Abstract
The white-winged duck (Cairina scutulata) is critically endangered. Breeding collections of this duck are established in the United Kingdom and the USA. Infection with Mycobacterium avium avium serotype 1 is a major cause of mortality in the UK collection. In this study, the aetiopathogenesis of deaths occurring in the US collection was studied. All ducks (n=21) that died over a 21-month period were examined. Mycobacteriosis was diagnosed in 20 ducks, killing 19 of them. Multifocal to diffuse granulomatous lesions, often with abundant intralesional organisms, were seen in all 20 ducks. Unusual manifestations of this disease were the extensive involvement of the respiratory system and the absence of multinucleated giant cells. Sequence analysis showed that the ducks were infected with a sequevar of M. a. avium that contains serotypes 2, 3, 4, and 9. Given that the long-term ingestion of metals affects immune function, we measured an array of such elements in the liver of six ducks. Concentrations were undetectable or low. The disseminated nature of the disease, high concentration of mycobacteria and absence of multinucleated giant cells within lesions suggest that these ducks were unable to effectively kill the mycobacteria and point to a possible defect or inhibition in cell mediated immunity. Taken together with previously reported UK data, these results suggest that captive white-winged ducks are highly susceptible to at least two sequevars of M. a. avium and that mycobacteriosis is a major threat to ex situ breeding. We hypothesize that the minimal heterozygosis previously shown in these ducks could be contributing to an apparently ineffective immune response.
Observations macroscopiques et microscopiques et investigation de l'étiopathogénie d'une mycobactériose dans une population de canards à aile blanche (Cairina scutulata) élevés en captivité
Le canard à aile blanche (Cairina scutulata) est en voie d'extinction. Des élevages de reproduction de ce canard se sont établis au Royaume Uni et aux USA. L'infection par Mycobacterium avium avium de sérotype 1 est une cause majeure de mortalité dans les élevages au Royaume Uni. Dans cette étude, l'étiopathogénie des mortalités apparues dans un élevage de collection au USA a été étudiée. Tous les canards (n =21) qui sont morts au cours d'une période de 2 ans ont été examinés. Une mycobactériose a été diagnostiquée chez 20 canards, tuant 19 d'entre eux. Des lésions granulomateuses multifocales à diffuses avec souvent des micro-organismes intralésionels nombreux, ont été observées chez les 20 canards. Les manifestations non habituelles de cette maladie ont été l'implication importante du système respiratoire et l'absence de cellules géantes multinuclées. L'analyse de séquence a montré que les canards étaient infectés par un sequevar de M. a. avium qui contient les sérotypes 2, 3, 4, et 9. Etant donné que l'investigation à long terme des métaux affecte la fonction immunitaire, nous avons mesuré un certain nombre de ces éléments dans le foie de 6 canards. Les concentrations étaient non détectables ou faibles.. La nature disséminée de la maladie, les concentration élevée de mycobactéries et l'absence de cellules géantes multinuclées dans les lésions suggèrent que ces canards ont été incapables d’éliminer les mycobactéries et indiquent un éventuel défaut ou une inhibition de l'immunité à médiation cellulaire. En prenant en compte des données antérieures rapportées au RU, ces résultats suggèrent que les canards à aile blanche en captivité sont hautement sensibles à au moins deux sequevars de M. a. avium et que la mycobactériose est une menace majeure pour l'élevage ex situ. Nous formulons l'hypothèse que l'hétérozygotisme minimal précédemment mis en évidence chez ces canards pourrait contribuer à une réponse immunitaire apparemment inefficace.
Untersuchungen zur Pathologie, Histopathologie und Ätiopathogenese der Mykobakteriose in einer in Gefangenschaft gehaltenen Population von Malaienenten (Cairina scutulata)
Die Malaienente (Cairina scutulata) ist eine vom Aussterben bedrohte Vogelart. In Großbritannien und den USA sind Zuchtprogramme für diese Ente etabliert worden. In der britischen Station ist die Infektion mit Mycobacterium avium avium-Serotyp 1 eine der Haupttodesursachen. In dieser Studie wurde die Ätiopathogenese der Todesfälle, die in der Station in den USA auftraten, untersucht. Alle Enten (n = 21), die in einen Zeitraum von zwei Jahren starben, wurden in die Untersuchung mit einbezogen. Bei 20 dieser Enten wurde Mykobakteriose diagnostiziert, die in 19 Fällen auch die Todesursache darstellte. Bei allen 20 Enten wurden multifokale bis diffuse, granulomatöse Läsionen oft verbunden mit hochgradigem Erregerbefall innerhalb der Veränderungen festgestellt. Eine ungewöhnliche Manifestierung der Erkrankung war die hochgradige Beteiligung des Respirationstrakts bei gleichzeitigem Fehlen von vielkernigen Riesenzellen. Die Sequenzanalyse ließ erkennen, dass die Enten mit einer Sequenzvariante des M. a. avium infiziert waren, die die Serotypen 2, 3, 4 und 9 enthielt. Angesichts der Tatsache, dass eine langfristige Aufnahme von Metallen zu einer Beeinträchtigung von Immunfunktionen führt, haben wir die Lebern von sechs Enten auf eine Reihe von diesen Elementen untersucht. Die Konzentrationen lagen jedoch unter der Nachweisgrenze oder waren gering. Die weite Verbreitung der Erkrankung, der starke Erregerbefall und die Abwesenheit von vielkernigen Riesenzellen lassen vermuten, dass diese Entenspezies nicht in der Lage ist, Mykobakterien wirksam zu bekämpfen, was möglicherweise auf einen Defekt oder eine Hemmung in der zellvermittelten Immunität zurückzuführen ist. Zusammen mit den kürzlich aus Großbritannien veröffentlichten Daten kann aufgrund dieser Ergebnisse angenommen werden, dass in Gefangenschaft gehaltene Malaienenten für mindestens zwei M. a. avium-Sequenzvarianten hochempfänglich sind und dass die Mykobakteriose ein erhebliche Bedrohung für eine ex situ -Zucht darstellt. Wir vermuten, dass die in diesen Enten beobachtete nur geringgradige Heterozygose zu der offensichtlich ineffektiven Immunantwort beitragen könnte.
Lesiones macroscópicas y microscópicas e investigación de la etiopatogenia de las micobacteriosis en poblaciones en cautividad de Patos de Jungla (Cairina scutulata)
El pato de Jungla (Cairina scutulata) está en grave peligro. En USA y Reino Unido se han creado criaderos. La infección con el serotipo 1 de Mycobacterium avium avium es la principal causa de mortalidad en los criaderos de Reino Unido. En este estudio, se investigó la etiopatogenia de las muertes ocurridas en criaderos de USA. Se estudiaron todos los patos (n =21) que murieron durante un periodo de dos años. Se diagnosticó micobacteriosis en 20 patos, siendo la causa de muerte en 19 de ellos. En los 20 patos se observaron lesiones granulomatosas multifocales a difusas, asociadas a menudo con gran cantidad de organismos. Algunas manifestaciones poco comunes de esta enfermedad observadas fueron la ausencia de células gigantes multinucleadas y la afectación extensa del sistema respiratorio. Los análisis de las secuencias mostraron que un secuevar de M. a. avium que contiene los serotipos 2, 3, 4, y 9 infectaba a los patos. Teniendo en cuenta que la ingestión de metales durante periodos prolongados afecta el funcionamiento del sistema inmune, se midieron un conjunto de estos elementos en el hígado de seis patos. Las concentraciones resultaron ser bajas o no detectables. La naturaleza sistémica de la enfermedad, la elevada concentración de micobacterias y la ausencia de células multinucleadas gigantes en las lesiones sugiere que estos patos eran incapaces de eliminar las micobacterias de forma efectiva e indica la posible inhibición o defecto de la inmunidad mediada por células. Estos resultados junto con otros previamente descritos en UK sugieren que los Patos de Jungla en cautividad son altamente susceptibles como mínimo a dos secuevares de M. a. avium y que la micobacteriosis es una de las principales amenazas de la cría ex situ. Hipotetizamos que la heterocigosis mínima previamente demostrada en estos patos podría contribuir a una respuesta inmune aparentemente ineficaz.
Introduction
The white-winged duck (Cairina scutulata) is a severely endangered Southeast Asian species (del Hoyo et al., Citation1992; Birdlife International, Citation2001, Citation2006). The main threats to these birds are loss of suitable habitat (wetlands and lowland tropical forest) and locally intense hunting pressure (del Hoyo et al., Citation1992; Birdlife International, Citation2001, Citation2006). Estimates of the global population range from less than 400 to as high as a “few thousand” individuals (Evans et al., Citation1997; Rose & Scott, Citation1997). Captive populations have been established at The Wildfowl and Wetlands Trust, Slimbridge, England, and at the Sylvan Heights Waterfowl Center, North Carolina, USA, in an effort to prevent the extinction of this species. The US breeding programme represents the only Anseriforme Species Survival Plan of the American Zoo and Aquarium Association. Currently, there are fewer than 100 white-winged ducks in North America and 39 in England (R. Cromie personal communication, 2006).
The survival of both captive populations has been jeopardized by the high susceptibility of this species to mycobacteriosis (Cromie et al., Citation2000; Riggs, Citation2005). Reports from the Wildfowl and Wetlands Trust show that during a period of 16 years up to 84% of captive white-winged duck deaths were the result of infection with Mycobacterium avium avium serotype 1 (Cromie et al., Citation1991, Citation1992). Mycobacteriosis has also been reported in captive birds in India (Birdlife International, Citation2006), and recently has been recognized in white-winged ducks in the Sylvan Heights Waterfowl Center collection (Riggs, Citation2005). Until now, the percentage of ducks dying with mycobacteriosis at the Sylvan Height Waterfowl Center, the nature and distribution of the lesions in these birds and the mycobacterial species causing this disease have not been reported.
The reasons for this apparently high susceptibility of white-winged ducks to avian mycobacteriosis are not known, but it has been speculated that low genetic diversity, management-related problems and environmental factors may all be predisposing factors (Cromie et al., Citation2000; Riggs, Citation2005). Additionally, it is possible that white-winged ducks may be more susceptible to mycobacterial infection because of immunosuppressive effects of environmental contaminants such as heavy metals (Stout et al., Citation2002; Fairbrother et al., Citation2004; Kalisinska et al., Citation2004; Braune & Malone, Citation2006).
A successful captive breeding programme for any severely endangered species depends on the development of effective disease control measures. Critical to the management of captive populations is a better understanding of the nature and severity of the disease caused by the infection, and an understanding of the factors that lead to the apparent susceptibility of these ducks to infection. Therefore, the main goals of this study were to: (1) determine the incidence of mycobacteriosis in white-winged ducks that died at the Sylvan Heights Waterfowl Center during a 21-month period; (2) identify, by DNA sequence analysis, the species and sequevar of Mycobacterium infecting the ducks; (3) describe the gross lesions and histopathological changes induced by mycobacteria in these ducks; (4) evaluate liver concentrations of several heavy metals in these birds; and (5) compare the results of this study with data reported elsewhere.
Materials and Methods
Specimens
All the white-winged ducks that died at Sylvan Heights Waterfowl Center from August 2004 to May 2006 (n=21) were submitted for necropsy. They were submitted frozen and had been refrigerated before freezing for variable times. Those birds that were received without having been soaked or wetted previously were weighed. The liver, spleen, lung, air sac, heart, kidney, pancreas, intestine, oesophagus, ventriculus, proventriculus, trachea and skeletal muscle were consistently examined during gross necropsy. The adrenal glands, gonads, bone marrow and thyroid glands were also examined in 7, 18, 13, and 15 birds, respectively (). The brain and cerebellum were not investigated in these birds due to severe postmortem autolysis and freezing changes. Specimens from these organs were formalin-fixed and paraffin-embedded. Sections were cut at 4 µm and stained with haematoxylin and eosin. A second section was stained with the Ziehl–Neelsen stain. Inflammatory lesions were subjectively graded as mild, moderate and severe based on the amount of inflammatory cells within the lesions and the area of tissue affected. The numbers of acid-fast bacteria were subjectively graded as none, few, moderate, many or massive. Congo Red staining and green birefringence was employed to investigate the presence of amyloid in liver and spleen. presents the organs examined histologically for each duck.
Table 1. Details results of examination of selected organs for macroscopic and microscopic lesions of avian mycobacteriosis in white-winged ducks (C. scutulata)
Detection of mycobacteria in tissues
Swabs from macerated tissues sections, either the liver or the lung, from all the ducks examined were inoculated into 5 ml Middlebrock 7H9 broth (Beckton Dickinson, Franklin Lakes, New Jersey, USA) containing 0.5% (v/v) glycerol and 10% (v/v) oleic acid–albumin, and were incubated at 39°C for up to 4 weeks. Cultures were inspected weekly for microbial growth and examined for the presence of mycobacteria by Ziehl–Neelsen staining.
Tissues were collected for polymerase chain reaction (PCR) using cleaned, autoclaved instruments that had been treated with bleach and formalin. A different set of instruments was used to collect tissues from each bird and organ to prevent DNA carryover. Tissues were either processed immediately or stored at −80°C until processing. Mycobacterial DNA was extracted from affected livers using the Puregene® Genomic DNA Purification Kit (Gentra Systems, Minneapolis, Minnesota, USA) following the instructions of the manufacturer. PCR screening for mycobacterial DNA was performed using primers T1 (5′-GGGTGACGCG(G/A)CATGGCCCA-3′) and T2 (5′-CGGGTTTCGTCGTACTCCTT-3′) for amplification of the 236-base-pair dnaJ gene (Morita et al., Citation2004). Amplified DNA was visualized after electrophoresis on a 1.5% ethidium bromide-stained agarose gel. PCR products were purified using the QIAquick PCR Purification Kit (Qiagen Inc., Valencia, California, USA). Sequencing reactions were performed using an ABI Prism® Big Dye Terminator v3.0 Cycle Sequencing Kit (Applied Biosystems Inc., Foster City, California, USA). Nucleotide sequences were determined with an ABI3100 automated DNA sequencer (Applied Biosystems Inc.). All sequences were aligned using Clustal X 1.81 (Thompson et al., Citation1997) and were compared with sequences retrieved from GeneBank (www.ncbi.nlm.nih.gov/Genbank/index.html).
Determination of liver heavy metal content
Heavy metal concentrations in liver samples for the first six white-winged ducks examined were determined by the technique described by Dehn et al. (Citation2006). Briefly, samples were chopped and homogenized. Approximately 0.1 g wet sample homogenate was weighed into tared, acid-washed 50 ml centrifuge tubes. Three millilitres of trace-metal-grade 69% to 71% nitric acid (Fisher Scientific Waltham, Maryland, USA) was added to each tube and the samples were allowed to stand overnight at room temperature. The next day samples were vortexed and heated in a microwave. Two millilitres of 30% ultrapure H2O2 (JT Baker UltrexII, Phillipsburgh, New Jersey, USA) and 1 ml of 37% to 38% trace-metal-grade HCl (EMD Chemicals Inc., Gibbstown, New Jersey, USA) were added and samples were again microwaved. Samples were then diluted with 18 MΩ/cm deionized water, and were analysed with a blank, a laboratory control sample, a sample duplicate, a spiked sample, and certified reference material (Bovine Liver 1577b; National Institute of Standards and Technology, Boulder, Colorado, USA). Metal concentrations were measured using a Spectro Ciros (Spectro Analytical Instruments Inc., Marlborough, Maryland, USA) inductively coupled plasma–optical emission spectrometer and by mass spectrometry. Values are expressed as micrograms per gram of wet tissue.
Results
Macroscopic findings
All 21 birds in the study were adults. Of the 20 birds with mycobacteriosis, 11 were females, seven were males and the sex of two birds was not determined. One bird was severely autolysed, complicating gross interpretation of changes. Twenty out of 21 (95.23%) ducks examined had extensive lesions consistent with mycobacteriosis (). All affected birds had multiple organ involvement. Avian mycobacteriosis was assumed to be the cause of death in 19 of the ducks because of the extent and severity of the lesions and the number of organs involved. One duck presented a craniocephalic trauma in absence of other lesions. Another duck had fractured cervical spine and characteristic gross lesions of avian mycobacteriosis; both of these ducks were assumed to have died as a result of trauma. All but one duck had marked atrophy of the pectoral muscles and loss of body fat. The mean body weight recorded for 11 ducks was 1315 g (standard deviation, ±262; range, 1110 to 2003 g) which was substantially below the weight range (2150 to 3855 g) reported previously for these ducks (Dunning, Citation1992).
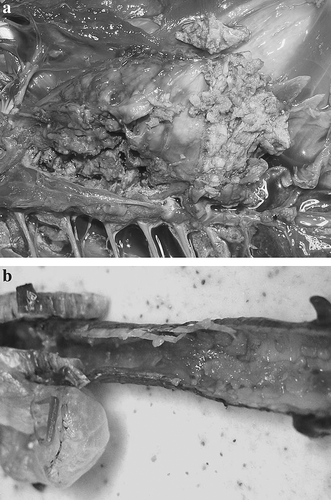
Hepatomegaly and splenomegaly, of up to two to three times the normal size, was common in these ducks. Gross lesions characteristic of mycobacteriosis in birds were found in the lung, liver, spleen, kidney and intestinal serosa of most ducks (). They consisted of random superficial and deep caseous, firm, irregular, grey or yellow nodules of variable size. Respiratory tract involvement was common. Multifocal coalescing granulomas were seen in the lungs of all ducks and were so extensive as to replace most of the lung in 18 ducks (a). Ten of 20 ducks had an extensive fibrinous air saculitis of all air sacs. The lesions were so severe in five ducks that exudate filled most of the air sac. A severe diffuse fibrinous tracheitis was present in seven out of 18 birds (b). Two ducks had granulomatous lesions in the subcutaneous cervical tissues. It is possible that these originated in the cervicocephalic air sacs; however, the extent of the lesion made its tissue of origin impossible to determine. Diffuse fibrinous to fibrous granulomatous inflammation of the serosa of the duodenum (five ducks), the pericardium (five ducks), and the ovary (three ducks) was also seen. Five ducks had diffusely enlarged livers without focal lesions. A yellow, caseous exudate distended the oviduct of one bird. One duck presented with a unilateral conjunctivitis with a thick fibrinous exudate covering the palbebral conjunctiva of the upper eyelid.
Microscopic findings
Lesions consistent with mycobacteriosis were not observed in one of the two ducks that died from trauma. Severe postmortem changes and freezing artefacts precluded a detailed analysis of some organs in some of the ducks. However, even in the birds with the most severe artefacts, acid-fast organisms and lesions consistent with mycobacterial infection could be found in most organs. The number and percentage of organs with microscopic lesions are presented ().
Microscopic lesions could be divided into two basic types, sometimes overlapping. The first type was discrete granulomas of variable size, with different degrees of central caseation necrosis and with a layer of surrounding histiocytes and lymphocytes and a fibrous capsule of varying thickness. These lesions were observed in the liver, spleen, kidney, lung, heart and on the intestinal serosa (). Spleens contained less defined granulomas compared with the other organs and had a diffuse histiocytic splenitis in the regions between the granulomas. Variable amounts of amyloid were observed around central veins and vessels and in the space of Disse in the liver as well in the spleen of nine ducks.
Table 2. Microscopic findings in organs of white-winged ducks (C. scutulata) with multifocal granulomatous inflammation
The second type of lesion was characterized by a more diffuse granulomatous inflammation, without the formation of discrete granulomas. The predominant cell types in these lesions were histiocytes and to a lesser degree lymphocytes and plasmacytes. These lesions were observed predominantly in the trachea (), oesophagus, proventriculus, bone marrow, air sacs, ovary, oviduct and on serosal surfaces (). Intestinal mucosal lesions were rare.
Figure 2. Microscopic lesion in white-winged ducks (Cairina scutulata) with avian mycobacteriosis. Severe diffuse fibrinous tracheitis with complete effacement of the mucosal epithelium.
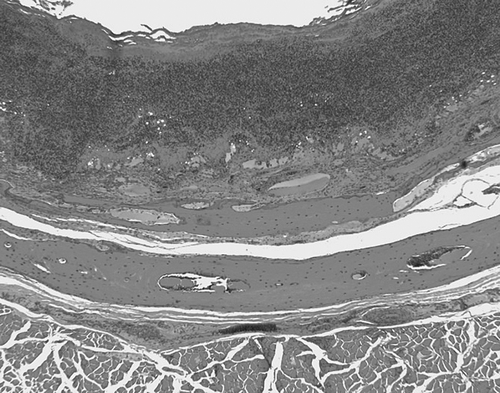
Table 3. Microscopic findings in organs of white-winged ducks (C. scutulata) with diffuse granulomatous inflammation
Multinucleated giant cells were absent from the lesions of all but one duck where they were present in granulomas of the liver, spleen, kidney and lung. In a second duck, some cells appeared agglomerated but without a clear syncitial pattern.
Results of culture and PCR
All cultures from the liver and/or the lung yielded mycobacteria after 3 to 4 weeks of incubation. An amplicon of expected molecular mass was amplified by PCR from the liver or lung from 19 birds histologically confirmed to have mycobacteriosis. The remaining bird with mycobacteriosis was not tested by PCR. The sequences of the amplified dnaJ gene for six isolates were identical and had 100% identity with the sequevar of Mycobacterium a. avium that contains serotypes 2, 3, 4 and 9 (Morita et al., Citation2004).
Liver heavy metal concentrations
Liver concentrations of silver, aluminium, antimony, arsenic, boron, beryllium, lithium, nickel, lead, mercury, selenium, thorium, uranium, and vanadium were below detectable concentrations by either mass spectrometry or optical emission spectrometer (data not shown). Liver concentrations of barium, cadmium, cobalt, chromium, molybdenum, strontium and thallium were below detection limits for optical emission spectrometer but detectable with mass spectrometry. However, all concentrations were very low, being just equal or less than three times the detection limit concentration (data not shown). One of the ducks had a barium liver concentration of 0.215 µg/g, almost four times the detection limit concentration (0.0542 µg/g). Another duck had a cadmium concentration of 0.131 µg/g, six times the minimal detectable concentrations (0.0216 µg/g), and a thallium concentration of 0.035 µg/g, 3.24 times the minimum detectable concentration (0.0108 µg/g).
Discussion
This report describes an investigation of the aetiopathogenesis of avian mycobacteriosis in white-winged ducks that died during a 21-month period from 2004 to 2006 at the Sylvan Heights Waterfowl Center in the USA. Mycobacteriosis was found in 20 of 21 ducks examined and was the confirmed cause of death for 19 of 21 ducks. The incidence of this disease in this population (95.23%) was higher than but similar in scale to the incidence reported in England (Cromie et al., Citation1991, Citation1992), indicating that mycobacteriosis is a significant impediment facing ex situ efforts to save this species.
Mycobacterium a. avium serotype 1 was the only mycobacterium isolated from white-winged ducks kept at the Wildfowl and Wetlands Trust, Slimbridge, England. These findings led investigators to suggest that M. a. avium serotype 1 may be particularly pathogenic to white-winged ducks. Serotyping of M. a avium isolates is no longer routinely done and has been largely replaced by genotyping of the isolates. Genotypes can be used to predict serotype in some, but not all, circumstances (Morita et al., Citation2004). The M. a. avium isolates from the white-winged ducks in this study were not serotype 1. Instead, comparative sequence analysis demonstrated that all the isolates were of a genotype that contained the serotypes 2, 3, 4 and 9. Thus, white-winged ducks are susceptible to infection with at least two genetic variants of M. a. avium. The high percentage of both captive populations of ducks dying of mycobacteriosis, caused by two different genetic strains of M. a. avium, might suggest that both organisms are particularly virulent in them. Nevertheless, susceptibility of both populations to different organisms may also point to a host factor predisposing to this increased susceptibility. High levels of contamination of the captive populations’ environment by mycobacteria were also considered. However, this possibility is not supported by the fact that different species of ducks at the Slimbridge collection in the United Kingdom also had different incidences of infection for avian mycobacteriosis (Cromie et al., Citation1991). Mycobacteriosis accounts for one-third of adult deaths in all the species of ducks (33%) in that facility, while in white-winged ducks, during a 16-year period, it averaged 84%. A much lower rate of mortality also occurs in other species of ducks in the Sylvan Heights collection (G. Riggs, unpublished data).A higher mortality in other species of ducks should be expected if environmental contamination is really the major cause of avian mycobacteriosis in these birds.
It has been suggested that the high incidence of mycobacteriosis in captive white-winged ducks could be explained by evolutionary and genetic characteristics of these species (Hillgarth & Kear, Citation1981; Cromie et al., Citation1991). In the wild, these ducks spend a considerable amount of time perching in trees, with little natural exposure to soil mycobacteria. As a result, they may show reduced natural immunity to these organisms (Hillgarth & Kear, Citation1981; Cromie et al., Citation1991). In captive birds, as a result of pinioning and lack of perching, a higher exposure to soil or water mycobacteria may occur (Hillgarth & Kear, Citation1981; Cromie et al., Citation1991). However, there are few reports of mycobacteriosis in Muscovy's ducks (Sabočanec et al., Citation2006), with similar habits and closely related to the white-winged duck (Cairinini tribe), despite its extensive use in agriculture and its common presence at zoos and waterfowl collections.
The current genetic diversity of captive white-winged ducks has been estimated as less than 63.5% (Riggs, Citation2005) and could be a more probable explanation for the susceptibility of captive white-winged ducks to mycobacteriosis. Increased susceptibility to infectious diseases caused by decreased genetic diversity has been recognized previously in wild animals (O'Brien et al., Citation1996; Keller & Waller, Citation2002; Acevedo-Whitehouse et al., Citation2003). Low heterozygosis at the level of the major histocompatibility complex has been recently demonstrated as a factor leading to higher susceptibility to diseases in wild mammals (Acevedo-Whitehouse et al., Citation2003) and wild birds (Miller & Lambert, Citation2004; Bonneaud et al., Citation2006).
The absence of multinucleated giant cells from the lesions of all but one white-winged duck in our series may provide a clue to the high susceptibility of the population in the United States. Multinucleated giant cells have been reported in other ducks with mycobacteriosis (Mallick et al., Citation1970; Thoen & Hines, Citation1976; Roffe, Citation1989). The granulomatous lesions seen in the white-winged ducks resemble the hyporeactive and poorly developed granulomas seen in humans with human immunodeficiency virus/acquired immune deficiency syndrome and tuberculosis (Smith et al., Citation2000). These poorly developed granulomas do not have multinucleated giant cells, have abundant acid-fast organisms and present areas of necrosis surrounded by histiocytes. In many cases they do not completely encircle the granuloma (Smith et al., Citation2000). In humans, the ability to form multinucleated giant cells is considered one indicator of an effective immune response to tuberculosis (Byrd, Citation1998; Smith et al., Citation2000). Multinucleated giant cells may limit the growth as well as the cell to cell spread of Mycobacterium tuberculosis (Byrd, Citation1998; North & Young, Citation2004; Dannemberg, Citation2006). The similarities to our findings in white-winged ducks are striking and suggest that a similar defect in the immune system may occur in these ducks.
Little is known about the mechanism of multinucleated giant cell formation in mammals or birds (Anderson, Citation2000; Smith et al., Citation2000; Okamoto et al., Citation2003). However, in humans, cytokines such as interleukin-3 and interferon gamma secreted by CD4 Th1 lymphocytes (Okamoto et al., Citation2003) are needed for cell fusion and to confine mycobacteria within these cells (Kunkel et al., Citation1998; Okamoto et al., Citation2003). Additionally, in humans, depletion of CD4 T cells results in reactivation of latent M. tuberculosis infections, impaired granuloma formation and macrophage activation, and a diminished CD8 cytotoxic T-cell response (Smith et al., Citation2000). The abundance of organisms and the absence of multinucleated giant cells in the lesions of these ducks may suggest a defect in the duck's ability to kill intracellular mycobacteria. It is possible that a defect in cell fusion and multinucleated giant cell formation may explain why white-winged ducks are so susceptible to mycobacterial infections. Nevertheless, immunology of mycobacterial infections is a little studied phenomenon in birds (Cromie et al., Citation2000; Tell et al., Citation2001), compared with humans, cattle and other animal models (Chacon et al., Citation2004; North & Young, Citation2004; Dannemberg, Citation2006; Flynn, Citation2006), and we are far from having a complete understanding of the immune response to this infection in most species of birds. The recent discovery of markers that can be used to identify CD4 and CD8 cells in mallard ducks (Kothlow et al., Citation2005) may help studies on immunity in other species of ducks.
Amyloidosis is a pathological condition characterized by the deposition of insoluble fibrillar proteins in various tissues and organs of the body following prolonged inflammation or infection (Cotran et al., Citation1999). Amyloid deposits have been reported previously in birds with chronic inflammatory diseases such as mycobacteriosis and aspergillosis. This lesion is particularly common in waterfowl (Montali et al., Citation1976; Schmidt et al., Citation2003; Meyerholz et al., Citation2005). Several forms of amyloid have been described in mammals, but only amyloid AA has been found in birds (Landman et al., Citation1998; Cotran et al., Citation1999; Schmidt et al., Citation2003). Amyloid AA is a product of the proteolytic cleavage of serum amyloid A, an acute phase-protein produced by hepatocytes (Landman et al., Citation1998). The concentration of serum amyloid A in the blood increases within several hours of the onset of injury, infection, or inflammation. Production of serum amyloid A is directly stimulated by the cytokines interleukin-1, interleukin-6 and tumour necrosis factor-α produced in response to tissue injury and inflammation (Petersen et al., Citation2004). The persistent inflammation caused by chronic mycobacteriosis is a probable cause of the deposition of amyloid in these ducks.
It is generally assumed that most M. a. avium infections in birds result from entry of the organisms into the body through the digestive tract (Montali et al., Citation1976; Cromie et al., Citation1991, Citation1992; Tell et al., Citation2001; Schmidt et al., Citation2003). Microbial shedding is also thought to occur through the faeces as many birds with mycobacteriosis have severe diffuse granulomatous enteritis. The lesion distribution in the white-winged ducks in this study, however, was somewhat unusual. In addition to the expected involvement of the liver and spleen, there was often massive involvement of the air sacs, other mesothelial surfaces, the lung and the upper digestive tract. Lesions in the intestinal mucosa were uncommon, an important difference from other reports of mycobacteriosis in birds (Montali et al., Citation1976; Tell et al., Citation2001; Schmidt et al., Citation2003). An aerogenic route of mycobacterial entry has been previously reported for dabbling ducks and other species (Cromie et al., Citation1991; Gerlach, Citation1997). Unfortunately, there are no previously reported descriptions of the pathology of mycobacteriosis in white-winged ducks affected by mycobacteriosis. This precludes comparison between these birds and our series. There are also very limited data available on the gross and microscopic pathology in other species of ducks with mycobacteriosis. The liver, spleen and kidneys were usually the only affected organs (Mallick et al., Citation1970; Thoen et al., Citation1976; Roffe, Citation1989; Cromie et al., Citation1991). Whether the high prevalence of respiratory lesions seen in our series is the result of inhalation or the result of preferred colonization of the respiratory tract by mycobacteria that enter through a different route is not known.
Heavy metal concentrations in the liver were determined because metals have the ability to cause immune suppression, and ducks, because of their feeding behaviours and aquatic habits, are likely to accumulate environmental toxins, especially metals (Di Giulio & Scanlon, Citation1984; Mateo & Guitart, Citation2003; Fairbrother et al., Citation2004). The absent or low concentrations of heavy metals in these ducks ruled out this possible explanation for their susceptibility to infection with mycobacteria. Slight elevations in liver barium concentrations in one duck and slight elevations in both cadmium and thallium in another were not considered significant since these concentrations were lower than those found in other species of ducks in previous reports (Cohen et al., Citation2000; Braune and Malone, Citation2006; Mochizuki et al., Citation2005).
In conclusion, this study demonstrated that disease caused by M. a. avium was the major cause of mortality in this population of white-winged ducks kept at Sylvan Heights Waterfowl Center. In conjunction with reports from the United Kingdom, it can be concluded that mycobacterial infections are the most significant factor limiting the ex situ recovery of this species. The reason for this high susceptibility to mycobacteriosis is unknown, but a defect in their ability to kill intracellular mycobacteria may be one possible explanation. The low heterozygosis of these ducks leading to immunodeficiency is possible (Cromie et al., Citation2000; Riggs, Citation2005), as occurs in other species of animals with low genetic diversity (Acevedo-Whitehouse et al., Citation2003; Bonneaud et al., Citation2006). If this supposition is correct, additional efforts to maintain this species with the currently available genetic stock may prove very difficult. M. a. avium is ubiquitous in the environment. Exposure to even low levels of the organism may ultimately result in infection and disease in the white-winged duck no matter what management efforts are undertaken. A more fruitful strategy to save this species may be to establish a new and genetically diverse captive breeding population of these ducks from the remaining wild population, together with a scientifically managed breeding programme assuring the maintenance of genetic diversity.
Acknowledgements
This study received generous support from The Schubot Exotic Bird Health Center, Sylvan Height Waterfowl Center, SSP-American Zoo and Aquarium Association, Association of Avian Veterinarians and Smokey Mountain Bird Club. The authors are grateful to Dr Patricia Gray, Dr Darrel Styles, Dr Elizabeth Tomaszewski and Ms Debra Turner (The Schubot Exotic Bird Health Center, College of Veterinary Medicine, Texas A&M University), Dr David McMurray and Dr Christine McFarland (College of Medicine, Texas A&M University), and Ms Deborah Dooley, Dr Bryan Brattin and Dr Bob Dalhaussen for their assistance with different aspects of this study.
Additional information
Notes on contributors
Miguel D. Saggese
College of Veterinary Medicine, Western University of Health Sciences, 309 E Second Street, Pomona, CA, 91766-1854. E-mail: [email protected]References
- Acevedo-Whitehouse , K. , Gulland , F. , Greig , D. and Amos , W. 2003 . Disease susceptibility in California sea lions . Nature , 422 ( 6 ) : 35
- Anderson , J.M. 2000 . Multinucleated giant cells . Current Opinion in Hematology , 7 : 40 – 47 .
- BirdLife International . (2001) . Threatened Birds of Asia: The BirdLife International Red Data Book (pp. 403 – 440 ). Cambridge : BirdLife International .
- BirdLife International . (2006) . Cairina scutulata. In IUCN 2006 Red List of Threatened Species . Available online at: www.iucnredlist.org (19 July 2006) .
- Bonneaud , C. , Peres-Tris , J. , Federici , P. , Chastel , O. and Sorci , G. 2006 . Major histocompatibility alleles associated with local resistance to malaria in a passerine . Evolution , 60 : 383 – 389 .
- Braune , B.M. and Malone , B.J. 2006 . Mercury and selenium in livers of waterfowl harvested in northern Canada . Archives of Environmental Contaminant Toxicology , 50 : 284 – 289 .
- Byrd , T.F. 1998 . Multinucleated giant cell formation induced by IFN-γ/IL-3 is associated with restriction of virulent Mycobacterium tuberculosis cell to cell invasion in human monocytes monolayers . Cellular Immunology , 188 : 89 – 96 .
- Chacon , O. , Bermudez , L.E. and Barletta , R.G. 2004 . Johne's disease, inflammatory bowel disease, and Mycobacterium paratuberculosis . Annual Review in Microbiology , 58 : 329 – 363 .
- Cohen , J.B. , Barclay , J.S. , Major , A.R. and Fisher , J.P. 2000 . Wintering Greater Scaup as biomonitors of metal contamination in federal wildlife refuges in the Long Island region . Archives of Environmental and Contamination Toxicology , 38 : 83 – 92 .
- Cotran , R.S. , Kumar , V. and Collins , T. 1999 . Robbins Pathologic Basis of Diseases , 6th edn , 251 – 259 . Philadelphia , PA : WB Saunders Co .
- Cromie , R.L. , Brown , M.J. , Price , D.J. and Stanford , J.L. 1991 . Susceptibility of captive wildfowl to avian tuberculosis: the importance of genetic and environmental factors . Tubercle , 72 : 105 – 109 .
- Cromie , R.L. , Brown , M.J. and Stanford , J.L. 1992 . The epidemiology of avian tuberculosis in white-winged wood duck Cairina scutulata at The Wildfowl and Wetland Trust, Slimbridge Centre (1976–1991) . Wildfowl , 43 : 211 – 214 .
- Cromie , R.L. , Ash , N.J. , Brown , M.J. and Stanford , J.L. 2000 . Avian immune response to Mycobacterium avium: the wildfowl example . Developmental and Comparative Immunology , 24 : 169 – 185 .
- Dannemberg , A.M. 2006 . Pathogenesis of Human Tuberculosis. Insights from the Rabbit Model , Washington , DC : ASM Press .
- Dehn , L.A. , Follman , E.H. , Rosa , C. , Duffy , L.K. , Thomas , D.L. , Bratton , G.R. , Taylor , R.J. and O′Hara , T.M. 2006 . Stable isotope and trace elements status of subsistence-hunted bowhead and beluga whales in Alaska and gray whales in Chukotka . Marine Pollution Bulletin , 52 : 302 – 319 .
- del Hoyo , J. , Sargatal , J. , Cabot , J. 1992 Handbook of the Birds of the World: Ostriches to Ducks Lynx Editions Barcelona , , Spain .
- Di Giulio , R.T. and Scanlon , P.F. 1984 . Heavy metals in tissues of waterfowl from the Chesapeake Bay, USA . Environmetal Pollution , 35 : 29 – 48 .
- Dunning , J.B. 1992 . CRC Handbook of Avian Body Masses , 21 Boca Raton , FL : CRC Press .
- Evans , T.D. , Robichaud , W.G. and Tizard , R.J. 1997 . The White-winged Duck Cairina scutulata in Laos . Wildfowl , 44 : 81 – 96 .
- Fairbrother , A. , Smits , J. and Grasman , K. 2004 . Avian inmunotoxicology . Journal of Toxicology and Environmental Health B Critical Review , 7 : 105 – 137 .
- Flynn , J.L. 2006 . Lessons from experimental Mycobacterium tuberculosis infections . Microbes Infections , 8 : 1179 – 1188 .
- Gerlach , H. 1997 . “ Bacteria ” . In Avian Medicine: Principles and Application , Edited by: Ritchie , B.W. , Harrison , G.J. and Harrison , L.R. Lake Worth , FL : Wingers Publishing .
- Hillgarth , N. and Kear , J. 1981 . Diseases of perching ducks in captivity . Wildfowl , 32 : 156 – 162 .
- Kalisinska , E. , Salicki , W. , Myslek , P. , Kavetska , K.M. and Jackowski , A. 2004 . Using the Mallard to biomonitor heavy metal contamination of wetlands in north-western Poland . Science Total Environment , 320 : 145 – 161 .
- Keller , F. and Waller , D.M. 2002 . Inbreeding effects in wild popualtions . Trends in Ecology and Evolution , 17 : 230 – 241 .
- Kothlow , S. , Mannes , N.K. , Schaerer , B. , Rebeski , D.E. , Kaspers , B. and Schultz , U. 2005 . Characterization of duck leucocytes by monoclonal antibodies . Developments in Comparative Immunology , 29 : 733 – 748 .
- Kunkel , S. , Lucaks , N. , Strieter , R. and Chensue , S. 1998 . Animal models of granulomatous inflammation . Seminars in Respiratory Infections , 13 : 221 – 228 .
- Landman , W.J.M. , Gruys , E. and Gielkens , A.L.J. 1998 . Avian amyloidosis . Avian Pathology , 27 : 437 – 449 .
- Mallick , B.B. , Chakrabarthy , R.I. and Chattopadhyay , S.K. 1970 . Some observations on the naturally occurring cases of avian tuberculosis in ducks . Indian Journal of Animal Health , 9 : 171 – 173 .
- Mateo , R. and Guitart , R. 2003 . Heavy metals in livers of Waterbirds from Spain . Archives of Environmental Contaminant Toxicology , 44 : 398 – 404 .
- Meyerholz , D.K. , Vanloubbeeck , Y.E. , Hoistetter , S.J. , Jordan , D.M. and Fales-Williams , A.J. 2005 . Surveillance of amyloidosis and other diseases at necropsy in captive trumpeter swans (Cygnus buccinator) . Journal of Veterinary Diagnostics and Investigation , 17 : 295 – 298 .
- Miller , H.C. and Lambert , D.M. 2004 . Genetic drift outweighs balancing selection in shaping post-bottleneck major histocompatibility complex variation in New Zealand robins (Petroicidae) . Molecular Ecology , 13 : 3709 – 3721 .
- Mochizuki , M. , Mori , M. , Akinaga , M. , Yugami , K. , Oya , C. , Hondo , R. and Fukiko , U. 2005 . Thallium contamination in wild ducks in Japan . Journal of Wildlife Diseases , 41 : 664 – 668 .
- Montali , R.J. , Bush , M. , Thoen , C.O. and Smith , E. 1976 . Tuberculosis in captive exotic birds . Journal of the American Veterinarian Medical Association , 169 : 920 – 927 .
- Morita , Y. , Maruyama , S. , Kabeya , H. , Nagai , A. , Kozawa , K. , Kato , M. , Nakajima , T. , Mikami , T. , Katsube , Y. and Kimura , H. 2004 . Genetic diversity of the dnaJ gene in the Mycobacterium avium complex . Journal of Medical Microbiology , 53 : 813 – 817 .
- North , R.J. and Jung , Y.J. 2004 . Immunity to tuberculosis . Annual Review in Immunology , 22 : 599 – 623 .
- O'Brien , S.J. , Martenson , J.S. , Miththapala , S. , Janczewski , D.N. , Pecon Slattery , J. , Johnson , W.E. , Gilbert , D.A. , Roelke , M.E. , Packer , C. , Bush , M. and Wildt , D.E. 1996 . “ Conservation genetics of the Felidae ” . In Conservation Genetics: Case Histories from Nature , Edited by: Avise , J.C. and Hamrick , J.L. 50 – 74 . New York : Chapman and Hall .
- Okamoto , H. , Mizuno , K. and Horio , T. 2003 . Monocyte-derived multinucleated giant cells and sarcoidiosis . Journal of Dermatological Sciences , 31 : 119 – 128 .
- Petersen , H.H. , Nielsen , J.P. and Heegaard , M.H. 2004 . Application of acute phase protein measurements in veterinary clinical chemistry . Veterinary Research , 35 : 163 – 187 .
- Riggs , G. (2005) . Mycobacterial infection in waterfowl collections: a conservation perspective . In Proceedings of the 26th Association of Avian Veterinarians Conference (pp. 70 – 76 ), Monterey, CA, USA .
- Roffe , T.J. 1989 . Isolation of Mycobacterium avium from waterfowl with polycystic livers . Avian Diseases , 33 : 195 – 198 .
- Rose , P.M. and Scott , D.A. 1997 . Waterfowl Population Estimates , 2nd edn , Wageningen , The Netherlands : Wetlands International .
- Sabočanec , R. , Konjević , D. , Čurić , S. , Cvetnić , Ž & Špičić , S. (2006) . Spontaneous Mycobacterium avium serovar 2 infection in a Muscovy Duck (Cairina moschata)— a case report . Veterinarski Archiv , 76 , 185 – 192 .
- Schmidt , R.E. , Reavill , D.R. and Phalen , D. 2003 . Pathology of Pet and Aviary Birds , Ames : Blackwell Publishing & Iowa State Press .
- Smith , M.B. , Boyars , M.C. , Veasey , S. and Woods , G.L. 2000 . Generalized tuberculosis in the acquired immune deficiency syndrome. A clinicopathologic analysis based on autopsy findings . Archives of Pathology and Laboratory Medicine , 124 : 1267 – 1274 .
- Stout , J.H. , Trust , K.A. , Cochrane , J.F. , Suydam , R.S. and Quakenbusch , L.T. 2002 . Environmental contaminants in four eider species from Alaska and artic Russia . Environmental Pollution , 119 : 215 – 226 .
- Tell , L. , Woods , L. and Cromie , R.L. 2001 . Mycobacteriosis in birds . Revue Scientifique et Technique. Office International des Epizooties , 1 : 180 – 203 .
- Thoen , C.O. and Himes , E.M. 1976 . Isolation of Mycobacterium avium serotype 3 from a white-headed tree duck (Dendrocygna viduata) . Avian Diseases , 20 : 587 – 592 .
- Thompson , J.D. , Gibson , T. , Plewniak , J.F. , Jeanmougin , F. and Higgins , D.G. 1997 . The CLUSTAL X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools . Nucleic Acid Research , 25 : 4876 – 4882 .