptible to the challenge dose. At 1, 2, and 3 weeks, the weight gain of INC and IRC birds was significantly greater than the UCC birds but not significantly different from the UUC controls. No significant difference was apparent in the weight gain of INC or IRC birds. It is concluded that, judged by weight gain, INC and IRC birds had acquired immunity to the challenge infection at 1, 2, and 3 weeks of age.
Table 2. Weight gain and oocyst production of birds reared in floor-pens on new or reused litter that were infected 18 h after hatch with 100 oocysts of E. maxima and challenged with 100 000 or 500 oocysts at 1, 2, and 3 weeks of age
INC and IRC birds produced significantly fewer oocysts than UCC birds at 1, 2, and 3 weeks, indicating that, judged by oocyst production, birds had acquired immunity to the challenge infection. INC birds produced significantly fewer oocysts than IRC birds at 1 week, but at 2 and 3 weeks this was reversed. No obvious reason for this inconsistency is apparent.
Oocysts in the litter
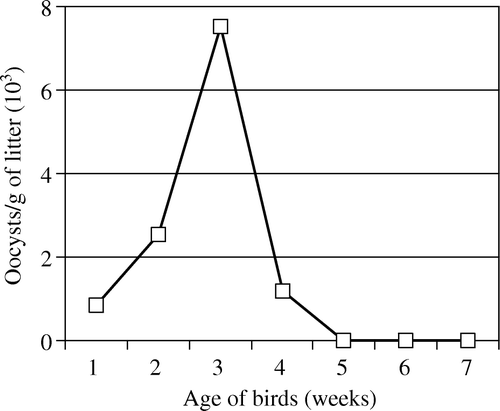
In the first experiment, no oocysts were detected in litter from pens 2 and 3 that contained birds not infected post-hatch (UCC and UUC). The number of oocysts present in the litter from pen 1 that contained birds infected post-hatch (IC) is shown in . Oocyst numbers initially increased with a peak at 3 weeks (). Thereafter, numbers declined and from 5 weeks after infection oocysts could not be detected. No oocysts could be detected at 7 weeks when birds of the second experiment were placed on the litter. In the second experiment, no significant differences in the number of oocysts in the litter at 1, 2, or 3 weeks from pens containing INC or IRC birds was observed (means were 1167, 2220, and 3750 oocysts/g for weeks 1, 2, and 3, respectively).
Litter moisture
Litter moisture in samples from Experiment 1, when birds were 2 and 5 weeks of age, was 15.6% and 19.3%, respectively.
Discussion
The practical use of coccidiosis vaccines in broilers has been facilitated by the development of methods for administering live oocysts of Eimeria, such as via spray cabinets, to newly hatched chicks. Most investigations of the immune response to Eimeria in poultry have been carried out with birds given a primary infection when at least 6 days old or much older (for example, Danforth et al., Citation1997; Rose, Citation1974; Williams & Catchpole, Citation2000). The protective efficacy of an attenuated anticoccidial vaccine was investigated when administered to chicks at 1 day of age (Crouch et al., Citation2003) but no other study has compared the rate of acquisition of immunity when 1-day-old chicks are given small numbers of oocysts and reared on new or reused litter. Experiments were conducted with E. maxima, the most immunogenic species of Eimeria that infects the fowl (Rose & Long, Citation1962). Birds were inoculated with 100 oocysts, a dose considered similar to that employed in commercial coccidiosis vaccines (Crouch et al., Citation2003).
In the experiments reported here, the acquisition of immunity to E. maxima in birds given 100 oocysts when newly hatched was investigated by challenging birds at weekly intervals following the primary infection. The results of the first experiment indicated that some degree of immunity had developed in birds challenged at 2 weeks, whether judged by weight gain following a large challenge dose of oocysts or oocyst production following a low challenge dose. However, immunity was not complete since IC birds produced some oocysts and their weight gain was significantly less than that of the UUC controls. Complete clinical protection, defined as no significant difference between the weight gains of IC and UUC birds, had developed in birds challenged at 3, 4, and 5 weeks of age. IC birds challenged at 3 and 5 weeks produced very few oocysts, indicating nearly solid immunity, but oocyst numbers of those challenged at 4 weeks were greater, suggesting immunity was incomplete judged by this criterion.
In the second experiment, in which birds were reared on new litter or reused litter from the first experiment, complete clinical protection, as defined above, had developed in birds challenged at 1, 2, and 3 weeks. According to Rose, immunity to E. maxima, judged by oocyst production resulting from a low-challenge inoculum, is evident as early as 3 days after an immunizing dose of 250 or 500 oocysts (Rose, Citation1974). These experiments were carried out when birds were 5 or 14 weeks of age, and complete protection (judged by no oocyst production) did not develop until 7 or 14 days after an initial inoculum of 500 or 250 oocysts, respectively (Rose, Citation1974). In the second experiment reported here, as in the first, INC and IRC birds produced some oocysts following challenge at 1, 2 and 3 weeks. According to Rose, clinical immunity precedes complete antiparasite immunity and these results support this conclusion (Rose, Citation1996).
It was previously found that birds reared in cages, in the absence of opportunity for re-infection, and infected post-hatch with 100 oocysts of E. maxima developed only partial protection when challenged at 4 weeks (Chapman et al., Citation2005). It was concluded, therefore, that re-infection was necessary for the establishment of solid immunity. Long et al., (Citation1986), however, found that chicks reared on wire floors and infected with 50 oocysts of E. maxima at 1 day of age developed substantial protection when challenged with 50 000 oocysts at 15 days of age. The authors point out, however, that it is possible immunity was reinforced by extraneous infection, which is difficult to prevent in battery cages (Long et al., Citation1986). Oocysts of E. maxima are first shed in the faeces 6 days after infection (Chapman et al., Citation2005) and are infective following sporulation, a process that takes approximately 24 h. Thus, in the second experiment, reinfection with oocysts produced following the initial inoculation of 100 oocysts could not have occurred prior to administration of the challenge dose when birds were 7 days old. Nevertheless, complete clinical protection developed following this challenge. It is possible that secondary infection could have occurred in the second experiment as a result of exposure to oocysts present in the new or reused litter prior to challenge at 1 week. In the second experiment, oocysts were not detected, however, when birds were placed on the litter at 1 day of age. Thus, in this study, re-infection may not have played an important role in the establishment of immunity.
The course of infection in the pens was determined by counting the number of oocysts present in the litter at weekly intervals. It should be noted that the design of the experiment, which necessitated the removal of birds to determine their immune status, resulted in a progressive reduction in stocking density, which would have affected the number of oocysts present in the litter. Thus no direct comparison with oocyst numbers observable in commercially raised birds is possible.
In the first experiment, peak numbers of oocysts were observed in the litter at 3 weeks followed by a rapid decline. Oocysts could not be detected at 5 and 6 weeks, or 1 week after birds had been removed at 6 weeks of age (minimum numbers that could be detected by the method used was approximately 250 oocysts/g litter). The single peak in oocyst production was similar to that observed in broilers vaccinated with a mixed species inoculum when 7 days old (Williams, Citation1994) although in field studies two peaks, the second higher than the first, are sometimes observed (Williams, Citation1992). Oocysts of E. maxima have been observed in faecal droppings from birds as late as 18 weeks of age (Williams, Citation1995a). A decline in oocyst numbers in litter following peak production has been attributed to their physical destruction in the litter accompanied by lack of replacement with new oocysts due to the acquisition of immunity by birds (Williams, Citation1995b). Dry litter adversely affects oocyst survival (Reyna et al., Citation1983). The moisture level found here (19.3% at 5 weeks), however, was similar to that reported for broiler flocks at 35 and 45 days of age (Chapman & Johnson, Citation1992; Williams, Citation2002; Williams & Gobbi, Citation2002) and is considered unlikely to have made an important contribution to the apparent absence of oocysts from 5 weeks of age. The rapid development of the immune response resulting in substantially reduced oocyst shedding in the faeces is probably the primary cause of the dramatic decrease in oocyst numbers observed in the litter.
In the second experiment, no significant difference in the number of oocysts in the new or reused litter was observed when birds were 1, 2 or 3 weeks old. No comparative information is available upon the number of oocysts of Eimeria species in flocks reared on new or reused litter and whether this has any affect upon the acquisition of immunity. In some countries the reuse of litter is widely practiced, and is often carried out for five or more flocks. In the present study, no significant difference was observed in immunity acquired to E. maxima by INC and IRC birds. The design of the second experiment was not intended to simulate commercial practice. Nevertheless, the results do suggest that, for E. maxima, vaccination of newly hatched chicks is likely to be equally effective whether birds are reared on new or reused litter.
Immunization of the chicken in the first few days of life was thought to be difficult because of the poor establishment of infection and the relative immaturity of the immune system (Rose, Citation1987). Results presented in this study, however, demonstrate the excellent immunizing ability of E. maxima when given in small numbers to newly hatched chicks. Investigations of species considered less immunogenic than E. maxima are desirable.
Acknowledgements
The authors would like to thank Dr Ray Williams for his helpful comments on the manuscript.
References
- Anonymous ( 1999 ). Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching 1st edn . Savoy , IL : Federation of Animal Science Societies .
- Chapman , H.D. 1992 . Research note: immunity to Eimeria in broilers reared on nicarbazin and salinomycin . Poultry Science , 71 : 577 – 580 .
- Chapman , H.D. 2000 . Practical use of vaccines for the control of coccidiosis in the chicken . Worlds Poultry Science Journal , 56 : 7 – 20 .
- Chapman , H.D. and Johnson , Z.B. 1992 . Oocysts of Eimeria in the litter of broilers reared to eight weeks of age before and after withdrawal of lasalocid or salinomycin . Poultry Science , 71 : 1342 – 1347 .
- Chapman , H.D. , Matsler , P.L. , Muthavarapu , V.K. and Chapman , M.E. 2005 . Acquisition of immunity to Eimeria maxima in newly hatched chickens given 100 oocysts . Avian Diseases , 49 : 426 – 429 .
- Crouch , C.F. , Andrews , S.J. , Ward , R.G. and Francis , M.J. 2003 . Protective efficacy of a live attenuated anticoccidial vaccine administered to 1-day-old chickens . Avian Pathology , 32 : 297 – 304 .
- Danforth , H.D. , Watkins , K. , Martin , A. and Dekich , M. 1997 . Evaluation of the efficacy of Eimeria maxima oocyst immunization with different strains of day-old broiler and roaster chickens . Avian Diseases , 41 : 792 – 801 .
- Long , P.L. 1962 . Observations on the duration of the acquired immunity of chickens to Eimeria maxima Tyzzer, 1929 . Parasitology , 52 : 89 – 93 .
- Long , P.L. , Johnson , J. , McKenzie , M.E. , Perry , E. , Crane , M. and Murray , P.K. 1986 . Immunisation of young broiler chickens with low level infections of Eimeria tenella, E. acervulina or E. maxima . Avian Pathology , 15 : 271 – 278 .
- Long , P.L. , Joyner , L.P. , Millard , B.J. and Norton , C.C. 1976 . A guide to laboratory techniques used in the study and diagnosis of avian coccidiosis . Folia Veterinaria Latina , 6 : 201 – 217 .
- McDougald , L.R. 2003 . “ Coccidiosis ” . In Diseases of Poultry , 11th edn , Edited by: Saif , Y.M. , Barnes , H.J. , Glisson , J.R. , Fadly , A.M. , McDougald , L.R. and Swayne , D.E. 974 – 991 . Ames : Iowa State University Press .
- Reyna , P.S. , McDougald , L.R. and Mathis , G.F. 1983 . Survival of coccidia in poultry litter and reservoirs of infection . Avian Diseases , 27 : 464 – 473 .
- Rose , M.E. 1974 . The early development of immunity to Eimeria maxima in comparison with that to Eimeria tenella . Parasitology , 68 : 35 – 45 .
- Rose , M.E. 1987 . “ Eimeria, Isospora, and Cryptosporidium ” . In Immunology, immunopathology and immunoprophylaxis of parasite infections , Edited by: Soulsby , E.J.L. Vol. 3 , 275 – 312 . Boca Raton , FL : CRC Press .
- Rose , M.E. 1996 . “ Immunity to coccidia ” . In Poultry immunology Poultry Science Symposium Series , Edited by: Davison , T.F. , Morris , T.R. and Payne , L.N. Vol. 24 , 265 – 299 . Abingdon : Carfax Publishing Company .
- Rose , M.E. and Long , P.L. 1962 . Immunity to four species of Eimeria in fowls . Immunology , 5 : 79 – 92 .
- SAS Institute ( 1991 ). SAS User's Guide: Statistics version 6.03 . Cary , NC : SAS Institute Inc .
- Williams , R.B. 1992 . The Development, Efficacy and Epidemiological Aspects of Paracox, a New Coccidiosis Vaccine for Chickens , Harefield : Pitman-Moore Europe .
- Williams , R.B. 1994 . Safety of the attenuated anticoccidial vaccine “Paracox” in broiler chickens isolated from extraneous coccidial infection . Veterinary Research Communications , 18 : 189 – 198 .
- Williams , R.B. 1995a . Epidemiological studies of coccidiosis in the domesticated fowl (Gallus gallus): IV. Reciprocity between the immune status of floor-reared chickens and their excretion of oocysts . Applied Parasitology , 36 : 290 – 298 .
- Williams , R.B. 1995b . Epidemiological studies of coccidiosis in the domesticated fowl (Gallus gallus): II. Physical condition and survival of Eimeria acervulina oocysts in poultry-house litter . Applied Parasitology , 36 : 90 – 96 .
- Williams , R.B. 2001 . Quantification of the crowding effect during infections with the seven Eimeria species of the domesticated fowl; its importance for experimental designs and the production of oocyst stocks . International Journal for Parasitology , 31 : 1056 – 1069 .
- Williams , R.B. 2002 . Anticoccidial vaccines for broiler chickens: pathways to success . Avian Pathology , 31 : 317 – 353 .
- Williams , R.B. and Catchpole , J. 2000 . A new protocol for a challenge test to assess the efficacy of live anticoccidial vaccines for chickens . Vaccine , 18 : 1178 – 1185 .
- Williams , R.B. and Gobbi , L. 2002 . Comparison of an attenuated anticoccidial vaccine and an anticoccidial drug program in commercial broiler chickens in Italy . Avian Pathology , 31 : 253 – 265 .