Abstract
Diabetes mellitus was diagnosed in a 5-year-old male chestnut-fronted macaw (Ara severa) and an 8-year-old female Military macaw (Ara militaris) based on persistent hyperglycaemia and glucosuria. Hepatic biopsies showed marked hepatic haemosiderosis, while pancreatic biopsies showed no inflammatory lesions. Repeatable and titratable responses to bovine or porcine protamine zinc insulin were recorded in both patients, who were followed up for more than 2 years. In addition, iron-elimination therapy was initiated by chelation or phlebotomy, and the birds’ diet was changed to low-iron content pellets. Both birds responded favourably to this therapy, showing a decreased demand for extrinsic insulin. Follow-up biopsies demonstrated marked reduction in hepatic haemosiderin. Plasma fructosamine and β-hydroxybutyric acid levels were measured periodically in both birds and compared with euglycaemic psittacines. Both tests appeared useful for monitoring treatment success. The potential association between diabetes mellitus and excessive iron storage in birds should be further investigated.
Diabète intercurrent d'une hémosidérose hépatique chez deux aras
Un diabète a été diagnostiqué chez un ara vert (Ara severa), mâle, âgé de 5 ans et chez un ara militaire (Ara militaris), femelle, âgé de 8 ans, sur la base d'une hyperglycémie persistante et d'une glycosurie. Des biopsies du foie ont montré une l'hémosidérose hépatique évidente, alors que les biopsies du pancréas n'ont pas mis en évidence de lésions inflammatoires. Des réponses, reproductibles et mesurables, vis-à-vis de l'insuline zinc protamine porcine ou bovine ont été enregistrées chez les deux sujets qui ont fait l'objet d'un suivi pendant plus de deux ans. De plus, une thérapie d'élimination du fer a été initiée par chélation ou phlébotomie, et le régime alimentaire des oiseaux a été modifié en leur donnant des granulés contenant une faible teneur en fer. Suite à cette thérapie le comportement des oiseaux a changé, en montrant une diminution de la demande en insuline extrinsèque. Les biopsies suivantes ont montré une réduction marquée de l'hémosidérine hépatique. Les niveaux plasmatiques en acide β-hydroxybutyrique et en fructosamine ont été mesurés périodiquement chez les deux oiseaux et ont été comparés à ceux de psittacidés ayant une glycémie normale. Les deux tests sont apparus utiles pour mesurer le succès du traitement. Les associations possibles entre le diabète et un stockage excessif de fer chez les oiseaux devraient être davantage investiguées.
Gleichzeitiges Auftreten von Diabetes mellitus und Leberhämosiderose bei zwei Aras
Bei einem fünf Jahre alten männlichen Rotbugara (Ara severa) und einem achtjährigen weiblichen Kleinen Soldatenara (Ara militaris) wurde aufgrund persistierender Hyperglykämie und Glukosurie Diabetes mellitus diagnostiziert. Durch eine Leberbiopsie wurde eine hochgradige Leberhämosiderose festgestellt, während das Biopsiegewebe aus dem Pankreas keine entzündlichen Veränderungen aufwies. Bei beiden Patienten, die über einen Zeitraum von zwei Jahren begleitet wurden, konnten wiederholbare und titrierbare Reaktionen auf die Applikation von Rinder- oder Schweine-Protamin-Zink-Insulin beobachtet werden. Außerdem wurde mittels Chelation oder Phlebotomie eine Eiseneliminationstherapie eingeleitet und die Vogelfütterung wurde auf Pellets mit niedrigem Eisengehalt umgestellt. Beide Vögel reagierten günstig auf diese Therapie, indem sich der Insulinbedarf verringerte. Durch nachfolgende Biopsien konnte eine deutliche Reduktion des Hämosiderin in der Leber festgestellt werden. Bei beiden Vögeln wurde regelmäßig der Plasma-Fruktosamin-und β-Hydroxybuttersäuregehalt bestimmt. Dabei erwiesen sich beide Tests bei der Überprüfung des Behandlungserfolges als sinnvoll. Über die mögliche Verbindung zwischen Diabetes mellitus und hochgradiger Eisenspeicherung bei Vögeln sollten weitere Untersuchungen durchgeführt werden.
Diabetes Mellitus Concurrente con hemosiderosis hepática en dos guacamayos
Se diagnosticó diabetes mellitus en un Guacamayo severo (Ara severa) macho de 5 años de vida y en una Guacamayo militar (Ara militaris) hembra en base a hiperglucemia persistente y glucosuria. Las biopsias de hígado mostraron marcada hemosiderosis hepática, mientras que las biopsias pancreáticas no mostraron lesiones inflamatorias. Se evaluaron las respuestas reproducibles y titulables frente a insulina-protamina cinc de bovino o porcino en ambos pacientes, que se monitorizaron durante más de dos años. Además, se inció una terapia de eliminación de hierro mediante quelación o flebotomía, y la dieta de las aves se cambió a pellets con bajo contenido en hierro. Las dos aves respondieron favorablemente a esta terapia, mostrando una reducción de la demanda de insulina externa. Las biopsias obtenidas tras la terapia demostraron una marcada reducción de la hemosiderina hepática. Se determinaron los niveles de fructosamina plasmática y de ácido β-hidroxibutírico periódicamente en las dos aves y se compararon con los de aves psitácidas con valores normales de glucosa en sangre. Se probó la utilidad de ambas pruebas para la monitorización del éxito del tratamiento. La asociación potencial entre diabetes mellitus y el almacenaje excesivo de hierro en las aves debería estudiarse en mayor profundidad.
Introduction
Diabetes mellitus (DM) is uncommonly diagnosed in large psittacine birds (Oglesbee et al., Citation1997; Lumeij, Citation1999). Most of our knowledge of the avian endocrine pancreas originates from experimental work with domestic granivorous species, chiefly Anseriformes (Laurent et al., Citation1981; Karmann & Mialhe, Citation1982). Some studies indicate that these species may differ from mammals in the physiology of blood glucose control. For example, it has been shown that their pancreatic and plasma glucagon levels are considerably higher than those in mammals (Sitbon et al., Citation1980). In addition, they found that some granivorous birds (e.g. domestic ducks) become hypoglycaemic rather than hyperglycaemic following total pancreatectomy (Sitbon et al., Citation1980). These findings have led some to believe that insulin plays a lesser role in glucose regulation in granivorous birds compared with mammals, and that DM in granivorous birds may not be caused by insulin abnormalities (Rae, Citation2000). In contrast to this view, other studies provide evidence that insulin does play a role in glucose control in granivorous birds. For example, administration of insulin-binding antibodies has been shown to cause transient DM in domestic ducks (Mirsky et al., Citation1964), while total pancreatectomy in these birds caused fasting hypoglycaemia in addition to glucose intolerance (Sitbon et al., Citation1980). The small number of existing clinical reports shows that diabetic birds can respond to insulin therapy (Douglass, Citation1981; Bonda, Citation1996; Oglesbee et al., Citation1997; Lumeij, Citation1999).
Excessive iron storage (EIS) is known to be a serious health problem of certain avian species, particularly toucans, mynahs, and birds of paradise, but has been less frequently described in psittacines (Oglesbee et al., Citation1997; Worell, Citation1997; Cornelissen & Ritchie, Citation1999; Lumeij, Citation1999; Rae, Citation2000). It has been suggested that these species develop EIS under captive conditions due to a highly efficient intestinal iron absorption (Oglesbee et al., Citation1997; Lumeij, Citation1999). More recently, it has been shown that iron metabolism in hill mynahs (Gracula religiosa) resembles that of human patients suffering from hereditary haemochromatosis (Mete et al., Citation2003), and that high levels of iron transporter proteins are expressed by the small intestine of these birds (Mete et al., Citation2005). In humans, EIS is strongly associated with DM (Wilson et al., Citation2003; Platis et al., Citation2004). To the authors’ knowledge, such an association has not been reported in avian species.
Here we describe two cases of concurrent DM and EIS in psittacine birds; a chestnut-fronted macaw (Ara severa) and a military macaw (Ara militaris).
Case 1
A 5-year-old male chestnut-fronted macaw was presented to the Avian and Exotic Pet Service at the Ontario Veterinary College (OVC), University of Guelph, for polyuria and polydypsia of 1 week duration accompanied by depression during the prior 24 h. On physical examination, a mildly decreased body condition and marked polyuria were noted. The bird was hospitalized and blood was drawn on the following day for a complete blood cell count (CBC), and a chemistry panel (unless stated otherwise, tests and reference intervals were provided by the Animal Health Laboratory, University of Guelph). Results revealed very mild leukocytosis (17.1×109/l; reference, 7 to 15×109/l) and heterophilia (12.8×109/l; reference, 3 to 11×109/l), a mild increase in creatine kinase (785 U/l; reference, <300 U/l), and marked hyperglycaemia (56.4 mmol/l; reference, 11.1 to 20 mmol/l). Whole-body radiographs showed no abnormal findings. Blood was submitted for determination of zinc levels and polymerase chain reaction testing for Chlamydophila psittaci, paramyxovirus, polyomavirus, psittacine herpesvirus-1, and herpesvirus (generic primers). Polymerase chain reaction assays were performed by an external laboratory (Healthgene Corp., Toronto, Ontario, Canada). Of these, only the latter yielded a positive result. Zinc levels were lower than the reference interval (0.56 mg/l; reference, 1.45 to 3.4 mg/l).
Over the following weeks the bird continued to lose body condition and was markedly polyuric. Hyperglycaemia persisted and was often accompanied by ketonaemia with plasma β-hydroxybutyric acid (BHBA) levels up to 7136 µmol/l (450 to 1422 µmol/l). The BHBA assay was performed using enzymatic oxidation of d-3-hydroxybutyrate (Ranbut, RB 1007; Randox Laboratories, Antrim, UK) on a Hitachi 911 automated chemistry analyser (Roche/Hitachi Diagnostics, Laval, Quebec, Canada). The reference interval is based on 20 euglycaemic psittacine birds. Repeated urinalysis using a urine dipstick (Chemstrip 9; Roche Diagnostics, Laval, Quebec, Canada) showed marked glucosuria (≥28 mmol/l) and ketonuria (at the middle or upper of three positive levels). Endoscopic examination was performed using the right caudal thoracic air sac approach. A miliary pattern of yellow to orange pigmentation was noted on the hepatic surface (a). Biopsies of the liver and the duodenal-pancreas were collected using 5-French (1.67 mm) biopsy forceps, and were submitted for histopathological examination. The liver biopsies revealed nodular hyperplasia and marked haemosiderosis (confirmed by Prussian-blue staining). Iron was present in large aggregates within macrophages as well as in the cytoplasm of the hepatocytes (b). The pancreatic biopsies did not contain endocrine tissue and showed some nodular exocrine hyperplasia as well as a mild haemosiderosis. Treatment was attempted using the oral hypoglycaemic drug glipizide (Glucotrol; Pfizer Inc., New York, USA) at 1.25 mg/kg every 24 h; however, the drug did not reduce the bird's blood glucose levels. Porcine protamine-zinc insulin (PZI) (Caninsulin; Intervet, Whitby, Ontario, Canada) therapy was initiated next and a dose of 0.5 to 0.67 IU/kg every 12 h was found to be effective in controlling hyperglycaemia and ketonaemia (c). In addition, the bird's diet was changed to a low-iron content (<80 parts/106) commercial pelleted diet with no additions, and eight courses of deferoxamine mesylate (Desferal; Novartis Canada Inc., Dorval, Quebec, Canada) by intramuscular injection at 50 mg/kg every 12h for 14 days were administered during the following 20 months.
Figure 1. Haemosiderosis (1a to 1d) in a chestnut-fronted macaw (A. severa) and (1e to 1h) in a military macaw (A. militaris) with concurrent diabetes mellitus. 1a: Endoscopic view of the liver from the left caudal thoracic air sac. A miliary distribution of yellow foci is visible on the hepatic surface. 1b: Liver biopsy at presentation showing coarse focal accumulation of iron within macrophages (blue staining, arrows), as well as diffuse iron staining within hepatocytes throughout the parenchyma (Perl's stain). 1c: Blood glucose curve following intramuscular administration of 0.55 IU/kg porcine PZI insulin. The plasma glucose nadir typically occurs between 1 and 3 h after administration, and the effect lasts approximately 8 h. 1d: Necropsy sample of liver collected 24 months after initial presentation. The bird had received eight courses of the iron chelator deferoxamine and its diet had been changed to a low-iron content pellet. Some coarse aggregates are still present (arrows) but iron staining of the hepatocytes is much reduced compared with (1b) (Perl's stain). 1e: Liver biopsy showing marked haemosiderosis (Perl's stain). 1f: A parallel section to (1e) showing increased collagen deposition (blue areas; Masson's Trichrome stain). 1g: Pancreatic biopsy showing moderate haemosiderosis (Perl's stain). 1h: A parallel section to (1g) showing increased in collagen deposition within the pancreatic interstitium (Masson's Trichrome stain).
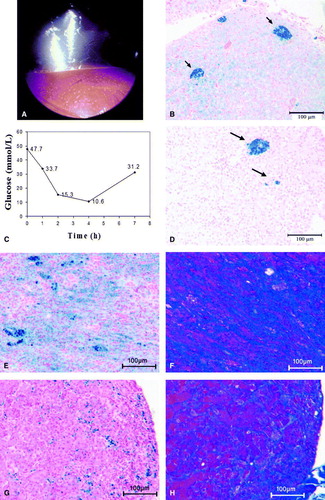
For more than 20 months post initial presentation, clinical signs as well as ketonaemia were generally controlled by this therapy, with occasional adjustments to the insulin dose. During each course of deferoxamine therapy, the insulin demand decreased and the hyperglycaemia and polyuria were better controlled. Endoscopy and collection of liver biopsies were repeated 14 months post presentation, revealing a decrease in hepatic haemosiderin. Therapy was continued until 22 months post presentation, when the bird had presented for a diabetic crisis and appeared to no longer respond to the porcine PZI. Treatment with bovine PZI (Summit Veterinary Pharmacy, Aurora, Ontario, Canada) was initiated and the bird's condition stabilized. However, two months later the bird again presented in crisis and died during routine anaesthesia for blood collection.
At necropsy the only macroscopic abnormalities were diffuse pallor of the liver and kidneys. Histopathological examination revealed mild hepatic and renal haemosiderosis, which was markedly less than that seen in the initial liver biopsies (d). The aorta showed moderate atherosclerosis. All other tissue types, including both endocrine as well as exocrine pancreatic tissue, appeared normal. Iron levels were determined by atomic absorption spectrophotometry and found to be 230 parts/106 (reference for poultry, 60–300 parts/106) and 150 parts/106 (reference for poultry, 45–100 parts/106) for the liver and kidney, respectively.
Case 2
A 9 year-old female military macaw was presented to the Avian and Exotic Pet Service at OVC for evaluation of decreased vocalization, weight loss, polyuria, polydypsia and polyphagia. The owner was unsure about the exact duration of the clinical signs, but they had been present for at least 1 month. The bird was purchased from a pet store and had been with the owner for 2 years. Its previous history was unknown. The bird's diet consisted of commercial pelleted food, nuts, and human foods including meat that was offered on a fairly regular basis.
On presentation the bird was bright alert and responsive, yet in thin body condition (body weight, 740 g) with moderate–severe pectoral muscle atrophy. Some stress bars on tail feathers and a dark discoloration of the wing coverts were noted, as was an overall loss of feather gloss. Focal hyperkeratosis was present on the right foot pad and the nails and beak were overgrown. Marked polyuria was noted. Blood was submitted for a CBC and chemistry panel, as well as bile acids, fructosamine and BHBA levels. Blood and a cloacal swab were sent for polymerase chain reaction testing for C. psittaci. Urine was tested by dipstick revealing marked glucosuria (≥28 mmol/l) and ketonuria (3 + ). The bird was hospitalized pending further test results.
Results of the CBC showed moderate, non-regenerative, anaemia (packed cell volume (PCV), 28%; reference, 40 to 55%), and heterophilia (19.2×109/l; reference, 3 to 11×109/l). The chemistry panel revealed marked hyperglycaemia (56.0 mmol/l; reference, 11.1 to 20 mmol/l), hypercholesterolaemia (13.3 mmol/l; reference, 1.6 to 8.0 mmol/l) and hypocalcaemia (1.76 mmol/l; reference, 2.0 to 2.9 mmol/l). A mild increase was also noted in γ-glutamyl transferase (21 U/l; reference, 0 to 15 U/l), while bile acid levels were within the reference range (17 µmol/l; reference, 11 to 81 µmol/l). The BHBA level was the highest the authors have recorded to date in a psittacine bird (11,126 µmol/l; reference, 450 to 1422 µmol/l), and fructosamine levels were also elevated (365 µmol/l; reference, 113 to 238 µmol/l). The fructosamine assay was based upon the fructosamine kit (11930010216; Roche Diagnostics, Indianapolis, Indiana, USA) on a Hitachi 911 automated chemistry analyser. The interval is based on 19 euglycaemic psittacine birds. The bird tested negative for C. psittaci.
The bird remained hospitalized for 1 week, during which it was started on bovine PZI insulin. Based on multiple glucose curves, intramuscular doses of 0.5 IU/kg in the morning and of 0.3 IU/kg in the afternoon were determined to be appropriate for controlling the bird's blood glucose levels. Blood drawn 6 days after starting this therapy revealed a normal heterophil count, and the blood smear showed significant polychromasia (16 to 18 polychromatic cells/high-power field) although the PCV remained low at 26%. The chemistry panel showed that calcium and γ-glutamyl transferase levels had returned to reference intervals, while cholesterol remained slightly elevated at 8.25 mmol/l and the BHBA levels had decreased to 1024 µmol/l, which is within the normal range. Ketones were not detectable on urine dipstick (Chemstrip 9) by day 3 of hospitalization. The bird was discharged with instructions to continue the insulin therapy at home.
Four weeks after the initial presentation, the bird had gained body weight (777 g) and was more active and vocal. A repeat CBC was normal with a PCV of 45%. Fructosamine and BHBA were within reference ranges. Endoscopic examination and biopsy of the liver and pancreas were performed via a right caudal thoracic air sac approach. Two 5-French biopsies of the liver and pancreas were collected. Histological examination of the biopsies revealed marked hepatic haemosiderosis and mild to moderate fibrosis (e,f). The pancreatic biopsies did not contain endocrine tissue and showed mild haemosiderosis and fibrosis (g,h). Based on these results, the bird was then treated by weekly phlebotomies, and its diet was changed to a commercial low-iron pelleted diet. Ten phlebotomies were performed, removing a volume equivalent to 1% body weight. These were well tolerated with the bird regenerating its PCV between treatments. After the fourth treatment the bird's insulin dose had to be reduced due to hypoglycaemic episodes and was set at 0.15 IU/kg in the morning and 0.1 IU/kg in the afternoon. In addition, the bird's bodyweight continued to increase during the treatment (to about 850 g) and its attitude improved according to the owner. The decreased insulin demand and clinical improvement persists to date (29 months post presentation). Follow-up liver biopsies were collected 12 months post presentation. These samples revealed marked reduction in hepatic haemosiderin compared with the original biopsies.
Discussion
Traditionally, two types of DM are recognized: type I, where a true deficiency of insulin exists, generally due to loss of pancreatic B cells; and type II, where a relative insulin deficiency or insulin resistance exists. In human medicine, the classification of DM into subtypes has undergone considerable modifications in recent years. Cases associated with EIS of any aetiology (i.e. genetic, acquired) were previously classified as type II DM, but are now placed in their own subclass (Expert Committee on the Diagnosis and Classification of Diabetes Mellitus, Citation2003).
Practically nothing is known about the typing of DM in avian species, except in cases where obvious destruction of pancreatic tissue has been documented. The number of reported cases of DM in psittacine birds is extremely limited. The disease appears to be uncommon in large psittacine species, but is more commonly encountered in the budgerigar (Melopsittacus undulatus) and cockatiel (Nymphicus hollandicus) (Oglesbee et al., Citation1997). Reported aetiologies include pancreatic neoplasia, pancreatitis (either primary or secondary to female reproductive pathology), pancreatic atrophy, and idiopathy (Oglesbee et al., Citation1997; Lumeij, Citation1999).
The association between DM and EIS has been well documented in humans (for example, Wilson et al., Citation2003; Platis et al., Citation2004), but not in birds. The pathophysiology behind this association is not fully understood. In humans, as well as in mice and rat models of haemochromatosis, iron has been shown to selectively accumulate in B cells, causing oxidative stress and apoptosis and interfering with insulin secretion (Iancu et al., Citation1990; Lu & Hayashi, Citation1994; Cooksey et al., Citation2004). Excessive iron levels have also been shown to increase peripheral insulin resistance and to increase circulating glucagon levels (Nelson et al., Citation1979). The treatment of choice in human patients suffering from concurrent EIS and DM includes weekly or bi-weekly phlebotomies and management of DM by either oral hypoglycaemic drugs or insulin. Chelation therapy, using deferoxamine mesylate, is indicated when phlebotomies cannot be performed (e.g. in anaemic patients), but carries the risk of systemic as well as local adverse drug reactions (Cutler, Citation1989; Whittington & Kowdley, Citation2002; Nielsen et al., Citation2003). Iron elimination therapy improves the control of DM in both insulin-dependent and insulin-independent cases; however, patients that are insulin dependent generally remain so despite treatment (Dooley, Citation2002).
Treatment recommendations for DM in birds are controversial. The oral hypoglycaemic drug glipizide has been reported effective in some DM cases, particularly in cockatiels and budgerigars (Wissman, Citation2003). Somatostatin, a potent suppressor of both insulin and glucagon secretion, has been used anecdotally to treat diabetic birds. Use of the drug was based on the belief that avian DM is caused by increased glucagon levels rather than by insulin deficiency or resistance to insulin (Bonda, Citation1996; Lumeij, Citation1999, Rae, Citation2000). Somatostatin was ineffective in controlling hyperglycaemia in a diabetic macaw (Bonda, Citation1996), while this bird and several others responded favourably to insulin therapy (Douglass, Citation1981; Bonda, Citation1996; Oglesbee et al., Citation1997; Lumeij, Citation1999). One of the two patients in the cases described above and one diabetic African grey parrot (Psittacus erithacus) also seen through the OVC failed to respond to the oral hypoglycaemic drug glipizide, but all three birds responded favourably to bovine or porcine PZI, and were maintained for long periods of time on this therapy. Effective doses were comparable with those used in dogs and cats and were titratable. Glucose curves showed the effect to last approximately 6 to 8 h, typically reaching the nadir after 1 to 3 h.
It is noteworthy that both diabetic psittacine birds treated by porcine PZI (the chestnut-fronted macaw from this report and the African grey parrot mentioned above) eventually became resistant to the drug, requiring its change to the bovine PZI product. Bovine and porcine insulin differ by two amino acids at locations 8 and 10 on the A-chain (Conlon, Citation2001), perhaps affecting the immune response to these peptides. The only psittacine insulin sequence available is from the budgerigar (Simon et al., Citation2004; Genbank accession numbers AAP45991 and AAP45963), and is partial (only 16 of 21 amino acids in the A chain). It differs from porcine and bovine insulin by three amino acids of the known 16 in the A chain, and by four of 30 in the B chain (bovine and porcine B chains are identical). The known segment of the budgerigar A-chain is identical to that of the domestic fowl (Gallus gallus) and rufous hummingbird (Selaphorus rufus), but differs by three amino acids from that of the mallard (Anas platyrhynchus). The budgerigar B-chain differs by one amino acid from that of the domestic fowl, by two from the mallard, and by three from the rufous hummingbird (Conlon, Citation2001). These interspecies differences underline the potential complexity of treating multiple avian species by extrinsic insulin; however, in reality, only a few (all mammalian) long-acting insulin products are commercially available, making the choice of drug limited.
Human recombinant neutral protamine hagedorn (NPH) or ultralente insulin products have successfully reduced blood glucose in some avian patients with DM (Douglass, Citation1981; Bonda, Citation1996; Oglesbee et al., Citation1997). However, other authors report failure to control avian DM with NPH insulin (Pilny & Luong, Citation2005). The authors have attempted to treat a diabetic African grey parrot with human recombinant NPH insulin, with no apparent response. The same bird responded in a predictable and titratable manner to porcine or bovine PZI products. The reason for this lack of consistency in response of diabetic birds to human recombinant NPH insulin is unknown. It may or may not be related to differences in amino acid sequence.
Chemical parameters such as fructosamine and BHBA appeared to correlate well with the birds’ state of glycaemic control; however, neither test has been validated for avian species and the timeframe to which the fructosamine measurement may relate is unknown. The clinical usefulness of these analytes for monitoring avian DM patients should be further evaluated.
The two cases described above are of special interest because of the concurrence of DM and EIS in psittacine species, and the fact that both birds responded clinically to iron-elimination therapy. Although an association between the two conditions has not been previously reported in birds, Worell (Citation2000) reports on the finding of iron deposition in the pancreas of diabetic toucans. The fact that both DM and EIS appear to be over-represented in toucans (Murphy, Citation1992; Worell, Citation1997; Cornelissen & Ritchie, Citation1999; Worell, Citation2000) is intriguing, as is the similarity of the two cases reported here. A search of the Veterinary Information Network (Citation2006) shows that three more cases of persistent hyperglycaemia have been reported in military macaws—a surprising finding considering the small number of cases reported in large psittacines. An additional case of DM in a chestnut-fronted macaw has been recently reported (Pilny & Luong, Citation2005). Histological findings from that bird included moderate hepatic haemosiderosis; however, lymphocytic pancreatitis and vacuolation of islet cells were also present, suggesting type I DM. The latter were not seen in the two cases described here.
In both cases described above, successful long-term control of DM was achieved by insulin therapy, while concurrent EIS was managed by iron elimination in conjunction with dietary changes. Iron elimination appeared to reduce the birds’ demand for extrinsic insulin and to ameliorate their clinical signs. The combination of iron elimination and restriction of iron intake was effective in reducing hepatic iron stores in both birds. Collection of hepatic and pancreatic biopsies should be considered part of the diagnostic plan of avian diabetic patients. The potential association between DM and EIS in birds should be further investigated.
Acknowledgements
The authors thank Ms Geraldine Higginson and Ms Ingrid Danylyk for their assistance in managing the clinical cases.
References
- Bonda , M. 1996 . “ Plasma glucagons, serum insulin, and serum amylase levels in normal and hyperglycemic macaw ” . In Proceedings of Annual Conference of the Association of Avian Veterinarians , 77 – 88 . Boca Raton , FL : Association of Avian Veterinarians .
- Conlon , J.M. 2001 . Evolution of the insulin molecule: insights into structure–activity and phylogenetic relationships . Peptides , 22 : 1183 – 1193 .
- Cooksey , R.C. , Jouihan , H.A. , Ajioka , R.S. , Hazel , M.W. , Jones , D.L. , Kushner , J.P. and McClain , D.A. 2004 . Oxidative stress, beta-cell apoptosis, and decreased insulin secretory capacity in mouse models of hemochromatosis . Endocrinology , 145 : 5305 – 5312 .
- Cornelissen , H. and Ritchie , B.W. 1999 . “ Ramphastidae ” . In Avian Medicine: Principles and Application , Edited by: Ritchie , B.W. , Harrison , G.J. and Harrison , L.R. 1276 – 1283 . Delray Beach : HBD International Inc .
- Cutler , P. 1989 . Deferoroxamine therapy in high-ferritin diabetes . Diabetes , 38 : 1207 – 1210 .
- Dooley , J.S. 2002 . Diagnosis and management of genetic haemochromatosis . Best Practica & Research Clinical Haematology , 15 : 277 – 293 .
- Douglass , E.M. 1981 . Diabetes mellitus in a toco toucan . Modern Veterinary Practice , 62 : 293 – 295 .
- Expert Committee on the Diagnosis and Classification of Diabetes Mellitus . ( 2003 ). Report of the expert committee on the diagnosis and classification of diabetes mellitus . Diabetes Care , 26 ( Suppl ), S5 – S20 .
- Iancu , T.C. , Ward , R.J. and Peters , T.J. 1990 . Ultrastructural changes in the pancreas of carbonyl iron-fed rats . Journal of Pediatric Gastroenterology and Nutrition , 10 : 95 – 101 .
- Karmann , H. and Mialhe , P. 1982 . Progressive loss of sensitivity of the A cell to insulin in geese made diabetic by subtotal pancreatectomy . Hormone and Metabolic Research , 14 : 452 – 458 .
- Laurent , F. , Gross , R. , Lakili , M. and Mialhe , P. 1981 . Effect of insulin on glucagon secretion mediated via glucose metabolism of pancreatic A cells in ducks . Diabetologia , 20 : 72 – 77 .
- Lu , J.P. and Hayashi , K. 1994 . Selective iron deposition in pancreatic islet B cells of transfusional iron-overloaded autopsy cases . Pathology International , 44 : 194 – 199 .
- Lumeij , J.T. 1999 . “ Endocrinology ” . In Avian Medicine: Principles and Application , Edited by: Ritchie , B.W. , Harrison , G.J. and Harrison , L.R. 582 – 606 . Delray Beach : HBD International Inc .
- Mete , A. , Hendriks , H.G. , Klaren , P.H. , Dorrestein , G.M. , van Dijk , J.E. and Marx , J.J. 2003 . Iron metabolism in mynah birds (Gracula religiosa) resembles human hereditary haemochromatosis . Avian Pathology , 32 : 625 – 632 .
- Mete , A. , Jalving , R. , van Oost , B.A. , van Dijk , J.E. and Marx , J.J. 2005 . Intestinal over-expression of iron transporters induces iron overload in birds in captivity . Blood Cells, Molecules & Diseases , 34 : 151 – 156 .
- Mirsky , I.A. , Jinks , R. and Perisutti , G. 1964 . Production of diabetes mellitus in the duck by insulin antibodies . The American Journal of Physiology , 206 : 133 – 135 .
- Murphy , J. ( 1992 ). Diabetes in toucans . In Proceedings of Annual Conference of the Association of Avian Veterinarians (pp. 165 – 170 ). Boca Raton , FL : Association of Avian Veterinarians .
- Nelson , R.L. , Baldus , W.P. , Rubenstein , A.H. , Go , V.L. and Service , F.J. 1979 . Pancreatic alpha-cell function in diabetic hemochromatotic subjects . The Journal of Clinical Endocrinology and Metabolism , 49 : 412 – 416 .
- Nielsen , P. , Fischer , R. , Buggisch , P. and Janka-Schaub , G. 2003 . Effective treatment of hereditary haemochromatosis with desferrioxamine in selected cases . British Journal of Haematology , 123 : 952 – 953 .
- Oglesbee , B.L. , Orosz , S. and Dorrestein , G.M. 1997 . “ The endocrine system ” . In Avian Medicine and Surgery , Edited by: Altman , R.B. , Clubb , S.L. , Dorrestein , G.M. and Quesenberry , K. 475 – 488 . Philadelphia : W.B. Saunders Company .
- Pilny , A.A. and Luong , R. 2005 . Diabetes mellitus in a chestnut-fronted macaw (Ara severa) . Journal of Avian Medicine and Surgery , 19 : 297 – 302 .
- Platis , O. , Anagnostopoulos , G. , Farmaki , K. , Posantzis , M. , Gotsis , E. and Tolis , G. 2004 . Glucose metabolism disorders improvement in patients with thalassaemia major after 24–36 months of intensive chelation therapy . Pediatric Endocrinology Reviews, Supplement , 2 : 279 – 281 .
- Rae , M. 2000 . “ Avian endocrine disorders ” . In Laboratory Medicine. Avian and Exotic Pets , Edited by: Fudge , A.M. 76 – 98 . Philadelphia : W.B. Saunders Company .
- Simon , J. , Laurent , S. , Grolleau , G. , Thoraval , P. , Soubieux , D. and Rasschaert , D. 2004 . Evolution of preproinsulin gene in birds . Molecular Phylogenetics and Evolution , 30 : 755 – 766 .
- Sitbon , G. , Laurent , F. , Mialhe , A. , Krug , E. , Karmann , H. , Gross , R. , Strosser , M.T. , Cohen , L. , Jean-Marie , P. , Foltzer , C. and Mialhe , P. 1980 . Diabetes in birds . Hormone and Metabolic Research , 12 : 1 – 9 .
- Veterinary Information Network . ( 2006 ). Search results for “diabetes” and “macaw” . Available (by membership) online at : http://www.vin.com ( accessed 19 May 2007 ).
- Whittington , C.A. and Kowdley , K.V. 2002 . Haemochromatosis . Alimentary Pharmacology & Therapeutics , 16 : 1963 – 1975 .
- Wilson , J.G. , Lindquist , J.H. , Grambow , S.C. , Crook , E.D. and Maher , J.F. 2003 . Potential role of increased iron stores in diabetes . The American Journal of the Medical Sciences , 325 : 332 – 339 .
- Wissman , M.A. Budgerigar and Cockatiel Clinics . ( 2003 ). Proceedings of the 75th Annual Western Veterinary Conference February 15–19, Las Vegas, Nevada . ( Article available at http://www.vin.com/Members/Proceedings/Proceedings.plx?CID_wvc2003&PID_pro3514&O_VIN ).
- Worell , A.B. 1997 . “ Toucans and mynahs ” . In Avian Medicine and Surgery , Edited by: Altman , R.B. , Clubb , S.L. , Dorrestein , G.M. and Quesenberry , K. 910 – 917 . Philadelphia : W.B. Saunders Company .
- Worell , A.B. 2000 . “ Ramphastids ” . In Avian Medicine , Edited by: Tully , T.N. , Lawton , M.P.C. and Dorrestein , G.M. 297 – 311 . Woburn : Butterworth-Heinemann .