Abstract
A real-time quantitative polymerase chain reaction was utilized to study the in vivo replication of Marek's disease vaccine viruses and of virulent oncogenic strains. In the first of four experiments, the growth of the herpes virus of turkeys (HVT) vaccine was detectable in various organs of infected chicken embryos, with the highest viral loads being present in the spleen. No evidence was obtained for replication of serotype-1 Marek's disease viruses in embryos. In the second experiment, viral loads were measured in several organs of chickens after administration of the Rispens and HVT vaccines immediately after hatching. Lowest levels were noted for the Rispens strain after 1 to 8 weeks. By contrast, HVT vaccine grew well in all tested organs, with the highest loads being present in the spleen. Highest loads were observed in unvaccinated birds after challenge with the highly virulent strain MPF57 at day 8, especially in lymphoid organs. A positive relationship was observed between viral load and clinical signs, including tumour formation. In a third study, viral loads were measured in the organs of chickens administered the Rispens vaccine on the day of hatch and challenged at day 8 with MPF57. High levels of clinical protection were afforded against MPF57 by the Rispens vaccine but, in confirmation of earlier findings, sterilizing immunity was not induced. In a fourth study, two experiments were conducted—in which viral loads were measured after challenge of chickens vaccinated with HVT in ovo or at day 1 after hatching. Similar protection was achieved in birds vaccinated in ovo on embryonic days 11 and 17, although protection was slightly, but not significantly, lower than for birds vaccinated at day 1.
Evaluation moléculaire des réponses à la vaccination et à l'épreuve par des virus de la maladie de Marek
La réaction de polymérisation en chaîne, quantitative en temps réel (QPCR), a été utilisée pour étudier la réplication in vivo des virus vaccinaux de la maladie de Marek et des souches virulentes oncogènes. Dans la première des quatre expérimentations, la multiplication de l'herpesvirus de la dinde (HVT) (vaccin) a été détectée dans divers organes d'embryons de poulet infectés; les charges virales les plus élevées étant observées dans la rate. Il n'a pas été mis en évidence de réplication des virus de la maladie de Marek (MDVs) de sérotype 1 dans les embryons. Dans la deuxième expérimentation, les charges virales ont été mesurées dans différents organes de poulets après l'administration des vaccins Rispens et HVT, immédiatement après l'éclosion. Les niveaux les plus faibles ont été enregistrés pour la souche Rispens après 1 à 8 semaines. Au contraire, le vaccin HVT s'est bien multiplié dans tous les organes testés, et la charge virale la plus élevée a été observée dans la rate. Les charges virales les plus élevées ont été enregistrées chez les sujets non vaccinés après épreuve avec la souche hautement virulente MPF57 à 8 jours, principalement dans les organes lymphoïdes. Une relation positive a été observée entre la charge virale et les symptômes, incluant la formation de tumeurs. Dans la troisième étude, les charges virales ont été mesurées dans les organes des poulets qui ont reçu le vaccin Rispens le jour de l'éclosion, et qui ont été éprouvés à 8 jours avec la souche MPF57. De hauts niveaux de protection clinique contre la souche MPF57 ont été conférés par le vaccin Rispens, mais en confirmation des premiers résultats, une immunité stérilisante n'a pas été induite. Dans une quatrième étude, deux expérimentations ont été conduites dans lesquelles les charges virales ont été mesurées après l'épreuve chez des poulets vaccinés avec la souche HVT in ovo ou à 1 jour (D-1) après l'éclosion. Une protection similaire a été obtenue chez les sujets vaccinés in ovo au 11ème et au 17ème jour embryonnaire, bien que la protection ait été légèrement, mais non significativement, inférieure à celle de sujets vaccinés à 1 jour.
Molekulare Bestimmung von Antworten auf eine Vakzination und Belastungsinfektion mit dem Virus der Marekschen Krankheit
Die Bestimmung der in vivo-Replikation von Impf- und virulenten onkogenen Virusstämmen der Marekschen Krankheit wurde mittels einer quantitativen Real Time-Polymerasekettenreaktion (QPCR) durchgeführt. In dem ersten von vier Experimenten konnte die Vermehrung eines Putenherpesvirus (PHV)-Impfstamms in verschiedenen Organen von infizierten Hühnerembryonen festgestellt werden, wobei die Milz den höchsten Virusgehalt aufwies. Eine Replikation von Serotyp-1-Virusstämmen der Marekschen Krankheit in Embryonen konnte nicht nachgewiesen werden. Im zweiten Versuch wurden die Virusgehalte in mehreren Organen von Hühnerküken gemessen, denen unmittelbar nach dem Schlupf Rispens- und PHV-Vakzine appliziert worden waren. Die niedrigsten Virusgehalte wurden für den Rispens-Stamm 1-8 Wochen nach der Impfung ermittelt. Das PHV-Impfvirus vermehrte sich in allen getesteten Organen gut, am besten jedoch in der Milz. Die höchsten Virusgehalte ließen sich bei nicht vakzinierten Tieren am 8. Tag nach der Infektion mit dem hoch virulenten Stamm MPF57 insbesondere in den lymphoiden Organen feststellen. Zwischen den Virusgehalten und den klinischen Symptomen einschließlich der Tumorbildung bestand eine positive Beziehung. Im dritten Versuch wurden die Virusgehalte in den Organen von Hühnerküken bestimmt, die am Schlupftag mit dem Rispens-Stamm vakziniert und am 8. Tag mit MPF57 belastungsinfiziert worden waren. Durch die Rispens-Vakzine wurde ein hohe klinische Schutzrate gegen den MPF57-Stamm erreicht, aber in Übereinstimmung mit früheren Untersuchungsergebnissen wurde keine sterile Immunität induziert. In der vierten Studie wurden zwei Experimente durchgeführt, in denen die Virusgehalte nach Belastungsinfektion von Hühnerküken, die entweder in ovo oder am Tag 1 (T-1) nach dem Schlupf mit PHV vakziniert worden waren, bestimmt wurden. Bei den am 11. und 17. Bebrütungstag in ovo-vakzinierten Küken wurde ein ähnlicher Impfschutz erreicht, wobei die Schutzwirkung geringfügig, aber nicht signifikant, schlechter war als bei den am T-1 vakzinierten Tieren.
Evaluación molecular de las respuestas a la vacunación y desafío con virus de la enfermedad de Marek.
Se usó una reacción en cadena de la polimerasa cuantitativa a tiempo real (QPCR) para evaluar la replicación in vivo de cepas vacunales y oncogénicas virulentas de virus de la enfermedad de Marek. En el primero de los cuatro experimentos, se detectó el crecimiento de la vacuna de herpes virus del pavo (HVT) en varios órganos de embriones de pollo infectados, siendo el bazo el que mostró cargas víricas mayores. No se obtuvo ninguna evidencia de la replicación de virus de de Marek (MDVs) del serotipo 1 en los embriones. En el segundo, se evaluaron las cargas víricas en diversos órganos de los pollos después de la administración inmediatamente tras el nacimiento de las vacunas HVT y Rispens. Los niveles más bajos se observaron con la cepa Rispens tras 1-8 semanas. En comparación, la vacuna HVT creció bien en todos los órganos evaluados, y las cargas víricas mayores se detectaron en el bazo. En las aves no vacunadas las mayores cargas se observaron especialmente en los órganos linfoides, a los 8 días tras el desafío con la cepa altamente virulenta MPF57. Se observó una relación positiva entre la carga vírica y los signos clínicos, incluyendo la formación de tumores. En el tercer estudio, se evaluaron las cargas víricas en los órganos de pollos vacunados con Rispens al día de nacimiento y desafiados a los 8 días con MPF57. Se consiguieron niveles de protección altos frente a MPF57 mediante la vacunación con Rispens, pero no se indujo inmunidad esterilizante, al igual que en observaciones previas. En el cuarto estudio, se realizaron dos pruebas, en las cuales las cargas víricas se midieron tras el desafío de pollos vacunados con HVT in ovo o al día 1 post nacimiento (D-1). Se consiguió una protección similar en las aves vacunadas in ovo a los 11 y 17 días de fase embrionaria (ED), aunque la protección fue levemente, aunque significativamente, menor que en las aves vacunadas a D-1.
Introduction
Marek's disease virus (MDV) is a highly infectious, oncogenic herpesvirus of chickens and is the aetiological agent of Marek's disease (MD), an economically significant disease of poultry. MDVs have been classified into three serotypes based on antigenic and genetic differences; some serotype-1 (MDV-1) viruses are responsible for oncogenic disease, while all serotype-2 (MDV-2) and serotype-3 (MDV-3) viruses are non-oncogenic. Since the introduction of vaccines in the early 1970s, consisting of mainly the serotype-3 herpesvirus of turkeys (HVT) (Okazaki et al., Citation1970; Witter et al., Citation1970), the incidence of MD has been greatly reduced. However, economic losses from MD have increased since the late 1970s due to the evolution and emergence of strains of increasing virulence (Witter, Citation1997). Responses to increases in virulence of field MDVs have included the use of combinations of serotype-2 and serotype-3 vaccine viruses (Calnek et al., Citation1983; Witter, Citation1982; Witter & Lee, Citation1984) and, more recently, attenuated serotype-1 vaccines such as the Rispens CVI988 strain (Rispens et al., Citation1972a,Citationb). More recently, recombinant vaccines prepared from fowlpox virus and HVT have been considered (Nazerian et al., Citation1992; Ross et al., Citation1996). The latest generation of recombinant MD vaccines is based on bacterial artificial chromosome and cosmid technologies (Tischer et al., Citation2002; Petherbridge et al., Citation2003; Cui et al., Citation2005).
Despite the effectiveness of vaccination, economic losses from MD caused by evolutionary changes to serotype-1 viruses are severe in many parts of the world. Comparatively little is known regarding the mechanism(s) of action of MD vaccines that are in widespread use, and there are many aspects of the pathogenesis of MDV that are not well understood. Most studies on pathogenesis were carried out at a time when infectious virus could only be estimated by traditional cell culture techniques, which are relatively insensitive, especially for highly virulent field strains (DeLaney et al., Citation1998). Cell culture-grown viruses probably only consist of a small proportion of the total viral population (Clementi et al., Citation1995) and may not be representative of viruses that cause disease in infected chickens. The role of vaccines in the prevention of MD is estimated from challenge with highly virulent MDV strains and the observation of clinical signs and histopathological changes over an extended period (Witter et al., Citation1995).
Assays using the real-time quantitative polymerase chain reaction (QPCR) have advanced significantly over recent years, facilitating measurement of the viral load and studies of viral pathogenesis (Mackay et al., Citation2002). Recent applications to MDV include the measurement of viral loads in feather tips, peripheral blood lymphocytes, lymphocyte organs of infected chickens and field dust samples (Baigent et al., Citation2005; Abdul-Careem et al., Citation2006; Islam et al., Citation2006; Renz et al., Citation2006). Baigent et al. (Citation2006) also used the QPCR to confirm successful vaccination of chickens by examining the CVI988 vaccine virus loads in feather tips.
In the present study, a QPCR was established to measure the growth kinetics of two currently used vaccine viruses and two highly virulent Australian challenge strains in the organs of infected chickens and chicken embryos. Data so obtained were then applied to study the role of vaccination in reducing viral loads following challenge and to examine the relationship between clinical signs, including tumour formation.
Materials and Methods
Viruses
HVT (cell-associated batch 1576050) and Rispens (CVI988, batch M02102) vaccines were obtained from Fort Dodge (Australia) Pty Ltd (Castle Hill, NSW, Australia) and Bioproperties (Australia) Pty Ltd (Ringwood North, Victoria, Australia). The highly virulent MPF57 (passage 12) and Woodlands (passage 14) challenge viruses were supplied by the Virology Laboratory, School of Applied Sciences, RMIT University (Bundoora, Victoria, Australia). The properties of these viruses have been described by DeLaney et al. (Citation1998).
Specific-pathogen-free eggs and chickens
Specific-pathogen-free (SPF) embryonated eggs and 1-day-old chickens (White-Leghorn strain) were obtained from SPAFAS (Australia) Pty Ltd (Woodend, Victoria, Australia). All chicken experiments were approved by the Animal Care and Ethics Committee of RMIT University.
DNA extraction
The commercial DNeasy® Tissue kit (QIAGEN Corporation, Germantown, MD, USA) was used to extract DNA from the tissues of chicken and chicken embryos in all experiments. A 25 mg tissue sample (up to 10 mg for spleen) was cut into small pieces, and mixed with 180 µl ATL buffer and 20 µl proteinase K. After incubation at 55°C until the tissue was completely lysed, 200 µl Al buffer was added and the mixture maintained at 70°C for 10 min. The mixture was then pipetted into the DNeasy spin column and centrifuged at 8000 rpm for 1 min. The tissue DNA was then eluted with 200 µl water followed by two washes with buffers AW1 and AW2, provided in the kit.
Real-time quantitative polymerase chain reaction
The MDV serotype-1 PCR assay utilized the forward (5′-CAT GCA AGT CAT TAT GCG TGA C-3′) and reverse (5′-TGT TTC CAT TCT GTC TCC AAG A-3′) primers designed by Zhu et al. (Citation1992), which amplified a 200 bp fragment of the glycoprotein C gene (location 696 to 895; Genbank AY 129979) from the nucleotide sequence described for MDV-1 (Shamblin et al., Citation2004). The MDV serotype-3 (HVT) PCR assay utilized the forward (5′-ATG GAA GTA GAT GTT GAG TCT TCG-3′) and reverse (5′-CGA TAT ACA CGC ATT GCC ATA CAC-3′) primers described by Handberg et al. (Citation2001) to amplify a 505 bp fragment from the US3 region (location 4222 to 4727; Genbank X68653) of the HVT sequence published by Zelnik et al. (Citation1993).
The above 200 and 505 bp PCR products were amplified from MDV-1 and MDV-3 DNAs, purified using a QIA quick PCR purification kit (QIAGEN Corporation, Germantown, MD, USA) and cloned into pGEM-T-easy vector (Promega Corporation, Madison, WI, USA) using standard procedures. After purification using Wizard® plus SV minipreps (Promega), the concentration of recombinant plasmids in the undiluted miniprep samples was determined by spectrophotometry at 260 nm. The copy number of the recombinant plasmid was calculated according to their concentration and relative molecular mass. The method for constructing a standard curve was similar to that described by Pevenstein et al. (Citation1999) and Markowski-Grimsrud et al. (Citation2002); 10-fold serial dilutions of each recombinant plasmid, comprising 1 to 100 copies, were represented in each standard curve. Each dilution was tested in duplicate.
The real-time QPCR was performed using the iCycler (BIO-RAD Laboratories, Hercules, CA, USA) in 50 µl SYBR® Green JumpStart™ Taq readyMix™ (Sigma Corporation, St. Louis, MO, USA), containing 0.2 µM each forward and reverse primer and 5 µl sample extract DNA. The mixture was denatured by one cycle at 94°C for 2 min and then amplified by 35 cycles, each consisting of a heating step at 95°C for 15 sec, a cooling step at 56°C for 1 min, and a further heating step at 72°C for 1 min. Following amplification, the PCR products were analysed by a melting curve programme consisting of one cycle of heating to 95°C, cooling to 65°C for 1 min and then heating to 95°C at a temperature transition rate of 0.3°C/sec. The products were then continuously held in one cycle at 4°C.
After completion of the QPCR, the copy number of the target sequence was calculated by plotting the logarithm of fluorescence versus the threshold cycle, and setting a baseline×axis. The baseline identified the cycle at which the log-linear signal could be distinguished from the background for each sample. The x-axis crossing point of each standard was measured and plotted against the logarithm of concentration to produce a standard curve. The concentrations of the target sequence in test samples prepared from 10% (w/v) organ extracts were determined by extrapolation from the curve. The limit of detection for the test was 10 MDV genome copies per sample and the test was equally sensitive for HVT and serotype-1 viruses (unpublished observations).
Experimental design
Experiment 1: growth on HVT and MDV-1 in chicken embryos
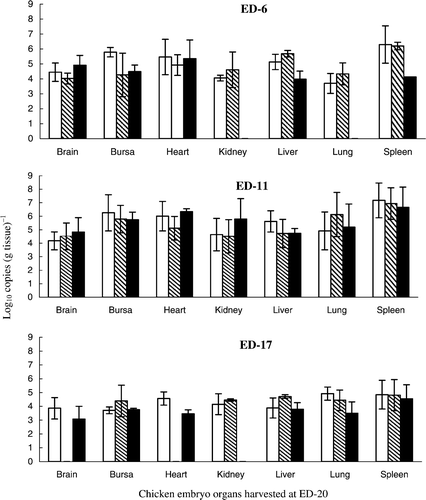
HVT was administered by inoculation via the yolk-sac at embryonic day (ED)-6, via the allantoic cavity at ED-11 or directly into the embryo at ED-17. The respective inoculation doses for each route were 57.2, 572 and 5720 plaque-forming units (PFU)/embryo in groups of four to six (see ). Following inoculation, any dead embryos were discarded within the first 24 h. A total of seven organs (brain, bursa, heart, kidney, liver, lung and spleen) from the surviving embryos at ED-20 were harvested and DNA extracts were prepared for testing of HVT viral load by QPCR.
Table 1. Survival rates (%) of the chicken embryos after inoculation with different HVT inocula
The viral loads of three serotype-1 MDVs (the virulent Woodlands and MPF57 strains and a commercial Rispens vaccine) were investigated following inoculation of chicken embryos. The inoculum dose per egg was 10, 60, 120 and 550 PFU for the Woodlands strain at passage 14 (Wp14), was 3×105 PFU for Wp78, was 200 PFU for MPF57, and was 6450 PFU for the Rispens vaccine. As with the previous experiment, chicken embryos were inoculated with each inoculum at ED-6, ED-11 and ED-17. Seven organs from the surviving embryos at ED-20 were harvested and weighed, and the DNA extracts were prepared for testing for HVT DNA load by QPCR.
Experiment 2: kinetics of MDV-1 and MDV-3 growth in chickens
One-day-old SPF chickens were divided into two groups, each of 24 birds, and vaccinated subcutaneously with cell-associated HVT (11 440 PFU/0.2 ml/dose/bird) and Rispens (6450 PFU/0.2 ml/dose/bird) vaccines. The remaining 28 unvaccinated chickens were challenged intra-abdominally with the MPF57 challenge virus (200 PFU/0.2 ml/bird) at day 8. Vaccinated and challenged groups were held in separate isolators and three chickens per group were culled each week until week 8. Their body, spleen and bursa weights were measured, and the viral DNA target load for each of the eight organs (brain, bursa, heart, kidney, liver, lung, spleen and thymus) was measured by QPCR. At the same time, isolations of HVT and the Rispens strain were attempted in SPF chicken embryo fibroblast and embryo kidney cultures, respectively. A group of non-inoculated chickens was included in Experiment 2, but no viral DNA could be detected.
Experiment 3: measurement of viral load in chickens administered the Rispens vaccine and subsequently challenged
Eighty-four 1-day-old SPF chickens were divided into three groups and kept in separate isolators. The three experimental groups comprised: (a) unchallenged vaccinated birds, (b) unvaccinated birds that were challenged, and (c) birds that were vaccinated and challenged. The Rispens vaccine was inoculated subcutaneously with the dose recommended by the manufacturer (6450 PFU/0.2 ml/bird) at day 1, and the challenge virus (MPF57, 200 PFU/0.2 ml/bird) was administrated intra-abdominally at day 8. The clinical condition of all birds was monitored daily and three birds per group were sacrificed each week throughout the 8-week period of the experiment. Each time, the whole body, spleen and bursa weights of individual birds were determined and the viral DNA loads in six organs (brain, bursa, liver, lung, spleen and thymus) were measured by QPCR.
Experiment 4: comparison of different routes for the administration of HVT vaccine
This experiment was conducted as two trials. In Trial 1, 58 SPF chicken embryos were vaccinated with HVT vaccine (11 440 PFU/0.2 ml/dose/egg) at ED-11 via the allantoic sac. After hatching, the chickens were divided into two groups. Twenty-three birds of the first group were tested for HVT viral load growth, while 24 birds from the second group were intra-abdominally challenged with MPF57 (200 PFU/0.2 ml/bird) at day 8. At the same time, 62 SPF embryos from same batch were vaccinated by inoculating directly to the embryo at ED-17. After hatching, 22 birds were used to study HVT growth and the remaining 29 birds were challenged with MPF57, as described above Two chickens from each group were culled each week and their bursa:body weight and spleen:body weight ratios were determined. The viral DNA load in five organs (brain, kidney, liver, lung and spleen) was measured by QPCR. Birds from a group of unvaccinated chickens hatched from the same batch of SPF eggs were challenged with MPF57 at day 8 and served as positive controls.
In Trial 2, SPF chicken embryos were divided into three groups. Thirty-one embryos from the first group and 33 embryos from the second were vaccinated, as described above, with the same dose of HVT used previously at ED-11 or ED-17. The third group of 47 embryos were unvaccinated during embryogenesis. After hatching, 23 chickens from the third group were subcutaneously vaccinated with HVT at day 1 (D-1), while another unvaccinated 16 birds served as controls. Birds from all groups were challenged with MPF57 at D-8, as described previously. They were kept in isolators for 8 weeks, the duration of the experiment, and only those showing clinical signs of MD were culled. At week 8, when the experiment was terminated, the body, bursa and spleen weights were determined on all dead and culled chickens, along with those that survived. The HVT and MPF57 DNA loads in the spleen were determined by QPCR.
Results
Distribution of MDV loads in chicken embryos infected with HVT and MDV-1
The results in show that all organs tested were positive and that, for all groups, the highest HVT DNA levels were present in the spleen, compared with other organs (Student's t-test, P < 0.05 when tested pairwise). Viral DNA was not detected in the kidneys and lungs of embryos inoculated at ED-6 with the lowest dose (57.2 PFU). Viral DNA was detected in all other groups but there were no significant differences between HVT DNA loads in the same organs of embryos that received different sized inocula at ED-6 or ED-11 (P > 0.1). For embryos inoculated at ED-6 or ED-11, the range of loads [expressed as log10 copies (g tissue)−1] were 4.1 to 4.9 and 4.2 to 4.8 (brain), 4.3 to 5.8 and 5.7 to 6.3 (bursa), 4.9 to 5.5 and 5.1 to 6.3 (heart), 4.1 to 4.6 and 4.5 to 5.8 (kidney), 4.0 to 5.7 and 4.7 to 5.6 (liver), 3.7 to 4.3 and 4.9 to 6.1 (lung), 4.1 to 6.3 and 6.7 to 7.2 (spleen), respectively. However, the HVT DNA target loads for both groups were significantly higher than for the group inoculated at ED-17 (brain, 3.1 to 3.9; bursa, 3.7 to 4.4; heart, 3.5 to 4.6; kidney, 4.2 to 4.5; liver, 3.8 to 4.7; lung, 3.5 to 4.9; and spleen, 4.5 to 4.8 log10 copies (g tissue)−1 (P < 0.05). The embryo survival rates for groups inoculated at ED-6, ED-11 and ED-17 were 38.5%, 89.5% and 94.1% ().
Figure 1.
Quantification of total HVT loads at ED-20 in organs of chicken embryos inoculated on different days of embryogenesis. Inoculum per bird: 5720 PFU, □; 572 PFU, ![]()
Increases in serotype-1 Woodlands, MPF57 and Rispens viral DNA could not be detected in any organs prepared from individual embryos at ED-20 [limit for detection 2.5 log10 copies (g tissue)−1, taking into account the tissue protein concentration of each reaction mixture], indicating that viral growth did not occur (data not shown). shows that the survival rates for embryos inoculated at ED-6, ED-11 and ED-17 with the same viruses were 75%, 78.6% and 93.3%.
Table 2. Survival rates (%) of chicken embryos after inoculation with different MDV-1 strains at different days of embryogenesis
MDV loads in the organs of chickens inoculated with HVT, the Rispens vaccine and MPF57
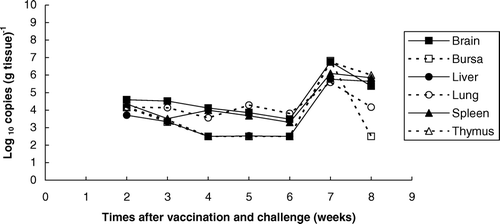
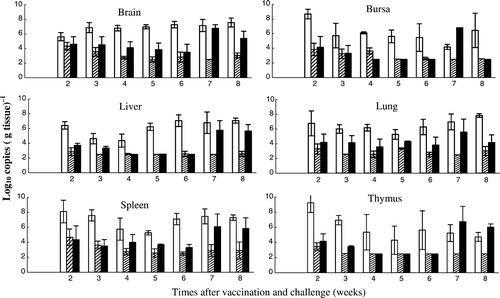
Serotype-3 HVT vaccine viruses grew in all organs tested following inoculation at day 1, with the highest viral copy number [5.0 to 6.6 log10 copies (g tissue)−1] being present in the spleen at weeks 2 to 8 p.i. (). Viral loads within the range of 3.1 to 6.2 log10 copies were present in other organs. By contrast, loads induced by the serotype-1 Rispens vaccine strain were much lower and were less than the detection limit of the QPCR for the heart, kidney, liver, lung and thymus. Despite its relatively poor growth, the highest viral DNA loads [2.5 to 4.7 log10 copies (g tissue)−1] were also observed in the spleen.
Figure 2. Growth of the HVT and Rispens vaccines and the virulent challenge virus MPF57 in the organs of chickens. Birds were inoculated by HVT or Rispens at day 1, challenged with MPF57 at day 8 and sacrificed at weekly intervals. Viral DNA was estimated by QPCR. The limit for detection of HVT, Rispens and MPF57 was 2.5 log10 copies (g tissue)−1. Data for each time point represent the mean DNA load for three organs.
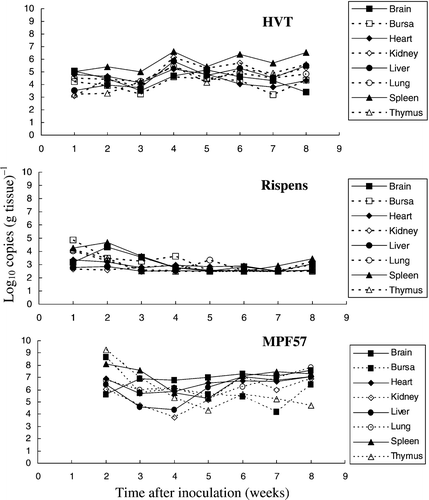
High levels of MPF57 target DNAs were detected in the lymphoid organs soon after challenge [8.2, 9.3 and 8.2 log10 copies (g tissue)−1 for the spleen, thymus and bursa]. Decreases then occurred, followed by increases in the brain, heart, kidney, liver and lungs following the development of tumours at weeks 5 to 6 p.i. All three MDVs were isolated from the lymphocytes, peripheral blood or spleen in cell culture from weeks 2 to 8 (data not shown).
Birds challenged with MPF57 grew slower than those administered either HVT or the Rispens vaccine, and clinical signs included depression, paralysis and death. The first peak in mortality occurred within 2 weeks p.i. in the absence of macroscopic lesions, whereas the second was observed at weeks 6 to 7 and was associated with the development of tumours. Pallor and a reduction in size were features of both the bursa and thymus of challenged control birds, which were scored visually according to the criteria of DeLaney et al. (Citation1998). The spleen:body weight ratios increased during the second peak of clinical signs following the development of tumours at week 6. In the challenge group, most birds (56.7%) showed clinical signs and gross tumours.
Protective responses induced by the Rispens vaccine
In the group given only the Rispens vaccine, none of the birds showed clinical signs at any stage, whereas in the unvaccinated, challenged group 56.7% of the birds had MD-specific clinical signs and an early peak in mortality was observed at week 2. By contrast, no birds in the vaccinated and challenged group showed clinical signs throughout the experiment. However, at postmortem, one bird was found to have an enlarged liver and another had an enlarged liver and spleen, with small tumours in the kidney at week 6 post-challenge. At the termination of the experiment (week 8) only 5.9% of the chickens that were vaccinated and challenged presented with clinical signs of MD. In the same group, some atrophy of the bursa and thymus was observed at week 6. Of particular significance, the bursa:body weight ratios for this group were similar to those of the vaccinated unchallenged group (P>0.1) but were higher than those of the unvaccinated challenged group. In addition, the spleen:body weight ratios were similar to those observed in the control vaccinated group but lower than for the challenge control group at week 4.
The total viral DNA loads (for both the Rispens and MPF57 viruses) in the vaccinated and challenged group were initially low in all organs tested [2.5 to 4.5 log10 copies (g tissue)−1] but increased to 4.2 to 6.8 log10 copies (g tissue)−1 from week 5 (). Of particular significance, the total viral DNA loads in all organs tested (brain, bursa, liver, lungs, spleen and thymus) for the vaccinated and challenged group [2.5 to 6.8 log10 copies (g tissue)−1] were much lower than for the unvaccinated group challenged with MPF57 [3.7 to 9.2 log10 copies (g tissue)−1], but were equal to or higher than for the group that received Rispens vaccine alone [2.5 to 4.9 log10 copies (g tissue)−1] (). Viral loads for the vaccinated and challenged birds in all organs throughout the experiment were significantly lower than the challenge control group (Student's t-test, P < 0.05, when tested pairwise). Differences between the challenge group and the group receiving Rispens vaccine, alone, were not significant (P > 0.1).
Responses to challenge following vaccination with HVT at different times of embryogenesis
Trial 1
Groups that were vaccinated with HVT at ED-11 and ED-17 had similar hatching rates of 81% (47/58) and 82.3% (51/62). Increases in body weight for both challenged groups 6 to 8 weeks after hatch were less than those observed in the two groups that received vaccine alone (data not presented). There were no significant differences in the bursa:body weight ratios between the four groups during the first 4 weeks, but some bursal atrophy was observed in the challenge groups after week 5 (data not presented). The spleens of birds in the challenge groups were either enlarged or had tumours and increased spleen:body weight ratios during the last 3 weeks of the experiment. The percentage of birds showing MD clinical signs in the group vaccinated at ED-17 (52.2%) was slightly higher than that of the MD-positive birds (45.8%) in the group vaccinated at ED-11. In addition, clinical signs of MD appeared earlier in the group vaccinated at ED-17 than in the group vaccinated at ED-11. All birds in the control challenge group were MDV-positive by QPCR and most birds showed clinical signs following the first peak in mortality after challenge (data not presented).
In the groups vaccinated at ED-11, similar HVT loads were observed in the organs tested, irrespective of whether the birds had been challenged. Actual levels observed were 5.2 to 8.2 log10 copies (g tissue)−1 for the challenged group and 5.2 to 8.0 log10 copies (g tissue)−1 in the vaccinated group for the brain, kidney, liver and lungs, and were 7.4 to 9.7 and 7.2 to 9.5 log10 copies (g tissue)−1 for the spleen. Similar results were found in the groups vaccinated at ED-17 with or without challenge [viral DNA levels of 4.5 to 7.0 and 4.1 to 6.4 log10 copies (g tissue)−1 for the brain, kidney, liver and lungs; and of 6.6 to 8.4 and 7.6 to 8.6 log10 copies (g tissue)−1 for the spleen]. The HVT viral DNA loads were higher following vaccination at ED-11 than at ED-17 (P < 0.05), irrespective of whether or not the birds had been challenged. Similar HVT loads were observed in spleen between the groups vaccinated at ED-11 and ED-17 following challenge.
The viral loads of birds challenged with MPF57 were similar but relatively low in the groups vaccinated at ED-11 and ED-17. Actual levels were 3.2 to 5.0 and 2.6 to 4.4 log10 copies (g tissue)−1 for the brain, kidney, liver and lungs, and were 3.5 to 6.9 and 3.2 to 7.0 log10 copies (g tissue)−1 for the spleen of vaccinated and unvaccinated birds, respectively.
Trial 2
Hatching rates for chickens vaccinated at ED-11 and ED-17 were 71% and 75.8%, and the rate for the unvaccinated group was 87.2%. MD-positive chickens in the challenge control group comprised 93.8% and most showed clinical signs during the first peak of mortality. Differences in the percentages of birds showing MD-specific clinical signs between the groups vaccinated at ED-11 or ED-17 (57.9% and 58.3%) were not significant, but more birds showed signs at an earlier stage for the group vaccinated at ED-17 compared with ED-11 ().
Table 3. Protective efficacy (%) of HVT vaccine administered at different times
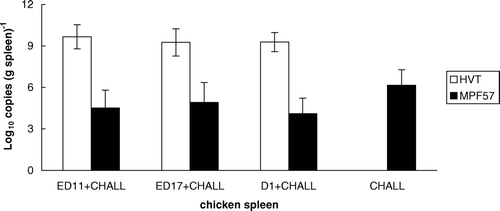
For the group vaccinated at D-1 and subsequently challenged, 52.2% of birds showed clinical signs, none of which were present during the earlier period of the experiment (). Bursal atrophy was observed in most birds of the challenge control group and only in some vaccinated at ED-11 and ED-17 (data not presented). However, only one bird showed bursal atrophy following challenge in the group vaccinated at D-1. Differences in the spleen:body weight ratios between the four groups were not significant, and very few MD-positive chickens from any group had enlarged spleens. The average HVT DNA loads were 9.7, 9.3 and 9.3 log10 copies (g spleen)−1 for the groups vaccinated at ED-11, ED-17 and D-1. The mean viral DNA levels of the three vaccinated groups after challenge with MPF57 were 4.5, 4.9 and 4.1 log10 copies (g tissue)−1, which were significantly lower than that of the control group (6.2 log10 copies (g tissue)−1; P<0.05) ().
Discussion
Real-time QPCR is a highly sensitive analytical tool that allows estimates of viral load for MDV in the organs of infected chickens (Gimeno et al., Citation2004; Baigent et al., Citation2005). Davidson et al. (Citation2002) reported that CVI 988 DNA was present in vaccinated chickens at low levels and was difficult to detect by conventional polymerase chain reaction. QPCR overcomes such difficulties and other problems associated with cell culture assays. It allows the direct measurement of MDV DNA in the spleen—the key target organ of infected chickens—thereby providing the basis of a model to measure responses to vaccination and challenge.
Increases in HVT DNA were shown in several organs of infected chicken embryos, with the highest copy number being present in the spleen, irrespective of the time of inoculation or the size of the inoculum used (). Inoculation of chicken embryos at ED-6 was associated with higher rates of mortality (38.5%), but at ED-11 the rates of survival (89.5%) were comparable with those obtained at ED-17 (94.1%), the normal time of embryo vaccination. However, growth in embryos of the Rispens vaccine strain or the virulent strain MPF57 (both serotype-1 viruses) could not be detected. St Hill and Sharma (Citation2000) reported that neither the pp38 gene nor infectious virus could be detected in chicken embryos infected with serotype-1 viruses by the amniotic route at ED-16. By contrast, replication of the same viruses could be detected 5 days after intravenous inoculation at ED-16. St Hill et al. (Citation2004) further showed that both HVT and serotype-1 DNA were detectable at ED-20 by in situ hybridization, following amniotic inoculation of virus at ED-17. However, only HVT virus could be recovered from the lungs of embryos at ED-20. These results can be explained in terms of variations to embryo susceptibility following infection by different routes and the relatively low sensitivity of methods used to detect virulent serotype-1 viruses in cell culture. The present study confirmed differences in the extent of virus replication in embryos infected with different serotypes and further demonstrates that chicken embryos are unsuitable for the growth and isolation of serotype-1 MDVs.
HVT vaccine viral DNA could be readily detected in the organs of chickens vaccinated at D-1, with the highest viral loads also being detected in the spleen (). By contrast, much lower loads were induced by the Rispens vaccine in most organs—except the spleen, where moderate loads were observed. For the virulent challenge virus MPF57, however, higher viral DNA copy numbers were observed in all test organs, compared with HVT and the Rispens vaccines (). Peak MPF57 loads for the spleen and other lymphoid organs were detected after 2 to 3 weeks, which confirms that the spleen is the best organ for the isolation of virulent isolates of serotype-1 viruses. These results indicate both differences in the pathogenesis of different serotypes of MDV and differences between serotype-1 viruses. A direct relationship between the viral DNA target copy number of the virulent challenge virus MPF57 in the organs of infected chickens and the development of clinical signs including tumour formation was also observed ().
The Rispens vaccine provided good protection when administered to 1-day-old chickens, which were subsequently challenged with MPF57 (). Reductions in the levels of the challenge virus in spleen and other organs, compared with unvaccinated controls, occurred over a period of 8 weeks (). These reductions were consistent with clinical measures of protection, including bursa:body weight ratios. Sterilizing immunity was not achieved by vaccination, even in the absence of clinical signs in the challenged birds. Similarly, viral DNA for the Rispens vaccine strain was present in all organs of vaccinated and unchallenged birds over the experimental period, although at reduced levels. These data support earlier approaches that measure vaccine efficacy based on the measurement of viraemia following vaccination (Karpathy et al., Citation2003). Of interest is the biphasic nature of growth for both the virulent challenge and Rispens vaccine viruses, with the initial peak occurring at 2 to 3 weeks and the second at 6 to 8 weeks (). These times coincide precisely with events in the pathogenesis of MDV involving an earlier lytic phase of B-cell infection, followed by a later phase of infection of T cells that is associated with the development of tumours (Shek et al., Citation1983; Calnek et al., Citation1984; Baigent et al., Citation1998).
Preliminary experiments of HVT growth in chicken embryos showed that an unacceptable number of deaths were associated with embryo vaccination at ED-6. Consequently, experiments were carried out to measure responses to embryo vaccination at ED-11 and ED-17. In Trial 1, the percentage of MD positives in the group vaccinated at ED-11 was slightly lower than that of the group vaccinated at ED-17, and HVT loads were found to be higher following vaccination at ED-11 than ED-17. These results suggest that HVT vaccination at ED-11 is more efficacious against MPF-57 challenge than at ED-17. However, for birds vaccinated in ovo at ED-11 and subsequently challenged, there was an indication in some that clinical signs could be observed later than in the ED-17 (data not presented) but numbers in both groups were small. Our data also confirm the findings of St Hill et al. (Citation2004) obtained by in situ hybridization—that embryonic lungs support the growth of HVT after in ovo vaccination.
In Trial 2 no significant differences in the percentages of MD-positive birds were observed between groups vaccinated at ED-11 and ED-17 and subsequently challenged with MPF57. Levels of protection were both slightly lower than for the group vaccinated at D-1 (). Moderate levels of clinical protection were achieved by all three vaccination regimes. HVT induced higher levels of viraemia but lower levels of protection than the Rispens vaccine, but both failed to induce sterilizing immunity to the MPF57 challenge MDV virus ( and ).
MD remains a continuing problem for the entire chicken industry and, in the absence of effective vaccination and management practices, has the capacity to induce disease in 15% to 20% of birds. The situation is complicated by the ability of serotype-1 viruses to undergo changes in virulence and by uncertainties in disease susceptibility that frequently occur following the introduction of new genetic stock. Despite these problems, the current principal control strategy involving intensive vaccination is likely to remain in place for the foreseeable future. The challenge model described above using QPCR to measure MDV viral loads should be a useful tool for the study and evaluation of new vaccines.
Acknowledgements
The authors thank Australian Rural Industries Research and Development Corporation (RIRDC) for supporting this project.
Additional information
Notes on contributors
Jianming Tan†
Present address: Virology and Immunology, Primary Industries Research Victoria, Department of Primary Industries, 475 Mickleham Road, Attwood, VIC 3049, AustraliaReferences
- Abdul-Careem , M.F. , Hunter , B.D. , Nagy , É. , Read , L.R. , Sanei , B. , Spencer , J.L. and Shayan , S. 2006 . Development of a real-time PCR assay using SYBR Green chemistry for monitoring Marek's disease virus genome load in feather tips . Journal of Virological Methods , 133 : 34 – 40 .
- Baigent , S.J. , Ross , L.J. and Davison , T.F. 1998 . Differential susceptibility to Marek's disease is associated with differences in number, but not phenotype or location, of pp38+ lymphocytes . Journal of General Virology , 79 : 2795 – 2802 .
- Baigent , S.J. , Petherbridge , L.J. , Howes , K. , Smith , L.P. , Currie , R.J.W. and Nair , V.K. 2005 . Absolute quantitation of Marek's disease virus genome copy number in chicken feather and lymphocyte samples using real-time PCR . Journal of Virological Methods , 123 : 53 – 64 .
- Baigent , S.J. , Smith , L.P. , Nair , V.K. and Currie , R.J.W. 2006 . Vaccinal control of Marek's disease: current challenges, and future strategies to maximize protection . Veterinary Immunology and Immunopathology , 112 : 78 – 86 .
- Calnek , B.W. , Schat , K.A. , Peckham , M.C. and Fabricant , J. 1983 . Field trials with a bivalent vaccine (HVT and SB-1) against Marek's disease . Avian Diseases , 27 : 844 – 849 .
- Calnek , B.W. , Schat , K.A. , Ross , L.J. and Chen , C.L. 1984 . Further characterization of Marek's disease virus-infected lymphocytes. II. In vitro infection . International Journal of Cancer , 33 : 399 – 406 .
- Clementi , M. , Menzo , S. , Manzin , A. and Bagnarelli , P. 1995 . Quantitative molecular methods in virology . Archives of Virology , 140 : 1523 – 1539 .
- Cui , X , Lee , L.F. , Hunt , H.D. , Reed , W.M. , Lupiani , B. and Reddy , S.M. 2005 . A Marek's disease virus vIL-8 deletion mutant has attenuated virulence and confers protection against challenge with a very virulent plus strain . Avian Diseases , 49 : 199 – 206 .
- Davidson , I. , Borenshtain , R. and Weisman , Y. 2002 . Molecular identification of the Marek's disease virus vaccine strain CVI988 in vaccinated chickens . Journal of Veterinary Medicine. B, Infectious Diseases and Veterinary Public Health , 49 : 83 – 87 .
- DeLaney , D.B. , Morrow , C.J. , Read , K.M. and Tannock , G.A. 1998 . The development and evaluation of two tissue culture-grown Marek's disease challenge viruses . Avian Pathology , 27 : 472 – 477 .
- Gimeno , I.M. , Witter , R.L. , Hunt , H.D. , Reddy , S.M. and Reed , W.M. 2004 . Biocharacteristics shared by highly protective vaccines against Marek's disease virus . Avian Pathology , 33 : 59 – 68 .
- Handberg , K.J. , Nielsen , O.L. and Jørgensen , P.H. 2001 . The use of serotype 1- and serotype 3-specific polymerase chain reaction for the detection of Marek's disease virus in chickens . Avian Pathology , 30 : 243 – 249 .
- Islam , A.F. , Walkden-Brown , S.W. , Islam , A. , Underwood , G.J. and Groves , P.J. 2006 . Relationship between Marek's disease virus loads in peripheral blood lymphocytes at various stages of infection and clinical Marek’ disease in broiler chickens . Avian Pathology , 35 : 42 – 48 .
- Karpathy , R.C. , Firth , G.A. and Tannock , G.A. 2003 . Field evaluations of safety and efficacy of an Australian Marek's disease vaccine . Australian Veterinary Journal , 81 : 222 – 225 .
- Mackay , I.M. , Arden , K.E. and Nitsche , A. 2002 . Real-time PCR in virology . Nucleic Acids Research , 30 : 1292 – 1305 .
- Markowski-Grimsrud , C.J. , Miller , M.M. and Schat , K.A. 2002 . Development of strain-specific real-time PCR and RT-PCR assays for quantitation of chicken anemia virus . Journal of Virological Methods , 101 : 135 – 147 .
- Nazerian , K. , Lee , L.F. , Yanagida , N. and Ogawa , R. 1992 . Protection against Marek's disease by a fowlpox virus recombinant expressing the glycoprotein B of Marek's disease virus . Journal of Virology , 66 : 1409 – 1413 .
- Okazaki , W. , Purchase , H.G. and Burmester , B.R. 1970 . Protection against Marek's disease by vaccination with a herpesvirus of turkeys . Avian Diseases , 14 : 413 – 429 .
- Petherbridge , L. , Howes , K. , Baigent , S.J. , Sacco , M.A. , Evans , S. , Osterrieder , N. and Nair , V. 2003 . Replication-competent bacterial artificial chromosomes of Marek's disease virus: novel tools for generation of molecularly defined herpesvirus vaccines . Journal of Virology , 77 : 8712 – 8718 .
- Pevenstein , S.R. , Williams , R.K. , McChesney , D. , Mont , E.K. , Smialek , J.E. and Straus , S.E. 1999 . Quantitation of latent varicella-zoster virus and herpes simplex virus genomes in human trigeminal ganglia . Journal of Virology , 73 : 10514 – 10518 .
- Renz , K.G. , Islam , A. , Cheetham , B.F. and Walkden-Brown , S.W. 2006 . Absolute quantification using real-time polymerase chain reaction of Marek's disease virus serotype 2 in field dust samples, feather tips and spleens . Journal of Virological Methods , 135 : 186 – 191 .
- Rispens , B.H. , van Vloten , H. , Mastenbroek , N. , Maas , J.L. and Schat , K.A. 1972a . Control of Marek's disease in Netherlands. Part I. Isolation of an avirulent Marek's disease virus (strain CVI988) and its use in laboratory vaccination trials . Avian Diseases , 16 : 108 – 125 .
- Rispens , B.H. , van Vloten , H. , Mastenbroek , N. , Maas , J.L. and Schat , K.A. 1972b . Control of Marek's disease in Netherlands. Part II. Field trials on vaccination with an avirulent strain (CVI 988) of Marek's disease virus . Avian Diseases , 16 : 126 – 138 .
- Ross , N. , O'Sullivan , G. and Coudert , F. 1996 . Influence of chicken genotype on protection against Marek's disease by a herpesvirus of turkeys recombinant expressing the glycoprotein B (gB) of Marek's disease virus . Vaccine , 14 : 187 – 189 .
- Shamblin , C.E. , Greene , N. , Arumugaswami , V. , Dienglewicz , R.L. and Parcells , M.S. 2004 . Comparative analysis of Marek's disease virus (MDV) glycoprotein-, lytic antigen pp38- and transformation antigen Meq-encoding genes: association of meq mutations with MDVs of high virulence . Veterinary Microbiology , 102 : 147 – 167 .
- St Hill , C.A. and Sharma , J.M. 2000 . Viral pathogenesis in chicken embryos and tumor induction in chickens after in ovo exposure to serotype 1 Marek's disease virus . Avian Diseases , 44 : 842 – 852 .
- St Hill , C.A. , Silva , R.F. and Sharma , J.M. 2004 . Detection and localization of avian alphaherpesviruses in embryonic tissues following in ovo exposure . Virus Research , 100 : 243 – 248 .
- Shek , W.R. , Calnek , B.W. , Schat , K.A. and Chen , C.H. 1983 . Characterization of Marek's disease virus-infected lymphocytes: discrimination between cytolytically and latently infected cells . Journal of the National Cancer Institite , 70 : 485 – 491 .
- Tischer , B.K. , Schumacher , D. , Beer , M. , Beyer , J. , Teifke , J.P. , Osterrieder , K. , Wink , K. , Zelnik , V. , Fehler , F. and Osterrider , N. 2002 . A DNA vaccine containing an infectious Marek's Disease virus genome can confer protection against tumorigenic Marek's disease in chickens . Journal of General Virology , 83 : 2367 – 2376 .
- Witter , R.L. 1982 . Protection by attenuated and polyvalent vaccines against highly virulent strains of Marek's disease virus . Avian Pathology , 11 : 49 – 62 .
- Witter , R.L. 1997 . Increased virulence of Marek's disease virus field isolates . Avian Diseases , 41 : 149 – 163 .
- Witter , R.L. and Lee , L.F. 1984 . Polyvalent Marek's disease vaccines: safety, efficacy and protective synergism in chickens with maternal antibodies . Avian Pathology , 13 : 75 – 92 .
- Witter , R.L. , Lee , L.F. and Fadly , A.M. 1995 . Characteristics of CVI988/Rispens and R2/23, two prototype vaccine strains of serotype 1 Marek's disease virus . Avian Diseases , 39 : 269 – 284 .
- Witter , R.L. , Nazeria , K. , Purchase , H.G. and Burgoyne , G.H. 1970 . Isolation from turkeys of a cell-associated herpesvirus antigenically related to Marek's disease virus . American Journal of Veterinary Research , 31 : 525 – 538 .
- Zelnik , V. , Darteil , R. , Audonnet , J.C. , Smith , G.D. , Riviere , M. , Pastorek , J. and Ross , L.J. 1993 . The complete sequence and gene organization of the short unique region of herpesvirus of turkeys . Journal of General Virology , 74 : 2151 – 2162 .
- Zhu , G.-S. , Ojima , T. , Hironaka , T. , Ihara , T. , Mizukoshi , N. , Kato , A. , Ueda , S. and Hirai , K. 1992 . Differentiation of oncogenic and nononcogenic strains of marek's disease virus type 1 by using polymerase chain reaction DNA amplification . Avian Diseases , 36 : 637 – 645 .