Abstract
Swollen head syndrome (SHS) associated with avian metapneumovirus (aMPV) subtype A or subtype B in broilers and broiler breeders has been reported worldwide. Data about pathogenesis of aMPV subtypes A and B in broilers are scarce. It has been difficult to reproduce swollen sinuses in chickens with aMPV under experimental conditions. In the field, SHS in broilers is suspected to be induced by combined infections with different respiratory pathogens. The objectives of the present study were to compare the pathogenesis of subtypes A and B aMPV in commercial broilers and to investigate the reproducibility of clinical disease. In two repeat experiments, commercial broilers free of aMPV maternal antibodies were inoculated with aMPV subtypes A and B of turkey origin. The clinical signs such as depression, coughing, nasal exudates, and frothy eyes appeared at 4 days post inoculation, followed by swelling of periorbital sinuses at 5 days post inoculation. Higher numbers of broilers showed clinical signs in subtype-B-inoculated compared with subtype-A-inoculated groups. Seroconversion to aMPV was detectable from 10 to 11 days post inoculation. The appearance of serum aMPV enzyme-linked immunosorbent assay antibodies and the clearance of the aMPV genome coincided. Subtype B aMPV showed a broader tissue distribution and longer persistence than subtype A. Histopathological changes were observed in the respiratory tract tissues of aMPV-inoculated broilers, and also in paraocular glands, such as the Harderian and lachrymal glands. Overall, our study shows that representative strains of both aMPV turkey isolates induced lesions in the respiratory tract, accompanied by swelling of infraorbital sinuses, indicating the role of aMPV as a primary pathogen for broilers.
Introduction
Avian metapneumovirus (aMPV) is a member of the subfamily Pneumovirinae in the family Paramyxoviridae (Pringle, Citation1998). Only one serotype of aMPV has so far been identified. Four different subtypes of aMPV have been differentiated by nucleotide sequence analysis on the basis of the attachment (G) protein (Juhasz & Easton, Citation1994) and neutralization tests using monoclonal antibodies (Collins et al., Citation1993; Cook et al., Citation1993a). While subtype A and subtype B viruses are mainly prevalent in Europe (Collins et al., Citation1993; Hafez et al., Citation2000), Asian countries (Mase et al., Citation2003), and the Middle East (Banet-Noach et al., Citation2005), subtype C is the only one prevalent in the USA (Seal, Citation1998, Citation2000). An additional subtype D has been reported in France (Bäyon-Auboyer et al., Citation2000).
aMPV causes turkey rhinotracheitis, an acute respiratory tract infection in turkeys of all ages. The virus is also associated with swollen head syndrome (SHS) in broilers and broiler breeders (O'Brien, Citation1985; Wyeth et al., Citation1987; Pattison et al., Citation1989; Jones et al., Citation1991; Gough et al., Citation1994) and egg production losses in layers (Cook et al., Citation1996, Citation2000; Sugiyama et al., Citation2006). It has been reported that chicken aMPV isolates are antigenically closely related to turkey isolates (Cook et al., Citation1993a), but in previous investigations aMPV isolates from turkeys did not induce clinical signs in chickens (Gough et al., Citation1988; Buys et al., Citation1989b). Chickens also remained clinically healthy when placed in contact with turkey poults naturally infected with aMPV (Alexander et al., Citation1986).
In the 1980s, swelling of periorbital sinuses was induced in layer-type chickens by direct intranasal inoculation with antibiotic-treated homogenates of the larynx, trachea and lung of SHS-affected chickens (Picault et al., Citation1987) or with filtrated sinus exudates of broilers with SHS (Buys et al., Citation1989a). With the exception of these two successful studies, it had been difficult to reproduce SHS in chickens under experimental conditions with the typical signs of swollen sinuses that had been observed in field outbreaks. The induction of microscopical lesions in the upper respiratory tract has been much easier to reproduce (Jones et al, 1987; Cook et al., Citation1993b; Majó et al., Citation1995; Catelli et al., Citation1998). Therefore, the experimental reproducibility of clinical SHS, specifically the induction of swollen sinuses, in chickens with aMPV subtypes A and B remains to be elucidated.
Comparative pathogenesis studies with aMPV subtypes A and B have mainly been conducted in turkey poults (Van de Zande et al., Citation1999; Toquin et al., Citation2006; Liman & Rautenschlein, Citation2007). Van de Zande et al. (Citation1999) found no differences in pathogenesis between the two subtypes in turkey poults. On the other hand, Toquin et al. (Citation2006) demonstrated that a higher percentage of turkeys exhibited clinical signs in the subtype-B-inoculated group than in the subtype-A-inoculated group. Although SHS associated with aMPV subtype A or subtype B had been reported in broilers and broiler breeders in many countries (Jones et al., Citation1991; Gough et al., Citation1994; Lu et al., Citation1994; Tanaka et al., Citation1995; Hafez et al., Citation2000; Mase et al., Citation2003; Banet-Noach et al., Citation2005), to our knowledge there is no report of comparative pathogenesis studies of aMPV subtypes A and B in broilers.
Previous pathogenesis studies in chickens have shown that mainly upper respiratory tract tissues, such as the nasal turbinate, infraorbital sinuses and trachea, develop microscopical lesions (Jones et al., Citation1987; Majó et al., Citation1995; Catelli et al., Citation1998). In addition to the upper respiratory tract tissues, Majó et al. (Citation1995) found infiltration of inflammatory cells in the submucosa of bronchi. There is no report on histopathological changes due to aMPV infection in paraocular glands, such as the Harderian and lachrymal glands in chickens, although studies with subtype C aMPV in turkeys indicate involvement of the Harderian glands in the pathogenesis (Chary et al., Citation2002). Owing to the localization of these glands as an important place of primary contact with antigens (Burns, Citation1976, Citation1977; Jeurissen et al., Citation1994) and as a site of defence against respiratory infections (Davelaar & Kouwenhoven, Citation1976; Survashe et al., Citation1979; Montgomery et al., Citation1991; Toro et al., Citation1996), we speculate that aMPV infection may also affect these glands in the early phase of infection in chickens.
The objectives of this study were to compare the pathogenesis of aMPV subtypes A and B of turkey origin in broiler-type chickens, and to investigate the reproducibility of the development of typical clinical signs of SHS with swelling of the periobital sinuses. We investigated also the development of histopathological lesions in different tissues of the respiratory tract and paraocular glands, and detected the distribution of the aMPV genome in different respiratory and systemic tissues.
Materials and Methods
Viruses
A virulent aMPV subtype A (BUT 1-8544 strain) and a virulent subtype B (Italian strain), both isolated from turkeys, were used in this study (kindly provided by Dr R.C. Jones, Liverpool, UK). Both strains were passaged several times in tracheal organ culture (TOC), and were then back-passaged in turkey poults to increase virulence. These passages were conducted by Dr R.C. Jones prior to arrival of these strains at our laboratory. We propagated and titrated these viruses for our in vivo studies again in chicken TOC (two or three passages for each virus). Tracheal rings were prepared from 19-day-old to 20-day-old specific pathogen free chicken embryos (Gough et al., Citation1998). No cross-contamination occurred between the two propagated virus subtypes as confirmed by subtype-specific reverse transcriptase (RT)-nested polymerase chain reaction (PCR). The median ciliostasis dose (CD50) was calculated using the method of Reed & Muench (Citation1938).
Chickens
One-day-old commercial Ross-type broilers were obtained from a local commercial hatchery (BWE-hatchery, Lower Saxony, Germany) and were raised in the isolation units of the Clinic for Poultry, University of Veterinary Medicine, Hannover, according to animal welfare guidelines and under strict biosecurity measures. Feed and drinking water were provided ad libitum.
Clinical score index
The clinical scores were determined according to the scoring system previously described by Jones et al. (Citation1992) and Naylor & Jones (Citation1994): score 0 = no clinical signs; score 1 = clear nasal exudates; score 2 = turbid nasal exudates; and score 3 = swollen infraorbital sinus and/or frothy eyes. The clinical score index of each group was calculated based on the sum of scores observed per total number of chickens/group investigated at each day post inoculation.
Histopathological evaluation
Collected tissue samples were fixed in 10% buffered formalin, embedded in paraffin wax, and processed by conventional methods. Tissue sections were stained by haematoxylin and eosin (H & E). Based on the microscopical findings, histopathological lesions in the nasal turbinate and trachea were scored as follows: score 0 = no significant finding; score 1 = mild intraepithelial or lamina propria infiltration of lymphocytes or heterophils, and three or more lymphoid cell foci; score 2 = massive infiltration of inflammatory cells in the epithelium or lamina propria focally or diffusely, and hypertrophy of mucous glands; score 3 = massive focal or diffuse infiltration of inflammatory cells in the epithelium or lamina propria, and sloughing off of ciliated respiratory epithelium.
Detection of the aMPV genome
Total RNA was isolated from individual tissue samples by TRIzol® reagent following the manufacturer's instruction (Invitrogen Life Technologies, USA). Isolated total RNA samples were pooled according to the organ, group and day (n = 5). As also observed previously by Ganapathy et al. (Citation2006), the sensitivity of the RT-nested PCR was not affected by pooling five samples for aMPV detection. The aMPV genome was detected by subtyping RT-nested PCR based on the attachment protein gene (G) sequence, which allowed differentiation of aMPV A and B subtypes (Cavanagh et al., Citation1999).
Detection of aMPV antibodies
A commercially available enzyme-linked immunosorbent assay (ELISA) system (Avian Rhinotracheitis Antibody Test Kit®, CK 120; BioCheck B.V., The Netherlands) was used for detection of maternal antibodies and aMPV seroconversion in serum samples of broilers. The coating antigen was aMPV subtype B (B. Van Dam; BioChek B.V., personal communication, 2007). Calculated aMPV antibody titres below 1158 are considered negative based on the manufacturer's recommendations.
Experimental design
Two separate experiments were conducted.
Experiment 1
One hundred and twenty 1-day-old commercial Ross-type broilers were included in Experiment 1. Fifteen serum samples were collected randomly at 7 and 12 days of age, and maternally derived aMPV antibody (MDA) titres were determined. At 16 days of age, when MDA titres had waned, the chickens were randomly divided into three groups (n=40/group). Chickens from the first group were inoculated oculonasally with 104 CD50 aMPV subtype A, and chickens of the second group were inoculated with 104 CD50 aMPV subtype B. The third group received virus-free TOC supernatant. All groups were maintained in separate isolation units. After inoculation, broilers were observed for clinical signs daily up to 24 days post inoculation (d.p.i.). Eight birds from each group were exsanguinated at the day of inoculation and at 3, 6, 10, 14, 17, 20, and 24 d.p.i., and aMPV seroconversion was determined by ELISA. Five birds from each group were bled at 3, 6, 11, 14, 17, 20, and 24 d.p.i. and macroscopical lesions were determined. Samples of the nasal turbinates, Harderian glands, lachrymal glands, the middle part of trachea, lung, spleen, and bursa cloacalis were collected for histopathological examination and also for detection of aMPV genome by RT-nested PCR.
Experiment 2
This experiment was a repeat of Experiment 1 with 96 1-day-old Ross-type broilers to confirm reproducibility of the clinical disease and pathogenesis. MDA titres were assessed by the commercial ELISA in 15 randomly collected serum samples at 7 and 12 days of age. At 14 days of age, when the MDA titres had waned, broilers were randomly divided into three groups (n=32/group). Two groups received oculonasally either 104 CD50 aMPV subtype A, or 104 CD50 aMPV subtype B. The third group was inoculated with virus-free TOC supernatant. Clinical signs were observed daily up to 21 d.p.i. Serum samples were collected from eight birds per group on the day of inoculation and at 3, 6, 10, 14, 17, and 21 d.p.i., and from seven birds per group at 24 d.p.i. The samples were tested for aMPV antibodies by ELISA. Five chickens from each group were exsanguinated at 3, 6, 10, 14, and 21 d.p.i. and macroscopical lesions were observed. Samples for histopathology and aMPV detection were collected as described in Experiment 1. In addition, caecal tonsils and cloacal swabs were collected for detection of the aMPV genome.
At 24 d.p.i., both experiments were terminated and all remaining birds were killed.
Statistical analysis
The data obtained were analysed by one-way analysis of variance, and the differences between means were compared by Duncan's multiple-range test using SPSS 11.5 (SPSS Inc., Chicago, IL, USA). P < 0.05 was considered statistically significant.
Results
Development of clinical signs
The percentage of chickens showing clinical signs and the clinical score indices observed in Experiments 1 and 2 are shown in a,b, respectively. Up to 3 d.p.i., infected broilers did not show any clinical signs. At 4 d.p.i., broilers began to show depression, coughing, and clear nasal exudate, followed by swelling of infraorbital sinuses at 5 d.p.i. On day 6 post inoculation the maximum number of birds showed swollen sinuses. In subtype-A-inoculated groups, four out of 37 birds and nine out of 27 birds showed swollen sinuses in Experiments 1 and 2, respectively. In subtype-B-inoculated groups, five out of 36 and 14 of 27 birds showed swollen sinuses in Experiments 1 and 2, respectively. Swelling was confined around the eye, and did not involve the entire head (a,b). Swelling became more prominent at 8 to 9 d.p.i. in both inoculated broiler groups. No turbid nasal exudate was recorded.
Figure 1. Percentage of broilers per group showing clinical signs and clinical score indices from (1a) Experiment 1 and (1b) Experiment 2. Bar graph shows the percentage of birds/group showing clinical signs, line graph shows clinical score indices of the groups. n = 5/group and experimental day. Clinical score index = the sum of scores observed/total number of chickens per group at each day point. Chickens that received virus-free TOC medium were free of clinical signs throughout both experiments.
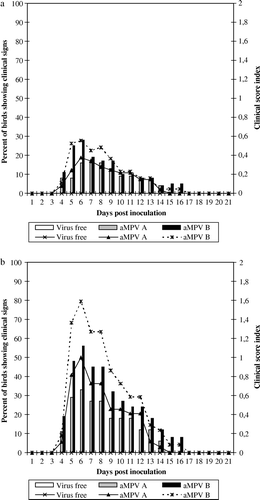
Figure 2. Clinical signs of aMPV-infected broilers at 9 d.p.i. 2a: Control broiler. 2b: aMPV subtype-B-inoculated broiler showing swelling of periorbital sinuses and the area around the eye (black arrow) and clear nasal exudate (white arrow). These clinical signs were also observed in aMPV subtype-A-inoculated birds (data not shown).

Pathological and histopathological findings
No other gross lesions were observed in either experiment, except swelling of the periorbital sinuses. Histopathological changes were found not only in the respiratory tract, but also in eye-associated lymphoid tissues of virus-inoculated broilers. No histopathological changes were detected in virus-free TOC-inoculated broilers.
The most significant histological lesions in the nasal turbinate were found in the ciliated columnar epithelium of the middle nasal turbinate in both experiments (). Thickening of the nasal turbinate epithelium was detected between 3 and 14 d.p.i., due to infiltration of inflammatory cells and formation of lymphoid follicles in the submucosa (a,b). Sloughing off of the epithelium, hypertrophy of the mucous glands and catarrhal exudate containing cell debris in the lumen were seen at 6 and 11 d.p.i. Regeneration of the epithelium with non-ciliated flat cells was first observed at 14 d.p.i. The regeneration process was not completed until 21 d.p.i.
Figure 3. Histopathological lesions in the nasal turbinate at 6 d.p.i. (H & E stain, 200x magnification). 3a: Intact ciliated epithelium of a control broiler (arrows). 3b: Nasal turbinate of an aMPV subtype-B-inoculated broiler showing infiltration of lymphoid cells in the epithelium (black arrow) and hypertrophy of the mucous gland (white arrow) (score 3). Nasal turbinates of aMPV subtype-A-inoculated broilers show similar lesions.
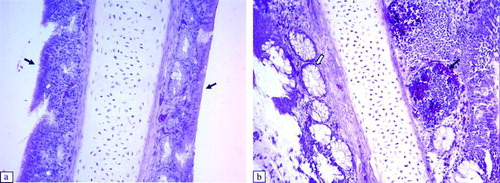
Table 1. Mean histopathological lesion scores in aMPV-inoculated and control broilers
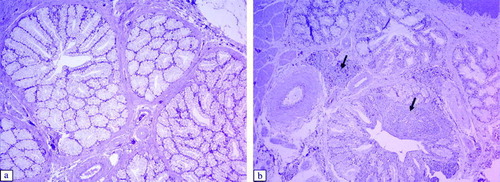
Significant histological lesions in the tracheae of virus-inoculated chickens were observed at 6 to 11 d.p.i. in Experiment 1, and at 6 to 14 d.p.i. in Experiment 2 (). The following lesions were detected: thickening of the epithelial layer due to massive infiltration of lymphocytes and heterophils, oedema (a,b), hypertrophy of mucous glands, and sloughing off of the epithelium. The damaged epithelium of the trachea showed the beginnings of regeneration with non-ciliated flat cells at 14 d.p.i. In other respiratory tract tissues, such as the infraorbital sinus, we observed infiltration of lymphoid cells and exfoliation or destruction of the ciliated epithelium between 6 and 11 d.p.i. (a,b). At the same time, we detected infiltration of mononuclear inflammatory cells and development of lymphoid foci in the submucosa of secondary bronchi in aMPV-inoculated broilers. The ciliated epithelium of the secondary bronchi was still intact (a,b).
Figure 4. Histopathological lesions of the trachea at 6 d.p.i. (H & E stain, 200x magnification). 4a: Intact ciliated epithelium of a control broiler (arrow). 4b: Trachea of an aMPV subtype-A-inoculated broiler with thickening due to oedema and infiltration of inflammatory cells in the epithelium (black arrow) and focal exfoliation (white arrow) (score 2). Tracheae of aMPV subtype-A-inoculated broilers show similar lesions.
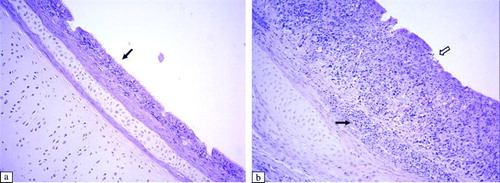
Figure 5. Histopathological lesions of the infraorbital sinus epithelium at 6 d.p.i. (H & E stain, 100x magnification). 5a: Control group. 5b: Infraorbital sinus epithelium of an aMPV subtype-B-inoculated broiler showing infiltration of lymphoid cells (black arrow) and destruction of the epithelium (white arrow). Infraorbital sinus epithelium of an aMPV subtype-A-inoculated broiler shows similar lesions.
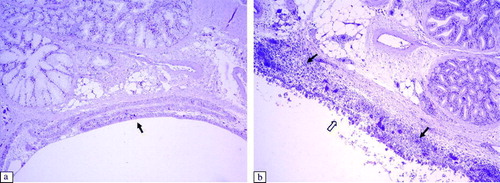
Figure 6. Histopathological lesions of the secondary bronchi of the lung, at 6 d.p.i. (H & E stain, 100x magnification). 6a: Control group. 6b: Secondary bronchi of an aMPV subtype-B-inoculated broilers showing formation of lymphoid follicles in the submucosa (arrows). Secondary bronchi of an aMPV subtype-A-inoculated broiler show a similar lesion.
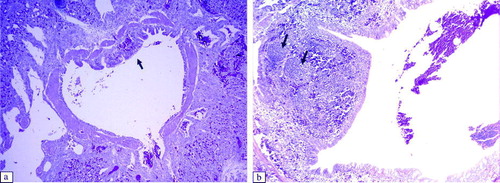
In the Harderian glands of virus-inoculated broilers, we observed infiltration of lymphocytes and development of lymphoid follicles in the interstitial tissues and around the secondary collecting ducts at 6 and 11 d.p.i. (a,b). In the lachrymal glands we detected infiltration of lymphoid cells and lymphoid follicles in the interstitial tissue and in the interlobular tissue between 3 and 11 d.p.i. (a,b). No histopathological changes were observed in the spleen and bursal cloacalis.
Figure 7. Histopathological lesions of the Harderian gland at 6 d.p.i. (H & E stain, 200x magnification). 7a: Control group. 7b: Harderian gland of an aMPV subtype-B-inoculated broiler showing infiltration of lymphocytes and lymphoid follicles around the secondary collecting duct (arrows). The Harderian gland of an aMPV subtype-A-inoculated broiler shows a similar lesion.
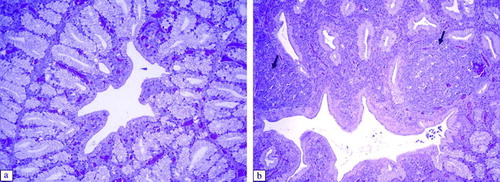
Figure 8. Histopathological lesions of the lachrymal gland at 6 d.p.i. (H & E stain, 100x magnification). 8a: Control group. 8b: Lachrymal gland of an aMPV subtype-B-inoculated broiler showing infiltration of lymphoid cells in the interstitial tissues and interlobular tissues (arrows). Lachrymal glands of an aMPV subtype-A-inoculated broiler show similar lesions.
Distribution of the aMPV genome
The distribution of the aMPV genome in different tissues of broilers from Experiments 1 and 2 is summarized in . Both aMPV subtypes were detected in the nasal turbinate and Harderian gland at 3 and 6 d.p.i. in Experiment 1. In the subtype-B-inoculated group the viral genome was detected up to 11 d.p.i. in the trachea in Experiment 1, and up to 10 d.p.i. in the Harderian gland in Experiment 2. In addition to the upper respiratory tract tissue, in subtype-B-inoculated broilers the aMPV genome was detected in both experiments in the lung, spleen, bursa cloacalis, and in Experiment 2 also in the caecal tonsils and cloacal swabs. No aMPV genome was detected after 10 or 11 d.p.i. in either infected group. No aMPV genome was detected in the control groups.
Table 2. Detection of the aMPV genome in broiler tissues by RT-nested PCR
aMPV maternal antibodies and seroconversion
The mean aMPV MDA levels at 7 days post hatch were 2735.50±420 and 2658.80±473 in Experiments 1 and 2, respectively. At 12 days post hatch, the mean MDA levels were 826.13±150 and 599.67±80 in Experiments 1 and 2, respectively. On the day of aMPV inoculation, randomly collected serum samples confirmed that the broilers were negative for aMPV MDA because all investigated samples had antibody levels below 1158 (data not shown). In Experiment 1, seroconversion was detected at 11 d.p.i. in both aMPV-inoculated groups. In Experiment 2, seroconversion was first detected at 6 d.p.i. in aMPV subtype-B-inoculated broilers and at 10 d.p.i. in subtype-A-inoculated broilers. TOC-supernatant-inoculated broilers from both experiments were free of aMPV antibodies throughout ().
Table 3. aMPV-antibody detection in broilers inoculated with aMPV subtype A or subtype B
Discussion
The objectives of the present study were to compare the pathogenesis of aMPV subtype A and aMPV subtype B of turkey origin in commercial broilers, and to investigate the reproducibility of clinical disease. It had been suggested after previous experimental studies that aMPV subtypes A and B of turkey origin do not induce respiratory signs in broilers or in layer-type chickens (Gough et al., Citation1988; Buys et al., Citation1989b). Chicken field isolates may represent a subpopulation of turkey rhinotracheitis virus that adapted to chickens (Buys et al., Citation1989a). Even with chicken isolates, which had been propagated under laboratory conditions, it had been difficult to reproduce under experimental conditions the typical swollen sinuses, which are observed in chickens in the field (Jones et al., Citation1987; Majó et al., Citation1995, Citation1997; Catelli et al., Citation1998; Nakamura et al., Citation1998). Our study now indicates that aMPV subtypes A and B of turkey origin were both pathogenic for commercial broilers, and induced swelling of periorbital sinuses and histopathological lesions in the upper respiratory tract. Swelling of infraorbital sinuses in both infected groups began at 5 d.p.i., and lasted up to 12 and 14 d.p.i. in subtype-A-inoculated and subtype-B-inoculated groups, respectively. This finding supports early reports of Picault et al. (Citation1987) and Buys et al. (Citation1989a), who demonstrated swelling of periorbital sinuses following inoculation of homogenized upper respiratory tract organs or sinus exudates of SHS-affected chickens.
We may speculate that a variety of reasons concerning both the virus and the host account for the difficulties in reproducing clinical disease with aMPV in chickens reported by other investigators. Depending upon the dose and virulence of the virus strain, we may expect that the severity of clinical signs and development of histopathological lesions in aMPV-infected chickens may vary. In previous studies with specific pathogen free light-breed chickens, in which swelling of periorbital sinuses was not reproduced in chickens under experimental conditions (Jones et al., Citation1987; Catelli et al., Citation1998; Nakamura et al., Citation1998), a challenge dose lower than 104 CD50 per bird was used. In our studies we used 104 CD50 per bird, which may explain the successful reproduction of clinical disease. Furthermore, the necessity to increase the dose of the challenge virus with increasing age of the bird in order to reproduce clinical disease has been shown in turkeys (Williams et al., Citation1991b).
It has been demonstrated that live attenuated aMPV strains may revert to virulence after repeated passages in turkey poults (Cook et al., Citation1989a; Catelli et al., Citation2006; Tiwari et al., Citation2006). In contrast to previous investigations, the virus strains we used in our experiments had been passaged in turkey poults to increase virulence and were subsequently propagated in TOC. Therefore, we may speculate that the virulence of our challenge virus may have been higher than in many other studies. In many studies aMPV had been solely propagated in vitro in cell culture of chicken embryo fibroblasts or Vero cells, and may had lost some of its virulence (Baxter-Jones & Jones, Citation1989; Williams et al., Citation1991a).
On the host side, the development and severity of clinical disease under experimental conditions may vary based on the immune status and age of the aMPV-infected birds. Although aMPV MDA cannot provide full protection against clinical disease (Cook et al, 1989aCook et al, Citation1989b), we may speculate that high levels of aMPV MDA may reduce the severity of the disease in chickens by limiting virus replication. Cook et al. (Citation1993b) found that older chickens were more susceptible to aMPV. The most susceptible age for aMPV-induced disease was observed to be between 2 and 6 weeks old under field conditions (Lister & Alexander, Citation1986; Hafez, Citation1993), which coincides with the age of the broilers used in our experiments.
In Experiment 2, we observed more severe clinical disease and slower recovery from histological lesions than in Experiment 1. We speculate that gender may also have an influence on the susceptibility of the birds, because in Experiment 2 we solely used male broilers while in Experiment 1 both genders were included.
The chronology of the development and disappearance of histological lesions correlated with the onset and recovery from clinical disease. The histopathological lesions observed in the nasal turbinate and trachea were similar to those previously described in experimental studies with chickens (Majó et al., Citation1995; Catelli et al., Citation1998). In addition we found lymphoid cell infiltration, development of lymphoid follicles and focal exfoliation of ciliated epithelium of infraorbital sinuses following inoculation with aMPV subtypes A and B, which supplements the observation of Catelli et al. (Citation1998). Confirming the observations of Majó et al. (Citation1995), we also detected infiltration of mononuclear cells and development of lymphoid follicles in the submucosa of secondary bronchi of aMPV-infected broilers. These observations indicate that both subtypes of aMPV affect all columnar ciliated epithelium of the respiratory tract tissue in broilers. Among the histopathological lesions in different respiratory tissues, the lesions in the lower respiratory tract were the least severe.
In addition to previous studies in chickens, we also investigated paraocular glands. Infiltration of lymphoid cells was detected in paraocular glands of subtype A and subtype B aMPV-inoculated broilers, as was previously found following subtype C aMPV infection of turkeys (Chary et al., Citation2002). It had been shown that antigenic material such as India ink and colloidal gold was readily transported to these glands via the main duct following eye-drop application (Burns, Citation1977). Lymphoproliferative changes typical of an immune response had been noted in the Harderian glands and lachrymal glands following ocular application of sheep red blood cells, infectious bronchitis virus and Newcastle disease virus (Davelaar & Kowenhoven, 1976; Survashe et al., Citation1979; Toro et al., Citation1996). In our study, both aMPV genomes were detected by RT-nested PCR in the Harderian gland of virus-infected chickens. The presence of the virus antigen in the Harderian glands may have stimulated lymphoid cell proliferation and aggregation in this gland.
Although the distribution pattern of the aMPV subtype A and subtype B genome slightly differed between the two experiments, we observed at each time in subtype-B-inoculated groups a wider aMPV tissue distribution and a longer period of aMPV-positive samples than in subtype-A-inoculated groups. The different distribution pattern of aMPV subtype A and aMPV subtype B may have been due to differences in the sensitivity of the RT-nested PCR for the two subtypes. Another explanation may be that subtype B is more invasive than subtype A. This speculation is supported by the higher numbers of chicken showing clinical signs in subtype-B-inoculated groups than in subtype-A-inoculated ones in both experiments. Similar observations of differences in virulence were made with the same aMPV strains as well as with other subtype A and subtype B strains in turkeys (Toquin et al., Citation2006; Liman & Rautenschlein, Citation2007). However, another comparative study of two subtypes in turkeys did not indicate differences between the two subtypes concerning respiratory signs (Van de Zande et al., Citation1999). Possibly, there is a large variation between strains, and virulence factors of aMPV need to be identified in the future.
No aMPV genome was detected at or after 14 d.p.i. in either group. This observation differs from studies with subtype C, where the aMPV C genome was detected by RT-nested PCR in the blood or lungs up to 15 d.p.i. (Shin et al., Citation2000). These differences may be due to variations in the invasiveness between subtypes A, B and C. Although the ciliated epithelium is the main target for aMPV infections, in our study the subtype B genome was also det ected by RT-nested PCR in the other systemic tissues, such as the spleen and bursa cloacalis. Macrophages may distribute aMPV from the site of replication in the respiratory tract to other peripheral tissues.
The appearance of detectable aMPV ELISA antibodies coincides with the clearance of the aMPV genome in different tissues after infection. Similar pattern of coincidence had been reported in the experimental infections of turkey poults with aMPV subtype C (Shin et al., Citation2000) and with aMPV subtypes A and B (Liman & Rautenschlein, Citation2007). We may speculate that, besides the occurrence of local antibody development, which was not investigated in the present study, some of the serum antibodies may also transudate onto mucosal surfaces and then contribute to viral clearance from respiratory tissue (Aitken & Parry, Citation1976; Toro et al., Citation1993; Suresh & Arp, Citation1995).
Overall, this is the first study in which clinical disease with swelling of infraorbital sinuses has been reproduced by experimental infection of commercial broilers with aMPV subtypes A and B of turkey origin. Both aMPV subtypes affected all ciliated columnar epithelium of respiratory tract tissues in broilers. Strong lymphoid cell infiltrations were detected in all respiratory mucosae following infection with both subtypes. These cells may participate in local immune responses. Cell infiltration was not only observed in the ciliated epithelium of the respiratory tract but also in paraocular glands, such as the Harderian and lachrymal glands. Our study clearly suggests the role of aMPV as a primary pathogen affecting the respiratory tract in commercial broilers.
Acknowledgements
The author would like to thank Kannan Ganapathy and Richard Jones from the Department of Veterinary Pathology, University of Liverpool, UK for their technical advice and support. They also would like to thank Christine Haase for her technical support, and Sonja Bernhard and Martina Koschorrek for their help in the animal work and necropsy. This work was supported by a scholarship (Y.H.A.) granted by the German Academic Exchange Service (DAAD).
References
- Aitken , I.D. and Parry , S.H. 1976 . Local immunity in the respiratory tract of the chicken I. Transudation of circulating antibody in normal and virus-infected birds . Immunology , 31 : 33 – 37 .
- Alexander , D.J. , Gough , R.E. , Wyeth , P.J. , Lister , S.A. and Chettle , N.J. 1986 . Viruses associated with turkey rhinotracheitis in Great Britain . Veterinary Record , 118 : 217 – 218 .
- Banet-Noach , C. , Simanov , L. and Perk , S. 2005 . Characterization of Israeli avian Metapneumovirus strains in turkeys and chickens . Avian Pathology , 34 : 220 – 226 .
- Baxter-Jones , C. and Jones , R.C. 1989 . “ Laboratory investigations with turkey rhinotracheitis (TRT) virus: virus isolation, maintenance and serology ” . In Poultry Science Symposium 21, Recent Advances in Turkey Science , Edited by: Nixey , C. and Grey , T.C. 224 – 233 . London , Boston : Butterworth .
- Bäyon-Auboyer , M.H. , Arnauld , C. , Toquin , D. and Eterradossi , N. 2000 . Nucleotide sequences of the F, L and G protein genes of two non-A/non-B avian pneumoviruses (APV) reveal a novel APV subgroup . Journal of General Virology , 81 : 2723 – 2733 .
- Burns , R.B. 1976 . The structure of Lachrymal glands of domestic fowl and of the duck . Research in Veterinary Science , 21 : 292 – 299 .
- Burns , R.B. 1977 . Possible route of antigen uptake by Harderian gland of domestic fowl . British Poultry Science , 18 : 407 – 408 .
- Buys , S.B. , Du Preez , J.H. and Els , H.J. 1989a . Swollen head syndrome in chickens: a preliminary report on the isolation of a possible aetiological agent . Journal of South African Veterinary Association , 60 : 221 – 222 .
- Buys , S.B. , Du Preez , J.H. and Els , H.J. 1989b . The isolation and attenuation of a virus causing rhinotracheitis in turkeys in South Africa . Journal of Veterinary Research , 56 : 87 – 98 .
- Catelli , E. , Cook , J.K.A. , Chesher , J. , Orbell , S.J. , Woods , M.A. , Baxendale , W. and Huggins , W. 1998 . The use of virus isolation, histopathology and immunoperoxidase techniques to study the dissemination of a chicken isolate of avian pneumovirus in chickens . Avian Pathology , 27 : 632 – 640 .
- Catelli , E. , Cecchinato , M. , Savage , C.E. , Jones , R.C. and Naylor , C.J. 2006 . Demonstration of loss of attenuation and extended field persistence of a live avian metapneumovirus vaccine . Vaccine , 24 : 6476 – 6482 .
- Cavanagh , D. , Mawditt , K. , Britton , P. and Naylor , C.J. 1999 . Longitudinal field studies of infectious bronchitis virus and avian pneumovirus in broilers using type-specific polymerase chain reactions . Avian Pathology , 28 : 593 – 605 .
- Chary , P. , Rautenschlein , S. , Njenga , M.K. and Sharma , J.M. 2002 . Pathogenic and immunosuppressive effects of avian pneumovirus in turkeys . Avian Diseases , 46 : 153 – 161 .
- Collins , M.S. , Gough , R.E. and Alexander , D.J. 1993 . Antigenic differentiation of avian pneumovirus isolates using polyclonal antisera and mouse monoclonal antibodies . Avian Pathology , 22 : 469 – 479 .
- Cook , J.K.A. , Ellis , M.M. , Dolby , C.A. , Holmes , H.C. , Finney , P.M. and Huggins , M.B. 1989a . A live attenuated turkey rhinotracheitis virus vaccine. 1. Stability of the attenuated strain . Avian Pathology , 18 : 511 – 522 .
- Cook , J.K.A. , Holmes , H.C. , Finney , P.M. , Dolby , C.A. , Ellis , M.M. and Huggins , M.B. 1989b . A live attenuated turkey rhinotracheitis virus vaccine. 2. The use of the attenuated strain as an experimental vaccine . Avian Pathology , 18 : 523 – 534 .
- Cook , J.K.A. , Jones , B.V. , Ellis , M.M. , Jing , L. and Cavanagh , D. 1993a . Antigenic differentiation of strains of turkey rhinotracheitis virus using monoclonal antibodies . Avian Pathology , 22 : 257 – 273 .
- Cook , J.K.A. , Kinloch , S. and Ellis , M.M. 1993b . In vitro and in vivo studies in chickens and turkeys on strains of turkey rhinotracheitis virus isolated from the two species . Avian Pathology , 22 : 157 – 170 .
- Cook , J.K.A. , Orthel , F. , Orbell , S. , Woods , M.A. and Huggins , M.B. 1996 . An experimental turkey rhinotracheitis (TRT) infection in breeding turkeys and the prevention of its clinical effects using live attenuated and inactivated vaccines . Avian Pathology , 25 : 231 – 243 .
- Cook , J.K.A. , Chesher , J. , Orthel , F. , Woods , M.A. , Orbell , S.J. , Baxendale , W. and Huggins , M.B. 2000 . Avian pneumovirus infection in laying hens: experimental studies . Avian Pathology , 29 : 545 – 556 .
- Davelaar , F.G. and Kouwenhoven , B. 1976 . Changes in the harderian gland of the chicken following conjunctival and intranasal infection with infectious bronchitis virus in one-and 20-day-old chickens . Avian Pathology , 5 : 39 – 50 .
- Ganapathy , K. , Cargill , P. & Jones , R.C. (2006) . Some observations on the detection of avian metapneumovirus and infectious bronchitis virus in clinical material . In Proceedings of the Vth International Symposium on avian Corona- and Pneumoviruses and complicating pathogens 14–16 May 2006 (pp. 251 – 256 ). Rauischholzhausen , Germany .
- Gough , R.E. , Collins , M.S. , Cox , W.J. and Chettle , N.J. 1988 . Experimental infection of turkeys, chickens, ducks, geese, guinea fowl, pheasants and pigeons with turkey rhinotracheitis virus . Veterinary Record , 123 : 58 – 59 .
- Gough , R.E. , Manvell , R.J. , Drury , S.E.N. and Pearson , D.B. 1994 . Isolation of an avian pneumovirus from broiler chickens . Veterinary Record , 134 : 353 – 354 .
- Gough , R.E. , Alexander , D.J. & Wyeth , P.J. (1998) . Avian rhinotracheitis (pneumovirus) . In A Laboratory Manual for the Isolation and Identification of Avian Pathogens 4th edn (pp. 164 – 168 ). American Association of Avian Pathologists, University of Pennsylvania, New Bolton Center, Kennett Square, PA, USA .
- Hafez , H.M. 1993 . The role of pneumovirus in swollen head syndrome of chickens . Archiv für Geflügelkunde , 57 : 181 – 185 .
- Hafez , H.M. , Hess , M. , Prusas , C. , Naylor , C.J. and Cavanagh , D. 2000 . Presence of avian pneumovirus type A in continental Europe during the 1980s . Journal of Veterinary Medicine , 47 : 629 – 633 .
- Jeurissen , S.H.M. , Vervelde , L. and Janse , E.M. 1994 . Structure and function of lymphoid tissues of the chickens . Poultry Science Reviews , 5 : 183 – 207 .
- Jones , R.C. , Baxter-Jones , C. , Savage , C.E. , Kelly , D.F. and Wilding , G.P. 1987 . Experimental infection of chickens with a ciliostatic agent isolated from turkeys with rhinotracheitis . Veterinary Record , 120 : 301 – 302 .
- Jones , R.C. , Naylor , C.J. , Bradbury , J.M. , Savage , C.E. , Worthington , K. and Williams , R.A. 1991 . Isolation of a turkey rhinotracheitis-like virus from broiler breeder chickens in England . Veterinary Record , 129 : 509 – 510 .
- Jones , R.C. , Naylor , C.J. , Al-Afaleq , A. , Worthington , K.J. and Jones , R. 1992 . Effect of cyclophosphamide immunosuppression on the immunity of turkeys to viral rhinotracheitis . Research in Veterinary Science , 53 : 38 – 41 .
- Juhasz , K. and Easton , A.J. 1994 . Extensive sequence variation in the attachment (G) protein gene of avian pneumovirus: evidence for two distinct subgroups . Journal of General Virology , 75 : 2873 – 2880 .
- Liman , M. and Rautenschlein , S. 2007 . Induction of local and systemic immune reactions following infection of turkeys with avian metapneumovirus (aMPV) subtype A and B . Veterinary Immunology and Immunopathology , 115 : 273 – 285 .
- Lister , S.A. and Alexander , D.J. 1986 . Turkey rhinotracheitis: a review . Veterinary Bulletin , 56 : 637 – 663 .
- Lu , Y.S. , Shien , Y.S. , Tsai , H.J. , Tseng , C.S. , Lee , S.H. and Lin , D.F. 1994 . Swollen head syndrome in Taiwan-isolation of an avian pneumovirus and serological survey . Avian Pathology , 23 : 169 – 174 .
- Majó , N. , Allan , M. , O'Loan , C.J. , Pages , A. and Ramis , A.J. 1995 . A sequential histopathologic and immunocytochemical study of chickens, turkey poults, and broiler breeder experimentally infected with turkey rhinotracheitis virus . Avian Diseases , 39 : 887 – 896 .
- Majó , N. , Gibert , X. , Vilafranca , M. , O'Loan , C.J. , Allan , G.M. , Costa , L.I. , Pages , A. and Ramis , A. 1997 . Turkey rhinotracheitis virus and Escherichia coli experimental infection in chickens: histopathological, immunocytochemical and microbiological study . Veterinary Microbiology , 57 : 29 – 40 .
- Mase , M. , Yamaguchi , S. , Tsukamoto , K. , Imada , T. , Imai , K. and Nakamura , K. 2003 . Presence of avian pneumovirus subtypes A and B in Japan . Avian Diseases , 47 : 481 – 484 .
- Montgomery , R.D. , Maslin , W.R. , Boyle , C.R. , Pledger , T. and Wu , C.C. 1991 . Effect of an Arkansas strain of infectious bronchitis vaccine on the head-associated lymphoid tissue (HALT) . Avian Diseases , 35 : 302 – 307 .
- Nakamura , K. , Mase , M. , Tanimura , N. , Yamaguchi , S. and Yuasa , N. 1998 . Attempts to reproduce swollen head syndrome in specific pathogen free chickens by inoculating with Escherichia coli and/or turkey rhinotracheitis virus . Avian Pathology , 27 : 21 – 27 .
- Naylor , C.J. and Jones , R.C. 1994 . Demonstration of a virulent subpopulation in a prototype live attenuated turkey rhinotracheitis vaccine . Vaccine , 12 : 1225 – 1230 .
- O'Brien , J.D.P. 1985 . Swollen head syndrome in broiler breeder . Veterinary Record , 117 : 619 – 620 .
- Pattison , M. , Chettle , N. , Randall , C.J. and Wyeth , P.J. 1989 . Observations on swollen head syndrome in broiler and broiler breeder chickens . Veterinary Record , 125 : 229 – 231 .
- Picault , J.P. , Giraud , P. , Drouin , P. , Guittet , M. , Bennejean , G. , Lamande , J. , Toquin , D. and Gueguen , C. 1987 . Isolation of a TRTV-like virus from chickens with swollen head syndrome . Veterinary Record , 121 : 135
- Pringle , C.R. 1998 . Virus taxonomy—San Diego 1998 . Archives of Virology , 143 : 1449 – 1459 .
- Reed , L.J. and Muench , H. 1938 . A simple method for estimating fifty percent endpoints . American Journal of Hygiene , 27 : 493 – 497 .
- Seal , B. 1998 . Matrix protein gene nucleotide and predicted amino acid sequence demonstrate that the first US avian pneumovirus isolate is distinct from European strains . Virus Research , 58 : 45 – 52 .
- Seal , B.S. 2000 . Avian pneumoviruses and emergence of a new type in the United States of America . Animal Health Research and Review , 1 : 67 – 72 .
- Shin , H.J. , McComb , B. , Back , A. , Shaw , D.P. , Halvorson , D.A. and Nagaraja , K.V. 2000 . Susceptibility of broiler chicks to infection by avian pneumovirus of turkey origin . Avian Diseases , 44 : 797 – 802 .
- Sugiyama , M. , Koimaru , H. , Shiba , M. , Ono , E. , Nagata , T. and Ito , T. 2006 . Drop in egg production in chickens by experimental infection with avian metapneumovirus strain PLE8T1 derived from swollen head syndrome and the application to evaluate vaccine . Journal of Veterinary Medical Science , 68 : 783 – 787 .
- Suresh , P. and Arp , L.H. 1995 . A time course study of the transfer of immunoglobulin G from blood to tracheal and lacrimal secretions in young turkeys . Avian Diseases , 39 : 349 – 354 .
- Survashe , B.D. , Aitken , I.D. and Powell , J.R. 1979 . The response of the Harderian gland of the fowl to antigen given by ocular route I. Histological changes . Avian Pathology , 8 : 77 – 93 .
- Tanaka , M. , Takuma , H. , Kokumai , N. , Oishi , E. , Obi , T. , Hiramatsu , K. and Shimizu , Y. 1995 . Turkey rhinotracheitis virus isolated from broiler chicken with swollen head syndrome in Japan . Journal of Veterinary Medical Science , 57 : 939 – 941 .
- Toquin , D. , Guionie , O. , Allee , C. , Morin , Y. , Le Coq , L. , Zwingelstein , F. , Jestin , V. & Eterradossi , N. (2006) . Compared susceptibility of SPF ducklings and SPF turkeys to the infection by avian Metapneumoviruses belonging to the four subgroups . In Proceedings of the Vth International Symposium on Avian Corona and Pneumoviruses and Complicating Pathogens (pp. 70 – 76 ). Rauischholzhausen , Germany .
- Toro , H. , Lavaud , P. , Vallegos , P. and Ferreira , A. 1993 . Transfer of IgG from serum to lachrymal fluid in chickens . Avian Diseases , 37 : 60 – 66 .
- Toro , H. , Godoy , V. , Larenas , J. , Reyes , E. and Kaleta , E.F. 1996 . Avian infectious bronchitis: viral persistence in the Harderian gland and histological changes after eyedrop vaccination . Avian Diseases , 40 : 114 – 120 .
- Tiwari , A. , Patnayak , D.P. and Goyal , S.M. 2006 . Attempts to improve on a challenge model for subtype C avian pneumovirus . Avian Pathology , 35 : 117 – 121 .
- Van de Zande , S. , Nauwynck , H. , De Jonghe , S. and Pensaert , M. 1999 . Comparative pathogenesis of a subtype A with a subtype B avian pneumovirus in turkeys . Avian Pathology , 28 : 239 – 244 .
- Williams , R.A. , Savage , C.E. and Jones , R.C. 1991a . Development of a live attenuated vaccine against turkey rhinotracheitis . Avian Pathology , 20 : 45 – 55 .
- Williams , R.A. , Savage , C.E. , Worthington , K.J. and Jones , R.C. 1991b . Further study on the development of a live attenuated vaccine against turkey rhinotracheitis . Avian Pathology , 20 : 585 – 596 .
- Wyeth , P.J. , Chettle , N.J. , Gough , R.E. and Collins , M.S. 1987 . Antibodies to TRT in chickens with swollen head syndrome . Veterinary Record , 120 : 286 – 287 .