Abstract
We previously developed a real-time polymerase chain reaction (PCR) assay for absolute quantitation of serotype 1 Marek's disease virus in feather tips of chickens, and this has been used clinically to monitor a flock's response following vaccination with CVI988, an attenuated serotype 1 strain. The level of vaccine virus in feather tips associated with protection against challenge by virulent virus is not known. Here, we used an experimental challenge model, in which one dose of vaccine gives over 90% protection against mortality, to investigate correlation between the CVI988 level in feathers and protection. One-day-old chickens were vaccinated with 1, 0.1 or 0.01 commercial dose of CVI988 vaccine, and were then challenged with a virulent strain (RB-1B) 14, 21 or 28 days later. Replication of CVI988 virus was followed in each bird by real-time PCR analysis of feather DNA samples. Since the PCR does not differentiate between CVI988 and RB-1B, samples were taken only prior to challenge to ensure that the virus being measured was CVI988. Administration of one dose of vaccine ensured a uniform, rapid and high replication amongst birds, while replication following administration of the 0.1 or 0.01 dose was very variable. However, given time, a low early level of vaccine virus eventually replicated to high levels in some birds. Both the dose of vaccine virus administered and the level of vaccine virus in feather tips at 13 days post vaccination showed significant correlation with protection against challenge. A level of CVI988 vaccine virus of 132 genome copies/10000 feather tip cells was calculated to be the level required for 90% protection in this experimental model. The potential of this assay, and its limitations for monitoring protection in the field, are discussed.
Corrélation entre la charge en virus vaccinal MDV au niveau des follicules plumeux et la protection, en utilisant un modèle expérimental d'épreuve
Antérieurement nous avons développé un test PCR en temps réel pour la quantification absolue du virus de la maladie de Marek dans les follicules plumeux des poulets, et ceci a été utilisé en clinique pour suivre les réponses des troupeaux après la vaccination avec la souche CVI988, une souche atténuée de sérotype 1. Le niveau de virus vaccinal dans les follicules plumeux associé à la protection vis-à-vis d'une épreuve avec un virus virulent n'est pas connu. Ici, pour étudier la corrélation entre le niveau de CVI988 dans les follicules plumeux et la protection, nous avons utilisé un modèle d'épreuve expérimentale dans lequel une dose de vaccin confère une protection supérieure à 90% contre la mortalité. Des poussins d'un jour ont été vaccinés avec 1, 0,1 ou 0,01dose de vaccin CVI988, puis ont été éprouvés avec une souche virulente (RB-1B) 14, 21 ou 28 jours plus tard. La réplication du virus CVI988 a été suivie chez chaque sujet à partir des échantillons d'ADN prélevés dans les plumes et analysé par PCR en temps réel. Du fait que la PCR ne peut pas différencier les souches CVI988 et RB-1B, les échantillons n'ont été prélevés qu'avant l'épreuve, pour s'assurer que le virus quantifié était bien le CVI988. L'administration d'une dose de vaccin assure une multiplication uniforme, rapide et élevée chez les sujets, alors que la multiplication suivant l'administration de 0,1 ou 0,01 dose a été très variable. Cependant, avec le temps, un niveau précoce et faible de virus vaccinal s'est finalement répliqué à des niveaux élevés chez quelques sujets. A la fois, la dose de virus vaccinal administré et le niveau du virus vaccinal dans les follicules plumeux 13 jours après vaccination ont montré une corrélation significative avec la protection contre l'épreuve. Un niveau de virus vaccinal CVI988 de 132 copies de génome pour 10000 cellules de follicules plumeux a été calculé pour être le niveau requis pour obtenir 90 % de protection dans ce modèle expérimental. Les possibilités de ce test et ses limites pour contrôler la protection sur le terrain sont discutées.
Korrelation von Impfvirusgenomgehalt des Virus der Marekschen Krankheit (MK) in Federspitzen mit der Schutzwirkung gegen MK bei der Testung in einem experimentellen Belastungsinfektionsmodells
Vor kurzem haben wir einen Real-Time-PCR-Test für die Quantifikation von Serotyp 1-Virus der Marekschen Krankheit in Federspitzen von Hühnern entwickelt. Dieser Test wurde für die Überprüfung einer Herdenantwort auf eine Vakzination mit dem attenuierten Serotyp 1-Stamm CV1988 verwendet. Bislang ist jedoch nicht bekannt, welche Impfvirusmenge in den Federspitzen mit einer Schutzwirkung gegen eine Belastungsinfektion verbunden ist. In dieser Studie benutzten wir ein experimentelles Belastungsinfektionsmodell, bei dem eine Impfstoffdosis zu einem über 90 %igen Schutz gegen Mortalität führt, um die Korrelation zwischen dem CV1988-Gehalt in Federn und der Schutzwirkung zu untersuchen. Eintagshühnerküken wurden mit einer ganzen, einer Zehntel oder einer Hundertstel Dosis der kommerziellen CV1988-Vakzine geimpft und 14, 21 oder 28 Tage später mit einem virulenten Stamm (RB-1B) belastungsinfiziert. Die Replikation des CV1988-Virus wurde in jedem Küken mittels Real-Time-PCR-Analyse von DNS-Proben aus den Federn verfolgt. Da diese PCR nicht zwischen den CV1988- und RB-1B-Stämmen differenziert, wurden die Proben nur bis zum Zeitpunkt der Belastungsinfektion entnommen, um sicher zu stellen, dass es sich bei dem gemessenen Virusgenom um das von CV1988 handelt. Die Verabreichung einer Impfstoffdosis führte bei den Küken zu einer einheitlichen, schnellen und hohen Virusreplikation, während die Vermehrung nach Applikation einer Zehntel oder Hundertstel Dosis sehr variabel war. Nach einer gewissen Zeit jedoch stieg bei einigen Küken der anfangs geringe Impfvirusgehalt zu hohen Werten an. Sowohl die verabreichte Impfvirusdosis als auch der Impfvirusgehalt in den Federspitzen 13 Tage nach der Vakzination korrelierte signifikant mit der Schutzwirkung gegen die Belastungsinfektion. Als erforderliche Menge für einen 90 %igen Schutz in diesem Infektionsmodell wurde eine Gehalt von 132 Genomkopien des CV1988-Impfvirus je 10000 Federspitzenzellen errechnet. Das Potential dieses Tests sowie seine Grenzen bei der Überprüfung von Schutzwirkungen im Feld werden diskutiert.
Correlación entre la carga de genoma de virus vacunal de MDV en los folículos de las plumas y protección, a través de un modelo de infección experimental
Anteriormente desarrollamos una PCR a tiempo real para la cuantificación absoluta del serotipo 1 del virus de la enfermedad de Marek en los folículos de las plumas de los pollos, y ésta se ha utilizado clínicamente para monitorizar la respuesta de los lotes tras la vacunación con CVI988, una cepa atenuada del serotipo 1. Se desconoce cuál es el nivel de virus vacunal en los folículos de las plumas asociado a protección frente a la infección con cepas virulentas. En este estudio, utilizamos un modelo de infección experimental, en el cual una dosis de vacuna ofrecía más de un 90% de protección frente a mortalidad, para investigar la correlación entre el nivel de CVI988 en las plumas y protección. Se vacunaron aves de un día de vida con 1, 0.1 o 0.01 dosis comerciales de la vacuna CVI988, y 14, 21 o 28 días después se desafiaron con la cepa virulenta (RB-1B). Se monitorizó la replicación del virus CVI988 en cada ave mediante la prueba de la PCR a tiempo real de muestras de DNA de las plumas. Dado que la PCR no diferencia entre CVI988 y RB-1B, las muestras se tomaron únicamente antes del desafío, para asegurar que el virus detectado era CVI988. La administración de 1 dosis de vacuna aseguró una replicación alta, rápida y uniforme entre las aves, mientras que la replicación tras la administración de 0.1 o 0.01 dosis fue muy variable. Sin embargo, tras un tiempo, niveles inicialmente bajos de virus permitieron, eventualmente en algunas aves, una replicación de éste a niveles mayores. Tanto la dosis de virus vacunal administrado como el nivel de virus vacunal en los folículos de las plumas a los 13 días post vacunación, mostraron una correlación significativa con la protección frente al desafío. Se determinó que 132 copias de genoma de virus vacunal por 10000 células del folículo de las plumas era el nivel necesario para una protección del 90% en este modelo experimental. Se discute el potencial de este ensayo y sus limitaciones para monitorizar la protección en el campo.
Introduction
Marek's disease (MD) is an economically important lymphoid neoplasm of chickens, caused by oncogenic serotype-1 strains of Marek's disease herpesvirus (MDV-1) (Calnek, Citation2001; Baigent & Davison, Citation2004). The resulting morbidity and mortality is responsible for decreased productivity and major economic losses. Vaccination against MD was introduced in 1970 and effectively protects against disease and mortality, the total annual worldwide vaccinations numbering over 20 billion. Vaccine viruses are attenuated or non-pathogenic MDV strains, generally administered as cell-associated live virus either to embryonated eggs, or to neonatal chicks using automated vaccinating machines. MD vaccine viruses establish a persistent infection which probably induces both cellular and humoral immune responses (Morimura et al., Citation1998; Baaten et al., Citation2004). Vaccination does not prevent super-infection by virulent challenge viruses, which can still replicate and be shed (albeit at a reduced level), but protects against tumours and mortality.
CVI988 (Rispens) strain, a naturally attenuated MDV-1 (Rispens et al., Citation1972; de Boer et al., Citation1986), has been used in European countries since 1973 and in the USA since 1994, and is the most effective vaccine against MD. However, vaccine breaks, excessive MD losses in a vaccinated flock, do occur and can have several causes: inhibition of vaccine virus replication by maternal antibodies (King et al., Citation1981; de Boer et al., Citation1986); suppression of the immune response to vaccine by environmental stresses or co-infections with immunosuppressive pathogens; infection with a virulent MDV strain prior to establishment of full vaccinal immunity; infection of vaccinated and fully immunocompetent birds with a highly virulent MDV able to break CVI988 immunity (Witter et al., Citation2005); and, finally, administration of insufficient vaccine dose that thus fails to induce protective immunity. The standard reference dose requirement for commercial CVI988 vaccine in Europe is ≥1000 TCID50 (which equates to approximately 1000 plaque-forming units (PFU), and has proved adequate in controlling disease), and in the USA it is 1500 PFU (Landman & Verschuren, Citation2003). However, improper storage, handling and reconstitution of vaccine stocks can mean that the actual amount administered to each chicken is lower than one standard dose. This can be a significant cause of MD vaccine breaks, as recently seen in CVI988-vaccinated flocks in The Netherlands. During those outbreaks, plaque-forming assays on vaccine ampoules and reconstituted vaccine showed the average MD vaccine dose was frequently significantly below 1000 PFU and, in one hatchery, was lower than 10 PFU (Landman & Verschuren, Citation2003).
Measurement of the TCID50 or PFU, per dose of reconstituted vaccine is clearly very useful to expose incidences of inappropriate storage and dilution. However, even if the dose of reconstituted vaccine is correct, an improper vaccination technique, coupled with the perceived advantage of processing large numbers of chicks as rapidly as possible, can result in administration of insufficient dose, with some chicks being missed completely. Although this can be addressed to a certain extent using dyes along with the vaccine to confirm injection, it is equally important to have a measure of vaccine virus level in the bird. CVI988 in vaccinated chicks has traditionally been detected and quantified by virus isolation following co-cultivation of blood lymphocytes with permissive cells (Rispens et al., Citation1972) and, more recently, by conventional end-point polymerase chain reaction (PCR) (Handberg et al., Citation2001; Davidson et al., Citation2002). We developed a highly sensitive real-time quantitative PCR (q-PCR) assay for absolute quantitation of serotype 1 MDV in the feather tips. This q-PCR cannot distinguish between CVI988 and virulent strains of MDV; however, in the absence of infection by virulent strains, it can be used to measure the levels of CVI988 in individual chickens following vaccination (Baigent et al., Citation2005a, Citationb).
The ‘CVI988 feather q–PCR assay’ is now run as a clinical test for routine flock monitoring, to confirm the success of vaccination programmes, to monitor a flock's response to vaccination, and thus to indicate whether these might be contributory factors in the event of a vaccine break (Baigent et al., Citation2006a, Citationb). The standard procedure is to feather-sample 50 birds per flock at 13 to 22 days post vaccination (d.p.v.) and then analyse the feather tip DNA by q-PCR to quantify the level of vaccine virus in each bird as number of genomes per 10 000 cells. However, at present, it is not known what level of CVI988 should be classed as “positive” in terms of protection against challenge, and we can only speculate how differences in CVI988 replication kinetics affect vaccinal protection. The experiment described herein was designed to determine the level of CVI988 in the feather tips following vaccination with different doses and to determine the relationship between this CVI988 level and protection against MD, in an experimental challenge model in which one dose of vaccine gives over 90% protection against mortality.
Materials and Methods
Experimental birds
Eighty 1-day-old MD-susceptible Rhode Island Red chicks, free from maternal antibodies against MDV, were divided at random between 10 cages in three rooms in the Experimental Animal House at IAH Compton. Room 1 contained eight birds in one cage, while Rooms 2, 3, and 4 each contained three cages of eight birds per cage. In order to obtain birds varying widely in their levels of CVI988 at the time of challenge, we adopted two strategies. We varied the dose of CVI988 vaccine given to birds, and we varied the time at which birds were challenged. The vaccination, challenge and sampling schedule is summarized in . All procedures were performed according to the guidelines of the UK Home Office.
Table 1. Experimental design
Vaccination
CVI988 vaccine Poulvac (Fort Dodge Animal Health), 1000 doses per vial, was diluted to 1 dose/100 µl. An aliquot of this sub-stock was serially diluted 10-fold to produce 0.1 dose/100 µl, and 0.01 dose/100 µl. To confirm the titre of these three sub-stocks, each was titrated from 10−1 to 10−3 onto chick embryo fibroblast (CEF) cell monolayers in duplicate six-well plates. After 5 days in culture, the CEFs were fixed, and MDV plaques stained using an antibody to detect MDV gB as previously described (Baigent et al., Citation2005b). Plaques were visualized and counted using an inverted microscope. The dilution at which plaque number was between 20 and 200 was used to calculate the vaccine titre as PFU/100 µl (the titre theoretically administered to the bird) taking the mean from duplicate samples. Birds were vaccinated as shown in .
Challenge
The stock of challenge virus (RB-1B) was prepared from CEFs co-cultured with splenocytes collected from an experimental chicken 12 days post injection with 1000 PFU cell-associated RB-1B virus. This stock virus was not subject to any further passes in cell culture. The pathogenicity of the stock was validated following injection of 12 15-day old non-vaccinated experimental chickens with 1000 PFU, via the intra-abdominal route: 100% of chickens died with gross lesions of MD, between 26 and 42 days post infection. The virulence of the stock was not tested in any vaccinated birds prior to the current experiment.
On each day of challenge, the virus stock was diluted such that the titre should be 1000 PFU/100 µl. This dilution was titrated onto a CEF cell monolayer. Birds were challenged as shown in . The challenge times used in this study (14, 21 and 28 d.p.v.) were selected to enable sampling of birds for vaccine virus measurement at several time-points prior to challenge, and are comparable with those used in previous studies on vaccinal protection (de Boer et al., Citation1981, Citation1987; Purchase et al., Citation1972). Birds were kept for a further 53 days after the final challenge, and were monitored for morbidity and mortality.
Feather sampling
Four pinfeathers were plucked from the axillary feather tract of each bird (Baigent et al., Citation2005a). At 10 and 13 d.p.v., all birds were sampled. Sampling was also done at 17 and 20 d.p.v. (Rooms 3 and 4), as well as at 24 and 27 d.p.v. (Room 4). To confirm successful administration and replication of the challenge virus, feather samples were taken at 10 days post challenge (d.p.c.) from representative non-vaccinated birds.
DNA preparation and q-PCR
Feather tips were cut into small pieces using a razor blade and DNA was prepared using the Nucleospin Tissue DNA kit (Macherey-Nagel) according to the manufacturer's instructions. The q-PCR for the specific amplification of the MDV meq gene was performed to quantify the virus genome, essentially as previously described (Baigent et al., Citation2005a) using 100 ng sample DNA in each reaction, and including 10 µg bovine serum albumin per reaction. For each sample, the MDV genome copy number per 10 000 cells was calculated. This q-PCR assay does not permit differentiation between CVI988 and virulent MDV-1 such as RB-1B. Therefore, to ensure that the virus being measured was CVI988, we took samples only prior to challenge.
Postmortem examination and sampling
Postmortem examination was performed on all birds that died or were killed at the humane end-point during the course of the experiment, and on all surviving birds at 81 d.p.v.
Statistical analyses
The arithmetic mean values for the CVI988 level (from groups of birds) were determined using the log10-transformed copy number for each individual sample, and were then back-transformed to obtain the actual values (as described in Baigent et al., Citation2005b). The effect of vaccine dose and the effect of time of challenge, on protection against MD, were examined using logistic regression (non-vaccinated control birds were excluded from these analyses, since all died from MD as expected). The effect of CVI988 vaccine dose on the level of CVI988 measured in feather tips was examined using analysis of variance (all birds were included in this analysis). The effect of CVI988 level in feathers on protection against disease was examined using logistic regression (all birds were included in this analysis).
The protective level of each vaccine dose was calculated using the following formula:
The effective level (EL) of CVI988 per 10 000 feather cells, at 13 d.p.v., required for 90% protection (EL90) and 50% protection (EL50) against MD was calculated using the following formula: log10 EL={ln[p/(1-p)] –a}/b, where p is the probability of survival (0.9 for EL90 and 0.5 for EL50), and a and b are coefficients determined from a binary logistic regression of log10 CVI988 level at 13 d.p.v. against survival.
Results
Confirmation of titre of vaccine and challenge virus sub-stocks administered to birds
The titres (per 100 µl dose) of the CVI988 vaccine virus sub-stocks were determined as: 540 PFU (1 dose), 47 PFU (0.1 dose), and 4.8 PFU (0.01 dose). For RB-1B challenge virus, the titres (per 100 µl dose) of the sub-stocks used at the three different challenge times were: 980 PFU at 14 d.p.v., 1105 PFU at 21 d.p.v., and 760 PFU at 28 d.p.v.
Confirmation of replication of challenge virus in birds
Feather samples taken 10 d.p.c. from four representative non-vaccinated birds all had high levels of virus. Although the virus level was quite variable between the birds (∼20 000 to 130 000 RB-1B genomes/10 000 cells), all four died with symptoms of MD.
Mortality
summarizes symptoms and mortality in each group of birds. Some birds died for reasons unrelated to MDV infection. Four birds from across the groups died post vaccination but prior to challenge, with ascitic fluid in the abdominal cavity and/or infection of the yolk sac, quite probably for non-specific reasons unrelated to vaccination. Two additional birds had to be killed for reasons unrelated to the experimental treatments. All of these birds were excluded from the group totals for statistical analyses. Birds that died post challenge fell into three categories. First, birds that died in the early stages of infection (0 to 10 d.p.c.) exhibited rapid onset paralysis of the neck, but no MD tumours; histological analysis showed some of these birds had small aggregates of lymphocytes in the brain, and all had early signs of lymphocyte infiltration into the liver. These symptoms are akin to the acute transient paralysis seen 9 to 10 d.p.c. with certain very virulent MDV strains in some breeds of chicken (Witter et al., Citation1999). Second, birds that died 24 to 35 d.p.c. during the tumour stage of the disease had grossly enlarged spleens with lymphoid hyperplasia, and lymphoid tumours in one or more visceral organs (heart, liver, kidney or gonad); two birds had skin lesions, and one had a swollen comb all associated with extensive lymphocyte infiltration at these sites. These birds did not have paralysis of the neck and, although some showed weakness of the legs, histological analysis showed no significant lymphocyte infiltration into the sciatic nerves. Finally, birds that became ill 23 to 35 d.p.c. but showed no obvious signs of neurological damage or MD tumours; two birds had excessive deposits of uric acid in the kidneys.
Table 2. Summary of vaccinal protection against MD
Time of challenge versus protection against challenge
The experimental design did not include unvaccinated chickens challenged at 21 and 28 d.p.v., and protection in all vaccinated groups was determined by comparison with the unvaccinated birds challenged at 14 d.p.v. Thus, the effects of age resistance, which becomes apparent at 2 to 6 weeks of age (Sharma, Citation1976) could enhance the level of vaccinal protection in birds challenged at later time-points. However, the time of challenge had no significant effect on protection, probably because challenge was done relatively late, allowing for induction of a good immune response even in birds challenged at the earliest time-point (14 d.p.v.). Nevertheless, the time of challenge did influence symptoms, since only those birds that were challenged at 14 d.p.v. showed neurological signs at the time of death. The interaction between the dose of vaccine and the time of challenge had no significant effect on protection.
CVI988 dose administered versus protection against challenge
All non-vaccinated birds died with signs of MD, while a high proportion of vaccinated chicks were protected, as expected following correct administration of CVI988 vaccine. The three birds that died without any obvious signs of MD were from the 0.1-dose and 1-dose groups. Dose of vaccine administered had a clear effect on protection, which approached significance (P=0.095). PD50 and PD90 (the number of PFU of vaccine virus, determined by in vitro assay, required to protect 50% and 90% of the MD-susceptible part of a group of chickens from clinical symptoms and macroscopical lesions of MD) were 1.63 PFU (0.003 dose), and 31.8 PFU (0.064 dose), respectively.
CVI988 dose administered versus CVI988 level in feather tips
The dose of CVI988 vaccine had a significant effect on the level of CVI988 measured in the feather tips. shows the calculated level of CVI988 (per 10 000 cells) in the feather tips of each individual bird prior to challenge. The baseline for accurate measurement of CVI988 was five copies of CVI988 genome per 10 000 cells: any samples with values below this baseline are considered negative. The non-vaccinated birds (data not shown) all gave values below the baseline.
Figure 1. Kinetics of CVI988 replication in feather tips of individual birds prior to challenge. The level of CVI988 vaccine virus per 10 000 feather tip cells (logarithmic scale) following vaccination is shown for each individual bird. Birds are grouped according to the dose of vaccine received and the time of challenge. Black lines (with either closed or open symbols), birds that survived until termination of the experiment (81 d.p.v.) with no clinical signs or macroscopic MD lesions; grey lines with closed symbols, birds that died with symptoms and lesions of MD; grey lines with open symbols, birds that died in the absence of MD symptoms and lesions. The baseline for accurate measurement was 5 CVI988 genomes/10 000 cells (dashed horizontal line), and values below this baseline are considered negative.
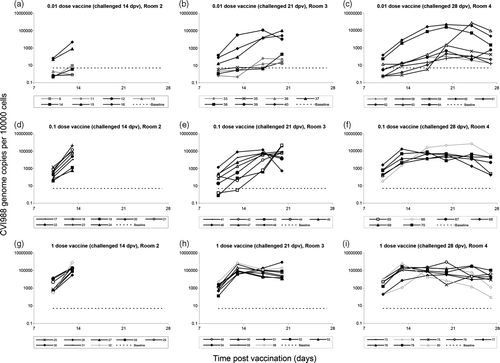
Most birds vaccinated with 1 dose CVI988 showed a uniform response. CVI988 rapidly replicated to high levels, peaking 13 d.p.v. (at approximately 100 000 genomes/10 000 cells), and then gradually decreasing to a lower level. There was no significant difference in the mean level of CVI988 between 1-dose birds in Rooms 2, 3 and 4. Birds vaccinated with 0.1-dose CVI988 gave a less uniform response but CVI988 eventually replicated to high levels, and the difference between 0.1-dose birds in Rooms 2, 3 and 4 was significant only at 13 d.p.v. For birds vaccinated with 0.01-dose CVI988 the level was, in many individuals, below the baseline at 10 d.p.v., and was not significantly different from that in non-vaccinated birds. There was wide variation among the 0.01-dose birds, which broadly fell into two groups: those that showed a reasonable response in terms of CVI988 replication, and those that showed a poor or negligible response. At each of the time-points, there was no significant difference in the mean level of CVI988 between 0.01-dose birds in Rooms 2, 3 and 4. In both the 0.1-dose and 0.01-dose groups, a low early level of CVI988 at 10 d.p.v. was associated either with a permanently low level or with a delayed peak of replication. In birds that showed a delayed peak, the level of this peak was often comparable with that seen in the 1-dose birds.
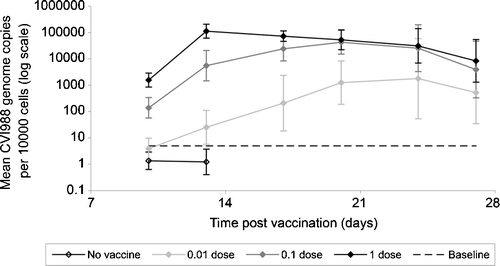
shows the mean values across all birds vaccinated with the same dose of CVI988. The mean CVI988 level in 0.01-dose birds was significantly lower than that in both 0.1-dose birds and 1-dose birds at 10, 13, 17 and 20 d.p.v. (P ≤ 0.001), but thereafter there was no significant difference. The mean CVI988 level in 0.1-dose birds was significantly lower than that in 1-dose birds at 10 and 13 d.p.v. (P<0.001) and 17 d.p.v. (P<0.05) but was, thereafter, very similar.
Figure 2. Mean level of CVI988 in feather tips from groups of birds prior to challenge. The mean level of CVI988 (logarithmic scale) is shown, with 95% confidence intervals, for all birds vaccinated with the same dose of CVI988 sub-stock: black line with open symbols, non-vaccinated; light grey line, 0.01-dose vaccinated; dark grey line, 0.1-dose vaccinated; black line with closed symbols, 1-dose vaccinated. Since feather tip samples for CVI988 measurement were only taken prior to challenge, and not after challenge, the number of birds sampled decreases at later time-points. For birds receiving 0.01, 0.1 or 1 dose vaccine, n=22 to 24 at 10 and 13 d.p.v., n=14 to 16 at 17 and 20 d.p.v., and n=6 to 8 at 24 and 27 d.p.v. All non-vaccinated birds (n=8) were challenged at 14 d.p.v., so the CVI988 level was not tested after this time. The baseline for accurate measurement was 5 CVI988 genomes/10 000 cells (dashed horizontal line).
CVI988 level versus protection against challenge
shows which birds survived challenge, and which died. In most cases, the level of CVI988 correlated well with survival. The relationship was examined between the CVI988 level at various times post vaccination and protection against challenge. The analysis covered all birds that died post challenge, including those three birds that had no obvious MD lesions at post mortem. The CVI988 level at the time of challenge was closely associated with protection against challenge (a) and had a significant effect on protection (P<0.001). All birds having a CVI988 level greater than 51 did not develop symptoms or lesions of MD after challenge. In the field, the time of challenge is variable and unknown. Therefore, it was considered more useful to examine the effect on protection of the CVI988 level at a defined early time-point (13 d.p.v.), which is within the time range during which commercial birds are feather-sampled for the clinical test. The CVI988 level at 13 d.p.v. had a significant effect on protection (P<0.001) (b). All birds having a CVI988 level greater than 64 did not develop symptoms or lesions of MD after challenge. We computed the EL of CVI988 per 10 000 feather cells, at 13 d.p.v., required for 90% protection (EL90) and 50% protection (EL50) against MD. The EL90 value was 132, and the EL50 value was 3.45. That is to say, a bird that has a level of 132 copies of CVI988 vaccine genome in the feather q-PCR test, at 13 d.p.v., has a 90% chance of surviving in this experimental model of MD challenge. Likewise, a bird that has a level of 3.45 copies of CVI988 genome in the feather q-PCR test, at 13 d.p.v., has a 50% chance of surviving in this experimental model of MD challenge.
Figure 3. Relationship between the CVI988 level and protection against challenge. The calculated level of CVI988 in feather tips at the time of challenge (3a) or at 13 d.p.v. (3b) is shown for each individual bird, independently sorted by CVI988 genome copy number (presented on a logarithmic scale). The baseline for accurate measurement was 5 CVI988 genomes/10 000 cells (dashed horizontal line), and values below this baseline are considered negative. Birds from the non-vaccinated group and from all vaccinated groups are included. Grey bars, birds that survived until termination of the experiment (81 d.p.v.) with no clinical signs of MD; hatched bars, birds that died with symptoms and lesions of MD; black bars, birds that died in the absence of MD symptoms and lesions.
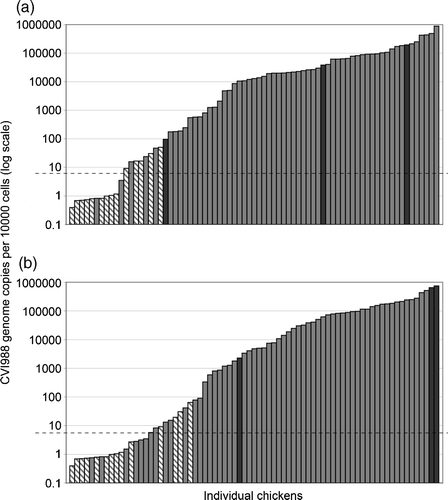
Some individual birds showed no obvious relationship between level of vaccine virus and protection. Birds 35 and 36 both had low levels of CVI988 at all times prior to challenge, but Bird 36 died, while Bird 35 survived. Two birds (Birds 74 and 80), which died without obvious signs of MD, had relatively low levels of CVI988 (compared with others in their group) at the time of challenge, but had high levels at earlier time-points. One bird that died post vaccination but prior to challenge (Bird 32), and two birds which died post challenge without signs of MD (Birds 56 and 80) had higher levels of CVI988 at 13 d.p.v. (>600 000) than all other birds.
Discussion
For effective monitoring of the success of a MD vaccination programme it is very useful to be able to measure the level of vaccine virus replication in birds, but until recently there were no practical field tests for this (Morrow & Fehler, Citation2004). We measured the response of chickens to CVI988 vaccination by quantifying CVI988 genomes in the feather tips using a real-time PCR assay. For the first time, we demonstrated that the CVI988 level in the feather tips is significantly influenced by the dose of CVI988 administered, and correlates with protection against virulent MDV in an experimental challenge model. We discuss the correlation of the CVI988 level in feather tips with protection in this experimental model, and discuss the potential and limitations for application of the assay to commercial poultry flocks.
Correlation of protection with CVI988 level in feathers
Good replication of vaccine virus in the spleen during the cytolytic phase is a key factor in its ability to elicit a strong immune response (Gimeno et al., Citation2004). We previously showed that, from 14 d.p.v., the CVI988 load in the spleen correlates well with that in feather tips (Baigent et al., Citation2005b). Thus, higher levels of CVI988 in the feather tips are likely to be associated with a better immune response. Consistent with this, our current study shows that higher levels of CVI988 in feather tips are associated with significantly greater protection against challenge. Although 0.1-dose and 1-dose vaccine were equally protective in this study, use of 1-dose vaccine ensured high, uniform and early CVI988 replication in the feather tips, which is likely to be associated with a reduced period before the onset of protective immunity. A low early level of CVI988 in feathers, a delayed peak, or a permanently low level will be associated with low level or delayed onset of immunity, which would be a great disadvantage in the commercial environment when chicks may be exposed to virulent strains in the first week of life.
The level of CVI988 measured in feather tips will be determined by a balance of the level of CVI988 replication and the strength of the host immune response. The maximum level of CVI988 in the feather tips was similar for all three doses of vaccine, and this might correspond to the systemic level that is required to stimulate a maximal immune response. This is consistent with the suggestion that efficacy of MD vaccines is limited by a biological threshold and that there may be a maximum limit for the host immune response to MD vaccine (Ball & Lyman, Citation1977; Witter & Kreager, Citation2004). If vaccination regimes and practices could be optimized to achieve this limit, it should be possible for all flocks to have maximum protection.
Even under our careful experimental conditions, birds that supposedly received the same dose of vaccine varied widely in the level of CVI988 in their feather tips, and in their response to challenge. Variability in the amount of CVI988 administered should have been minimal, since vaccine was carefully administered by hand to each bird, but no bird will receive the exact intended dose, since it is difficult to maintain uniformity in dilute stocks. There is likely to be variable replication of CVI988 between birds, particularly at low levels, as Rhode Island Reds are not highly inbred so may show some genetic variability in response to vaccination (Bacon & Witter, Citation1993, Citation1994).
Several birds survived, despite having low levels of CVI988. This again probably reflects genetic differences between birds rather than variability in amount of challenge virus administered. While CVI988 is safe in commercial birds, higher doses can sometimes be mildly pathogenic in highly-susceptible laboratory birds, including Rhode Island Reds (von Bulow, Citation1977; Pol et al., Citation1986). This might explain the deaths of two of the birds that died post-challenge without signs of MD, since these birds had higher levels of CVI988 at 13 d.p.v. than all other birds.
Although it appears that protection against challenge is associated with the peak level of CVI988 reached at any time prior to challenge, a clinical test requires measurement of CVI988 at a defined early time-point. We selected 13 d.p.v.—the earliest time-point at which feathers can be practically sampled from all flocks, the time at which CVI988 is approaching its peak in correctly vaccinated birds, and the time from which CVI988 levels in the feather tips and the spleen show good correlation. We calculated the EL90 value (the effective level of CVI988 genomes per 104 feather tip cells, at 13 d.p.v., required for protection of 90% birds in this experimental challenge model) as 132.
Potential and limitations of the feather EL90 assay as a field test for vaccine efficacy
Responses of chicks to MD vaccines, including CVI988 (de Boer et al., Citation1981, Citation1986, Citation1987) and HVT (Purchase et al., Citation1972; Basarab & Hall, Citation1976; Eidson et al., Citation1978), have previously been measured by viraemia assay to confirm replication of vaccine virus in the blood, by PD50 assay to show immune protection against challenge virus, and by serological assay to demonstrate antibodies against vaccine virus. Our test quantifies CVI988 in chicks by measurement of viral DNA in feather tips. This is much more sensitive, and much less laborious, than measurement of infectious virus in blood, since feather tips harbour high levels of MDV DNA for many weeks after vaccination, can be easily and regularly sampled, and are easy to store and transport.
In our study, PD50 and PD90 values were approximately 10-fold lower than values obtained by de Boer et al. (Citation1981, Citation1986). Differences in calculated protective doses, both within and between different laboratories, can be attributed to several variables, including the actual dose of vaccine administered, age at vaccination, batch of vaccine, maternal antibody status, and breed of chick. Thus, measurements of PD50 are inaccurate and are not an ideal way to compare vaccinal protection between flocks. Both PD50 and EL90 values are influenced by the strain of challenge virus, route and timing of challenge. However, EL90 provides valuable information, which cannot be obtained from PD50 studies, on the relationship between the levels of vaccine virus in the bird and the level of protection. Levels of vaccine virus will be a function of both the amount of vaccine administered and the level of replication of vaccine virus within the bird (influenced by the genetic background of the bird and by the presence of maternal antibodies), so these variables are already factored into the EL90 value.
The feather q-PCR assay is clearly a valid test to demonstrate the effect of the vaccine dose and the time of challenge on vaccine efficacy. Furthermore, it is proving valuable in the field to confirm successful vaccination, and successful replication of vaccine virus in chickens. We now know that merely having a detectable level of CVI988 is not sufficient for protection, and the EL90 value can be used to predict protection against challenge, in this experimental model.
However, it is not correct to assume that results of laboratory experiments can be duplicated in the field under natural exposure conditions, and it is important to emphasize that the EL90 value calculated here relates specifically to use of Fort Dodge CVI988 vaccine in this experimental challenge model where the vaccine is able to protect over 90% of birds against challenge. Thus, the test in its current form has limitations in terms of predicting protection against challenge in the field. In controlled studies, different doses of vaccine can induce similar levels of protection, as seen with 0.1-dose and 1-dose in this study. However, under field conditions, the higher dose is required to establish earlier infection and more effectively overcome maternal antibodies, as the intensity and earliness of the CVI988 replication peak is important for immunity. It is impossible to accurately simulate challenge in the field under experimental conditions. Under our experimental conditions, highly MD-susceptible, maternal-antibody-negative chickens were given a high dose of known challenge virus, at a defined (and relatively late) time, and via an unnatural (intra-abdominal) route. In the field, the genetic background of birds can differ widely, as can maternal antibody status. Maternal antibodies have an adverse effect on replication of CVI988 and other serotype-1 vaccine viruses, reducing protective efficacy and increasing PD50 (Witter & Lee, Citation1984; de Boer et al., Citation1986). Although it is unlikely that these antibodies affect protection when CVI988 is administered at the correct dose, the effects of maternal antibody could be significant if the vaccine dose is too low. In the field, chicks may be exposed to challenge virus from 1 day of age; exposure is continuous, the dose is variable, and infection is via the respiratory route. It is impossible to predict the virulence of viruses in the field. Following challenge with some very virulent MDVs, there is no correlation between replication of vaccine virus and level of protection, since such viruses can overcome the immune response induced by even high doses of CVI988.
Our q-PCR assay cannot distinguish between CVI988 and virulent challenge strains that are of the same serotype (MDV-1), and development of an assay that fulfils this requirement will not be easy since genomic differences are limited (see Baigent et al., Citation2006a), Measurements of the CVI988 level are only totally accurate when challenge virus is not present. In our study we achieved this by taking samples only prior to challenge. Clearly, in the field, birds may be exposed to virulent virus at an earlier age and, because the ideal sampling time to measure CVI988 in feathers is 13 to 22 d.p.v., challenge virus could theoretically be present in addition to the vaccine virus. Absence of virulent MDV in feather DNA samples can be confirmed using conventional PCR to amplify the 132-base-pair repeat genomic region, in which CVI988 and virulent strains differ (Becker et al., Citation1992; Silva, Citation1992; Baigent et al., Citation2005b). Nevertheless, a q-PCR assay to distinguish CVI988 from virulent strains will be essential to maximize the potential of this test for use in the field.
In conclusion, we have developed a test that can be used to demonstrate the effect of MD vaccine dose on vaccine efficacy, and used to confirm successful vaccination and successful replication of vaccine virus, in the absence of challenge virus, in the field. Host and environmental variables will always limit the universality of the test for monitoring protection against MD in the field. However, application to the field could be expanded by development of a CVI988-specific q-PCR assay, which would provide a valid indicator of whether a low level of vaccine virus is a potential contributory factor in the event of a vaccine break.
Acknowledgements
The authors would like to thank the staff of the Experimental Animal House for everyday care of the birds. They also thank Mr A. Leidi for advice on statistical analyses, Mrs S. Hacker and Mrs H. Eburne for processing of histological samples, and Mr M. Gill for digital imaging and graphics.
References
- Baaten , B.J. , Butter , C. and Davison , T.F. 2004 . Study of host-pathogen interactions to identify sustainable vaccine strategies to Marek's disease . Veterinary Immunology and Immunopathology , 100 : 165 – 177 .
- Bacon , L.D. and Witter , R.L. 1993 . Influence of B-haplotype on the relative efficacy of Marek's disease vaccines of different serotypes . Avian Diseases , 37 : 53 – 59 .
- Bacon , L.D. and Witter , R.L. 1994 . B haplotype influence on the relative efficacy of Marek's disease vaccines in commercial chickens . Poultry Science , 73 : 481 – 487 .
- Baigent , S.J. and Davison , T.F. 2004 . “ Marek's disease virus: biology and life cycle ” . In Marek's Disease, An Evolving Problem , Edited by: Davison , F. and Nair , V. 62 – 77 . Oxford , , UK : Elsevier .
- Baigent , S.J. , Petherbridge , L.J. , Howes , K. , Smith , L.P. , Currie , R.J. and Nair , V.K. 2005a . Absolute quantitation of Marek's disease virus genome copy number in chicken feather and lymphocyte samples using real-time PCR . Journal of Virological Methods , 23 : 53 – 64 .
- Baigent , S.J. , Smith , L.P. , Currie , R.J. and Nair , V.K. 2005b . Replication kinetics of Marek's disease vaccine virus in feathers and lymphoid tissues using PCR and virus isolation . Journal of General Virology , 86 : 2989 – 2998 .
- Baigent , S.J. , Nair , V.K. and Currie , R.J. 2006a . “ Real-time quantitative PCR for Marek's disease vaccine virus in feather samples: applications and opportunities ” . In Developments in Biologicals, New Diagnostic Technology: Applications in Animal Health and Biologics Controls , Edited by: Vannier , P. and Espeseth , D. 271 – 281 . Basel : Karger .
- Baigent , S.J. , Smith , L.P. , Nair , V.K. and Currie , R.J. 2006b . Vaccinal control of Marek's disease: Current challenges, and future strategies to maximize protection . Veterinary Immunology and Immunopathology , 112 : 78 – 86 .
- Ball , R.F. and Lyman , J.F. 1977 . Revaccination of chicks for Marek's disease at twenty-one days old . Avian Diseases , 21 : 440 – 444 .
- Basarab , O. and Hall , T. 1976 . Comparisons of cell-free and cell-associated Marek's disease vaccines in maternally immune chicks . Veterinary Record , 99 : 4 – 6 .
- Becker , Y. , Asher , Y. , Tabor , E. , Davidson , I. , Malkinson , M. and Weisman , Y. 1992 . Polymerase chain reaction for differentiation between pathogenic and non-pathogenic serotype 1 Marek's disease viruses (MDV) and vaccine viruses of MDV-serotypes 2 and 3 . Journal of Virological Methods , 40 : 307 – 322 .
- Calnek , B.W. 2001 . “ Pathogenesis of Marek's disease virus infection ” . In Current Topics in Microbiology and Immunology , Edited by: Hirai , K. 25 – 55 . Berlin , , Germany : Springer .
- Davidson , I. , Borenshtain , R. and Weisman , Y. 2002 . Molecular identification of the Marek's disease virus vaccine strain CVI988 in vaccinated chickens. Journal of Veterinary Medicine. B . Infectious Diseases and Veterinary Public Health , 49 : 83 – 87 .
- de Boer , G.F. , Orthel , F.W. , Krasselt , M. , Oei , H.L. , Pereboom , W.J. and Barendregt , L.G. 1981 . Comparative 50% protective dose assays (PD50) of Marek's disease virus strain CVI988 . Journal of Biological Standards , 9 : 15 – 22 .
- de Boer , G.F. , Groenendal , J.E. , Boerrigter , H.M. , Kok , G.L. and Pol , J.M. 1986 . Protective efficacy of Marek's disease virus (MDV) CVI-988 CEF65 clone C against challenge infection with three very virulent MDV strains . Avian Diseases , 30 : 276 – 283 .
- de Boer , G.F. , Pol , J.M. and Oei , H.L. 1987 . Biological characteristics of Marek's disease vaccine CVI-988 clone C1 . The Veterinary Quarterly , 9 ( Suppl 1 ) : 16S – 28 .
- Eidson , C.S. , Page , R.K. and Kleven , S.H. 1978 . Effectiveness of cell-free or cell-associated turkey herpesvirus vaccine against Marek's disease in chickens as influenced by maternal antibody, vaccine dose, and time of exposure to Marek's disease virus . Avian Diseases , 22 : 583 – 597 .
- Gimeno , I.M. , Witter , R.L. , Hunt , H.D. , Reddy , S.M. and Reed , W.M. 2004 . Biocharacteristics shared by highly protective vaccines against Marek's disease . Avian Pathology , 33 : 59 – 68 .
- Handberg , K.J. , Nielsen , O.L. and Jorgensen , P.H. 2001 . The use of serotype 1- and serotype 3-specific polymerase chain reaction for the detection of Marek's disease virus in chickens . Avian Pathology , 30 : 243 – 249 .
- King , D. , Page , D. , Schat , K.A. and Calnek , B.W. 1981 . Difference between influences of homologous and heterologous maternal antibodies on response to serotype-2 and serotype-3 Marek's disease vaccines . Avian Diseases , 25 : 74 – 81 .
- Landman , W.J. and Verschuren , S.B. 2003 . Titration of Marek's disease cell-associated vaccine virus (CVI 988) of reconstituted vaccine and vaccine ampoules from Dutch hatcheries . Avian Diseases , 47 : 1458 – 1465 .
- Morimura , T. , Ohashi , K. , Sugimoto , C. and Onuma , M. 1998 . Pathogenesis of Marek's disease (MD) and possible mechanisms of immunity induced by MD vaccine . The Journal of Veterinary Medical Science , 60 : 1 – 8 .
- Morrow , C. and Fehler , F. 2004 . “ Marek's disease: A worldwide problem ” . In Marek's Disease, An Evolving Problem , Edited by: Davison , F. and Nair , V. 49 – 61 . Oxord , , UK : Elsevier .
- Pol , J.M. , Kok , G.L. , Oei , H.L. and de Boer , G.F. 1986 . Pathogenicity studies with plaque-purified preparations of Marek's disease virus strain CVI-988 . Avian Diseases , 30 : 271 – 275 .
- Purchase , H.G. , Okazaki , W. and Burmester , B.R. 1972 . The minimum protective dose of the herpesvirus turkeys vaccine against Marek's disease . Veterinary Record , 91 : 79 – 84 .
- Rispens , B.H. , Van Vloten , J. , Mastenbroek , N. , Maas , H.J.L. and Schat , K.A. 1972 . Control of Marek's disease in the Netherlands. I. Isolation of an avirulent Marek's disease virus (strain CVI 988) and its use in laboratory vaccination trials . Avian Diseases , 16 : 108 – 125 .
- Sharma , J.M. 1976 . Natural genetic resistance to Marek's disease at hatching in chickens lacking maternal antibody, and relationship between this early resistance and resistance acquired with age . Avian Diseases , 20 : 311 – 323 .
- Silva , R.F. 1992 . Differentiation of pathogenic and non-pathogenic serotype 1 Marek's disease viruses (MDVs) by the polymerase chain reaction amplification of the tandem direct repeats within the MDV genome . Avian Diseases , 36 : 521 – 528 .
- von Bulow , V. 1977 . Further characterization of the CVI988 strain of Marek's disease virus . Avian Pathology , 6 : 394 – 403 .
- Witter , R.L. and Kreager , K.S. 2004 . Serotype 1 viruses modified by backpassage or insertional mutagenesis: approaching the threshold of vaccine efficacy in Marek's disease . Avian Diseases , 48 : 768 – 782 .
- Witter , R.L. and Lee , L.F. 1984 . Polyvalent Marek's disease vaccines: safety, efficacy and protective synergism in chickens with maternal antibodies . Avian Pathology , 13 : 75 – 92 .
- Witter , R.L. , Gimeno , I.M. , Reed , W.M. and Bacon , L.D. 1999 . An acute form of transient paralysis induced by highly virulent strains of Marek's disease virus . Avian Diseases , 43 : 704 – 720 .
- Witter , R.L. , Calnek , B.W. , Buscaglia , C. , Gimeno , I.M. and Schat , K.A. 2005 . Classification of Marek's disease viruses according to pathotype: philosophy and methodology . Avian Pathology , 34 : 75 – 90 .