Abstract
This paper describes two experiments. In each experiment, 1-day-old specific pathogen free chicks were divided into three groups. In Experiment 1—[avian metapneumo virus (aMPV) challenge]—one group served as unvaccinated controls; the second group was vaccinated with live aMPV (subtype B) vaccine only, and the third group received the aMPV vaccine in combination with live Newcastle disease virus (NDV) vaccine (VG/GA strain). Oropharyngeal swabs, tissues and blood samples were collected before and after challenge with a virulent subtype aMPV at 21 days post vaccination. Chicks were monitored for post-challenge clinical signs. Swabs and tissues were examined for the detection of challenge aMPV by virus isolation and by reverse-transcriptase polymerase-chain reaction. Sera were assayed for antibodies against aMPV and NDV. The single and combined vaccinated chicks were all protected against clinical signs and no challenge virus was isolated from either of the vaccinated-challenged groups. In Experiment 2 (NDV challenge), as in Experiment 1, chicks were divided into three groups where one group remained as unvaccinated control and the other two groups were vaccinated as above, except that the second group received live NDV vaccine only, instead of aMPV. At 21 days post vaccination, 15 chicks from each of the three groups were removed to a different site and challenged with a virulent NDV (Texas GB strain). Re-isolation of the challenge virus was not attempted. All chicks in both NDV-vaccinated challenged groups were protected against clinical signs and mortality. These results show that, based on parameters monitored for the respective challenge virus, simultaneous application of live aMPV and NDV vaccines did not affect the efficacy of either vaccine.
Protection des poulets exempts de microorganismes pathogènes spécifiés avec des vaccins à virus vivant de la métapneumovirose aviaire et de la maladie de Newcastle administrés seuls ou en association
Cet article décrit deux expérimentations. Pour chacune, des poussins d'un jour exempts de microorganismes pathogènes spécifiés (SPF) ont été répartis en trois groupes. Dans la première expérimentation [épreuve avec un métapneumovirus aviaire (aMPV)], un groupe a servi de témoins non vaccinés, le deuxième groupe a seulement reçu un vaccin vivant aMPV (sous type B) et le troisième groupe a reçu le vaccin aMPV en association avec un vaccin vivant de la maladie de Newcastle (NDV) (souche VG/GA). Des écouvillons oropharyngés, des échantillons de tissus et de sang ont été prélevés avant et après l'épreuve réalisée avec un sous type virulent d'aMPV 21 jours après la vaccination (d.p.v.). Les poussins ont fait l'objet d'une observation des symptômes après l'épreuve. La détection du virus d'épreuve aMPV à partir des écouvillons et des tissus a été réalisée par isolement (VI) et par la réaction de transcription inverse suivie d'une réaction de polymérisation en chaîne (RT-PCR). Les anticorps sériques anti aMPV et anti NDV ont été recherchés. Les poussins ayant reçu le vaccin aMPV et ceux ayant reçu les vaccins aMPV et ND n'ont pas présenté de symptômes et le virus d'épreuve n'a pas été réisolé quel que soient les groupes vaccinés éprouvés. Dans la deuxième expérimentation (épreuve avec le ND), comme dans la première expérimentation les poussins ont été répartis en trois groupes. Un groupe a servi de témoins non vaccinés et les deux autres groupes ont été vaccinés (Cf. supra) excepté que le deuxième groupe a seulement reçu le vaccin vivant ND à la place du vaccin aPMV. Vingt-et-un jours d.p.v., 15 poussins de chacun des trois groupes ont été transférés sur un autre site et ont été éprouvés avec la souche virulente NDV (souche Texas). Le réisolement du virus d'épreuve n'a pas été tenté. Tous Aucun des poussins des deux groupes éprouvés ND n'a présenté de symptômes ou de mortalité. Ces résultats montrent que sur la base des paramètres suivis pour les virus d'épreuve respectifs, l'administration simultanée des vaccins aPMV et ND n'a pas affecté l'efficacité de l'un ou l'autre des vaccins.
Schutz spezifisch-pathogen-freier Hühnerküken durch aviäre Metapneumo- und Newcastle Krankheit-Lebendvirusimpfstoffe nach Einzel- oder Kombinationsapplikation
In diesem Artikel werden zwei Experimente beschrieben. In jedem Experiment wurden spezifisch-pathogen-freie (SPF) Hühnereintagsküken in drei Gruppen aufgeteilt. Im ersten Versuch [Belastungsinfektion mit aviärem Metapneumovirus (aMPV)] diente eine Gruppe als nicht vakzinierte Kontrolle, die zweite Gruppe wurde nur mit aMPV (Subtyp B)-Lebendimpfstoff vakziniert und die dritte Gruppe erhielt dieselbe aMPV-Vakzine in Kombination einer Newcastle Krankheit-Lebendvirus (NKV)-Vakzine (VG/GA-Stamm). Vor und nach der Belastungsinfektion mit einem virulenten aMPV-Subtyp am 21. Tag nach der Vakzination (d.p.v.) wurden Schnabelhöhlen-Rachentupfer, sowie Gewebe- und Blutproben entnommen. Die Küken wurden nach der Belastungsinfektion auf klinische Symptome hin beobachtet. Der Nachweis des aMPV-Challengevirus wurde in den Tupfer- und Gewebeproben mittels Isolation (VI) und Reverse Transkriptase-Polymerasekettenreaktion (RT-PCR) versucht. Die Seren wurden auf Antikörper gegen aMPV und NKV getestet. Die einzeln und kombiniert geimpften Küken waren gegen das Auftreten von klinischen Symptomen geschützt und das Virus der Belastungsinfektion konnte aus keiner der geimpften und belasteten Gruppen reisoliert werden. Im zweiten Versuch (NDV-Belastungsinfektion) wurden die Küken wie im Experiment I in drei Gruppen unterteilt, wobei eine Gruppe zu Kontrolle unvakziniert blieb, und die anderen beiden Gruppen wie oben beschrieben geimpft wurden, nur mit dem Unterschied, dass die zweite Gruppe anstelle der aMPV- die NKV-Lebndvakzine erhielt. Am 21. d.p.v. wurden 15 Küken aus jeder der drei Gruppen umgestallt und mit einem virulenten NKV (Texas GB-Stamm) belastungsinfiziert. Es wurde nicht versucht, das Challengevirus zu reisolieren, aber in den beiden NKV-geimpften Gruppen waren alle Küken gegen das Auftreten von klinischen Symptomen und Mortalität geschützt. Diese Ergebnisse aus der Überprüfung der genannten Parameter für das entsprechende Challengevirus zeigen, dass eine simultane Applikation von aMPV-und NKV-Lendvakzinen ihre Wirksamkeit nicht beeinträchtigt.
Protección de pollos libres de patógenos específicos con vacunas vivas frente a metapneumovirus aviar y virus de la enfermedad de Newcastle administradas individualmente o en combinación
Este artículo describe dos experimentos. En cada uno, pollos libres de patógenos específicos (SPF) se distribuyeron en tres grupos. En el experimento 1 [desafío con metapneumovirus aviar 8aMPV)], se utilizó un grupo como controles no vacunados; el segundo grupo se vacunó con una vacuna frente a aMPV (subtipo B) solamente y el tercer grupo recibió una vacuna frente a aMPV junto con la vacuna viva frente al virus de la enfermedad de Newcastle (NDV) (cepa VG/GA). Se obtuvieron hisopos orofaríngeos, tejidos y muestras de sangre antes y después del desafío con un subtipo virulento de aMPV a los 21 días post vacunación (d.p.v.). Se monitorizaron los signos clínicos post desafío de las aves. Se determinó la presencia del aMPV de desafío en hisopos y tejidos mediante aislamiento vírico (VI) y transcriptasa reversa-reacción en cadena de la polimerasa (RT-PCR). Se determinó la presencia de anticuerpos frente a aMPV y NDV en los sueros. Tanto las aves vacunadas con una sola vacuna o con la combinación de ambas vacunas estuvieron protegidas frente a signos clínicos y no se aisló virus de ninguno de los grupos vacunados y desafiados. Al igual que en el experimento 1, en el experimento 2 (desafío con NDV), los pollos se distribuyeron en tres grupos, de los cuales, uno se mantuvo como control no vacunado, y los otros dos se vacunaron como en el ensayo anterior, excepto que el segundo grupo recibió la vacuna viva de NDV solamente, en lugar de la de aMPV. A los 21 d.p.v., 15 aves de cada uno de los tres grupos se alojaron en un sitio distinto y se desafiaron con un NDV virulento (cepa Texas GB). No se realizó reaislamiento del virus de desafío. Todas las aves en ambos grupos vacunados y desafiados estuvieron protegidas frente a signos clínicos y mortalidad. Estos resultados muestran que en base a los parámetros monitorizados para cada uno de los virus respectivos, la aplicación simultánea de vacunas vivas frente a aMPV y NDV no afectaron la eficacia de ninguna de las vacunas.
Introduction
Turkey rhinotracheitis is an important respiratory disease in turkeys caused by avian metapneumovirus (aMPV), and in chicks the virus is sometimes associated with swollen head syndrome (Cook, 2000; Cook & Cavanagh, Citation2002). Newcastle disease is caused by paramyxovirus serotype 1 (APMV-1) and is an economically important disease worldwide in chickens and turkeys (Alexander & Jones, Citation2001; Alexander, Citation2003). Live and inactivated vaccines are used to prevent both of these diseases (Alexander & Jones, Citation2001; Alexander, Citation2003).
The interactions between live aMPV and Newcastle disease virus (NDV) vaccines when given simultaneously in specific pathogen free (SPF) chicks were reported by Ganapathy et al. (Citation2005). The main findings included increased humoral and local immune responses to NDV vaccine in the presence of aMPV vaccine, decreased humoral immune response to aMPV in the presence of NDV, but similar levels of local aMPV-specific IgA in lachrymal fluids, irrespective of single or dual vaccination.
In those experiments, no challenge was carried out to assess protection against virulent aMPV or NDV. This paper reports on the protection conferred by aMPV (NEMOVAC®) or NDV (AVINEW®) vaccines applied singly or dually in White Leghorn SPF chicks against virulent aMPV or NDV challenge.
Materials and Methods
Chickens
In each of the two experiments, White Leghorn 1-day-old SPF chicks (Lohmann Animal Health, Cuxhaven, Germany) were randomly allocated into three groups and were placed in separate isolation rooms in an experimental house. Food and water were provided ad libitum.
Vaccines
As in the previous report (Ganapathy et al., Citation2005), commercial vaccines of NDV (AVINEW®) and aMPV (NEMOVAC®) were used. The vaccine reconstitution and mixture preparation were the same as described previously (Ganapathy et al., Citation2005). The dosages of vaccines are presented in .
Table 1. Experimental design for protection against virulent aMPV or NDV challenge in SPF chicks offered by live aMPV or NDV vaccines given either alone or simultaneously
Experimental design
Two experiments were carried out. In each experiment, chicks were allocated into three groups ().
Experiment 1 (aMPV challenge)
Chicks were randomly allocated to three groups of 24 each (). The control (unvaccinated) group was sham-vaccinated with sterile water. Each chick received 50 µl ocularly and another 50 µl orally. Using the same route of application and volumes, aMPV vaccine was administered to chicks in the single aMPV vaccination group. For the dual-vaccinated aMPV + NDV group, sterile water containing both the aMPV and NDV vaccines were administered in the same way. Dosages received by each bird were as recommended by the manufacturer, and are presented in . The chicks were monitored daily for clinical signs. At 21 days post vaccination (d.p.v.), 12 birds from each group were transferred to another isolation room, and were challenged with virulent aMPV (subtype B), which was propagated in tracheal organ cultures (TOCs) and the virus titre determined using the same culture system. TOCs were prepared from SPF chick embryos after 19 to 20 days incubation following the method of Cook et al. (Citation1976). Each bird received 0.1 ml of 4.5 log10 median ciliostatic doses50 of the challenge virus via the ocular route.
Post-challenge clinical signs
After challenge, birds were monitored daily for clinical signs and the severity of the clinical signs was scored as described by Jones et al. (Citation1992). Briefly, a score of 0 = no signs, 1 = clear nasal exudates, 2 = turbid nasal exudates, and 3 = frothy eyes and/or swollen infraorbital sinuses in conjunction with nasal exudate.
Oropharyngeal swabs
Prior to challenge, 10 oropharyngeal (OP) swabs were collected from each group. After challenge, oropharyngeal samples were obtained from eight chicks in each group at 3, 6 and 9 days post challenge (d.p.c.) using dry swabs. In addition, another set of swabs was taken from the same chicks but using swabs previously moistened in TOC medium (Eagle's serum-free modified essential medium with glutamine, 50 µg/ml streptomycin and 50 IU/ml penicillin). OP swabs were also randomly collected from the unchallenged groups but only from five chicks. The dry and wet swabs were processed for reverse transcriptase-polymerase chain reaction (RT-PCR) and virus isolation, respectively. Swabs from the challenged birds were processed individually while the other swabs were pooled.
Sera
Blood samples were obtained at 21 days (pre-challenge) and 32 days (10 d.p.c.) of age from eight chicks per group for detection of antibodies against aMPV and NDV.
Tissues
At 7d.p.v. and at 5 and 10 d.p.c., four birds per group were humanely killed and pieces of the turbinate, trachea and lung were collected for aMPV RT-PCR and virus isolation.
Experiment 2 (NDV challenge)
The grouping was the same as Experiment 1 except that chicks in the single vaccination group received live NDV instead of aMPV vaccine. The volume and administration of inocula were the same as described above and the dosages per bird are presented in . The chicks were monitored daily for clinical signs and mortality. At 21 d.p.v., 15 birds from each group were brought to the OIE/FAO/EU Reference Laboratory for Avian Influenza and Newcastle Disease, Veterinary Laboratory Agency, Weybridge, Surrey. The chicks were housed in isolation rooms for 16 h and then were challenged with a virulent NDV (Texas GB strain). Prior to challenge, OP swabs were collected from 10 chicks in each group. Each bird received 0.2 ml of 4.0 log10 median embryo infectious doses50 of the challenge virus via the ocular route.
Post-challenge clinical signs
After challenge, birds were monitored daily for clinical signs and mortality.
Sera
Blood samples were obtained from all the groups (unvaccinated or vaccinated-unchallenged) at 21 days old (pre-challenge) and 28 days old (7 d.p.c.). Eight sera per group were tested for antibodies against aMPV and NDV.
Detection of viruses
For Experiment 1 only, OP swabs and selected homogenized tissues from the chicks were examined for aMPV challenge virus by isolation in TOCs (Cook et al., Citation1976; Ganapathy et al., Citation2005). OP swabs and tissues were also examined for aMPV by RT-PCR as described previously (Ganapathy et al., Citation2005).
Detection of vaccinal antibodies
NDV antibodies were detected by haemagglutination-inhibition (HI) (Allan & Gough, Citation1974). For aMPV antibodies, sera were tested by an in-house enzyme-linked immunosorbent assay (ELISA) as described by Worthington et al. (Citation2003), but the coating antigen was subtype B aMPV (Ganapathy et al., Citation2005). In both assays, the mean titre at each sampling point was calculated. For the aMPV ELISA, titres above 6 (log2) are considered significant for the presence of the aMPV antibodies.
Statistics
The mean antibody titres at each sampling point were compared using Student's t-test.
Results
Clinical signs and postmortem lesions
Experiment 1 (aMPV challenge)
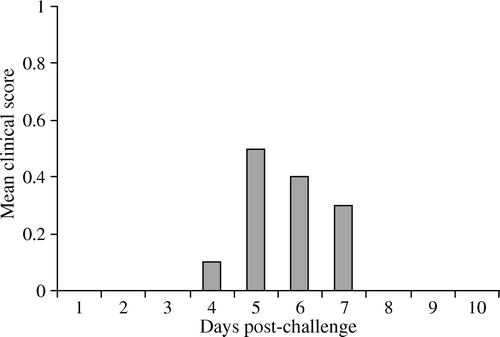
After vaccination, single (aMPV) and dual (aMPV + NDV) vaccinates showed no clinical signs. Following aMPV challenge, chicks in both vaccinated groups (aMPV-Ch or aMPV + NDV-Ch) remained free of clinical signs. The unvaccinated-unchallenged chicks remained normal throughout. In the unvaccinated-challenged (Unvac-Ch) group, a clear to turbid nasal discharge was seen at different times in chicks. shows the mean clinical scoring in this group. No postmortem lesions were found in any birds examined at 7 d.p.v. or 10 d.p.c. For the necropsy carried out at 5 d.p.c., three chicks of the Unvac-Ch birds showed a bilateral turbid nasal discharge. In the vaccinated-challenged birds, clear nasal discharge was seen in two and three chicks in the aMPV-Ch group and the aMPV + NDV-Ch group, respectively.
Experiment 2 (NDV challenge)
In the Unvac-Ch chicks, signs of dullness, depression, ruffled feathers, watery eyes, inappetence and paralysis were recorded 3 days after NDV challenge. All birds in this group died or were humanely killed by 7 d.p.c. Chicks in the NDV-vaccinated groups showed no clinical signs and none died. No necropsy was carried out.
Detection of a MPV virus
Experiment 1 (aMPV challenge)
All OP swabs from the unvaccinated, aMPV-vaccinated and aMPV + NDV-vaccinated birds were negative for aMPV by RT-PCR and virus isolation (VI) (data not shown). For the Unvac-Ch birds, eight, seven and eight swabs were positive for aMPV by RT-PCR at 3, 6 and 9 d.p.c., respectively, but virus was isolated from all swabs at 3 d.p.c. and not later (). In the aMPV-Ch and aMPV + NDV-Ch chicks, seven and eight swabs, respectively, were RT-PCR positive at 3 and 6 d.p.c., and this was reduced to three or four swabs at 9 d.p.c. All of these swabs were negative by VI.
Table 2. Experiment 1: detection of aMPV virus in the swabs and tissues by RT-PCR and passage in tracheal organ cultures
When the tissues were examined, no virus was detected in the trachea in any group by either method. Using RT-PCR, virus was detected in turbinates (5 and 10 days) in the Unvac-Ch group (). Both of the other groups were negative.
Table 3. Experiments 1 and 2: aMPV and NDV mean antibody titres in groups of chicks vaccinated at 1 day old with each vaccine alone or with both vaccines
Experiment 2
OP swabs taken at 21 d.p.v. (pre-challenge) from the unvaccinated, aMPV-vaccinated and aMPV + NDV-vaccinated birds were negative for aMPV by RT-PCR.
Serology
Experiment 1 (aMPV challenge)
There were no significant differences in the pre-challenge aMPV ELISA antibody titres between the aMPV-vaccinated groups (). The post-challenge antibody titres in each group were significantly higher than the respective unchallenged groups.
For NDV, HI titres in the control and single aMPV-vaccinated groups remained below the detectable level (). In the combined vaccination group, there were no significant differences in the pre or post aMPV-challenged NDV HI titres.
Experiment 2 (NDV challenge)
For NDV HI antibodies, at 21 d.p.v. (pre-challenge) the titre remained below detectable level in the unvaccinated group () and there were no significant differences between the two NDV-vaccinated groups. The post-challenge antibody titres in each group were significantly higher than the respective unchallenged groups.
For aMPV ELISA antibodies, there were no significant differences in the pre (7.90±1.56) and post (9.27±0.97) NDV-challenge titres in the simultaneously vaccinated chicks.
Discussion
This study was designed to complement our previous work (Ganapathy et al., Citation2005) where interactions between live aMPV and NDV vaccine viruses in SPF chicks were examined. In that work, systemic vaccine virus distribution and immune responses were studied but no challenge was carried out with virulent aMPV or NDV. From findings reported in this paper, it is clear that simultaneous vaccination with aMPV and NDV confers protection against virulent aMPV challenge that is no less than the level provided by aMPV vaccine alone. The aMPV-vaccinated chicks were not only protected against clinical signs but the challenge virus was not isolated, even though most unvaccinated-challenged chicks were positive by RT-PCR. This may suggest that, in the aMPV-vaccinated chicks, the challenge virus was unable to colonize the respiratory epithelium. However, necropsy at 5 d.p.c. revealed nasal discharge in few of the aMPV-vaccinated-challenged chicks, which may suggest that sterile vaccination was not completely achieved. The difficulty in recovering infectious virus from aMPV-vaccinated chicks is well known but factors such as sampling time and low virus titre may have influenced the outcome. While our results appear to indicate a discrepancy between the molecular detection and virus isolation, Hess et al. (Citation2004) have shown that the aMPV genome was still detectable as late as 28 d.p.c., long after the infectious virus was eliminated.
As for NDV, no clinical signs or mortality were seen in NDV-vaccinated chicks (alone or dually with aMPV) but all the unvaccinated-challenged chicks died or were humanely killed (due to illness) within 7 days of challenge. This result shows that, irrespective of single or dual NDV vaccination, complete protection was induced against virulent NDV challenge.
With respect to the serological results, in contrast to our previous study (Ganapathy et al., Citation2005), there were no significant differences in the levels of antibodies with respect to each vaccine between the single and dual vaccinated groups. The reasons for this discrepancy are not clear but factors such as group size (larger here compared with the small and reducing numbers in the previous work), perhaps the male–female ratio (unknown in both), and other unknown factors may have influenced the outcome. For aMPV, previous studies have emphasized that local and cell-mediated immune responses are important in resisting and clearing the virus (Cook et al., Citation1989; Jones et al., Citation1992; Khehra et al., Citation1999; Ganapathy et al., Citation2005). For NDV, levels of protection closely correlate with the levels of humoral HI antibodies (Alexander, Citation2003). In this study, at the time of NDV challenge, the mean NDV HI titres in the single-vaccinated and dual-vaccinated groups were log2 4.75 and 5.06, respectively. All birds in these groups were protected against clinical signs and mortality.
In conclusion, as judged by post-challenge clinical signs and virus detection, the efficacy for live aMPV (NEMOVAC®) vaccine was not compromised with simultaneous application of live NDV (AVINEW®) vaccine in SPF chicks. In the NDV challenge study, due to logistical reasons, detection of residual viruses was not carried out. However, based on post-challenge clinical signs and mortality, we have shown that the dual vaccination did not interfere with the efficacy of NDV (AVINEW®) vaccine.
References
- Allan , W.H. and Gough , R.E. 1974 . A standard haemagglutination-inhibition test for Newcastle disease. (1) A comparison of macro and micro methods . Veterinary Record , 95 : 120 – 123 .
- Alexander , D.J. 2003 . “ Newcastle disease ” . In Diseases of Poultry , 11th edn , Edited by: Saif , Y.M. , Barnes , H.J. , Glisson , J.R. , Fadly , A.M. , McDougald , L.R. and Swayne , D.E. 64 – 87 . Ames , IA : Iowa state University Press .
- Alexander , D.J. and Jones , R.C. 2001 . “ Paramyxoviridae ” . In Poultry Diseases , 5th edn , Edited by: Jordan , F.T.W. , Pattison , M. , Alexander , D.J. and Faragher , T. 257 – 272 . London : W.B. Saunders, Harcourt Publishers Ltd .
- Cook , J.K.A. 2002 . Avian pneumovirus infections of turkeys and chickens . Veterinary Journal , 160 : 118 – 125 .
- Cook , J.K.A. and Cavanagh , D. 2002 . Detection and differentiation of avian pneumoviruses (metapneumoviruses) . Avian Pathology , 31 : 117 – 132 .
- Cook , J.K.A. , Darbyshire , J.H. and Huggins , M.B. 1976 . The use of chicken tracheal organ cultures for the isolation and assay of avian infectious bronchitis virus . Archives of Virology , 50 : 109 – 118 .
- Cook , J.K.A. , Ellis , M.M. , Dolby , C.A. , Holmes , H.C. , Finney , P.M. and Huggins , M.B. 1989 . A live attenuated turkey rhinotracheitis virus vaccine. 1. Stability of the attenuated strain . Avian Pathology , 18 : 511 – 522 .
- Ganapathy , K. , Cargill , P. , Montiel , E. and Jones , R.C. 2005 . Interaction between live avian pneumovirus and Newcastle disease virus vaccines in specific pathogen free chicks . Avian Pathology , 34 : 297 – 302 .
- Hess , M. , Huggins , M.B. , Mudzamiri , R. and Heincz , U. 2004 . Avian metapneumovirus excretion in vaccinated and non-vaccinated specified pathogen free laying chicks . Avian Pathology , 33 : 35 – 40 .
- Jones , R.C , Naylor , C. , Al-Afaleq , A. , Worthington , K. and Jones , R. 1992 . Effect of cyclophosphamide immunosuppression on the immunity of turkeys to viral rhinotracheitis . Research in Veterinary Science , 53 : 38 – 41 .
- Khehra , R.S. and Jones , R.C. 1999 . Investigation into avian pneumovirus persistence in poults and chicks using cyclosporin A immunosuppression . Research in Veterinary Science , 66 : 161 – 163 .
- Worthington , K.J. , Sargent , B.A. , Davelaar , F.G. and Jones , R.C. 2003 . Immunity to avian pneumovirus infection in turkeys following in ovo vaccination with an attenuated vaccine . Vaccine , 21 : 1355 – 1362 .