Abstract
Broiler chickens are intensively selected for productive traits. The management of these highly productive animals must be optimal to allow their full genetic potential to be expressed. If this is not done, inefficient production and several metabolic diseases such as ascites become apparent. The causes of the ascites are multifactorial but diet and, particularly, interactions between diet, other environmental and genetic factors play an important role. The relatively high heritability estimates for ascites-related traits and the significance of maternal genetic effects for most of the traits indicate that direct and maternal genetic effects play an important role in development of the ascites syndrome. An imbalance between oxygen supply and the oxygen required to sustain rapid growth rates and high food efficiencies causes ascites in broiler chickens. Because of the relationship to oxygen demand, ascites is affected and/or precipitated by factors such as growth rate, altitude (hypoxia) and environmental temperature. As the high metabolic rate (fast growth) is a major factor contributing to the susceptibility of broilers to ascites, early-age feed or nutrient restriction (qualitative or quantitative) or light restriction in order to slow down the growth rate seem practically viable methods, since final body weight is not compromised. Manipulation of the diet composition and/or feed allocation system can have a major effect on the incidence of ascites. Optimization of the house temperature and ventilation in cold weather seem helpful practices to decrease ascites incidence.
Syndrome ascite chez le poulet de chair: perspectives physiologique et nutritionnelle
Les poulets de chair sont intensivement sélectionnés pour les caractères de production. Le management de ces animaux très hautement productifs doit être optimum pour permettre l'expression de la totalité de leur potentiel génétique. Si ceci n'est pas fait, une production inefficace et différentes maladies métaboliques, telle l'ascite, apparaissent. Les causes de l'ascite sont multifactorielles mais l'alimentation et particulièrement les interactions entre l'aliment et d'autres facteurs, génétiques et environnementaux, jouent un rôle important. Les estimations de l'héritabilité relativement élevées pour les caractères liés à l'ascite et l'importance des effets génétiques maternels pour la plupart des caractères ont indiqué que les effets génétiques maternels et directs jouent un rôle important dans le développement du syndrome ascite. Un déséquilibre entre l'oxygène apporté et l'oxygène requis pour soutenir des taux de croissance rapides et une efficacité alimentaire élevée entraîne de l'ascite chez les poulets de chair. Du fait de la relation vis-à-vis de la demande en oxygène, l'ascite est affectée et/ou aggravée par des facteurs tels le taux de croissance, l'altitude (hypoxie), et la température environnementale. Comme le taux élevé du métabolisme (croissance rapide) est un facteur majeur contribuant à la sensibilité des poulets de chair à l'ascite, la restriction alimentaire ou nutritionnelle (qualitative ou quantitative) pendant le jeune âge ou la restriction lumineuse dans le but de diminuer le taux de croissance semblent être des méthodes susceptibles d’être mises en pratique, puisque le poids final du corps n'est pas compromis. La manipulation de la composition de l'aliment et/ou un système d'alimentation régulé peuvent avoir un effet majeur sur l'incidence de l'ascite. L'optimisation de la température du bâtiment et la ventilation en période froide semblent être des pratiques utiles pour diminuer l'incidence de l'ascite.
Aszites-Syndrom in Broilern: Physiologische und ernährungsbedingte Perspektiven
In der Broilerzucht steht die Selektion auf Produktionsmerkmale an erster Stelle. Zur vollständigen Erreichung der Ausprägung dieses genetischen Potentials muss jedoch das Management der Hochleistungstiere optimal sein. Wenn das nicht der Fall ist, kommt es zu einer ineffizienten Produktion und dem Auftreten verschiedener metabolischer Erkrankungen wie die Aszites. Die Ursachen für Aszites sind multifaktoriell, aber das Futter und insbesondere die Interaktionen zwischen Futter und anderen haltungsbedingten sowie genetischen Faktoren spielen eine bedeutsame Rolle. Die relativ hohe Heritabilität für Aszites bezogene Merkmale und die Signifikanz der maternalen genetischen Effekte für die meisten dieser Merkmale zeigen, dass direkte und maternale genetische Effekte eine große Bedeutung für die Entstehung des Aszites-Syndroms haben. Eine Imbalanz zwischen der Sauerstoffversorgung und dem Sauerstoffbedarf für schnelles Wachstum und hohe Futterverwertungen verursacht Aszites in Broilern. Wegen dieser Beziehung zum Sauerstoffbedarf wird die Aszites von Faktoren wie der Wachstumsrate, Höhenlage (Hypoxie) und Umgebungstemperatur beeinflusst und/oder herbeigeführt. Da eine hohe Metabolisierungsrate (schnelles Wachstum) ein Hauptfaktor für die Empfänglichkeit von Broilern für die Aszites ist, scheinen eine Restriktion des Starterfutters oder der Nährstoffe (qualitativ oder quantitativ) oder eine Restriktion des Lichts praktikable Methoden zu sein, die Wachstumsrate zu verlangsamen, da das Mastendgewicht nicht gefährdet ist. Die Veränderung der Futterzusammensetzung und/oder der Futterbereitstellung kann einen großen Einfluss auf das Auftreten der Aszites haben. Auch die Optimierung der Stalltemperaturen und der Lüftung bei kaltem Wetter scheinen sinnvolle Maßnahmen zu sein, um das Aszites-Vorkommen zu reduzieren.
Síndrome ascítico en pollos de engorde: perspectivas nutricionales y fisiológicas
Los pollos de engorde sufren una intensa selección genética de sus caracteres productivos. El manejo de estos animales altamente productivos debe ser óptimo para permitir que se exprese todo su potencial genético. Si esto no ocurre, se observa una producción ineficiente y aparecen alteraciones metabólicas como la ascitis. La etiología de la ascitis es multifactorial pero la dieta, y en particular, las interacciones entre la dieta y otros factores ambientales y genéticos parece ser que juegan un papel importante. La relativamente elevada heredabilidad estimada para los caracteres relacionados con la ascitis y la importancia de los efectos genéticos maternos de la mayoría de los caracteres indicaron que los efectos maternos y directos juegan un papel importante en el desarrollo del síndrome ascítico. Un desequilibrio entre el aporte de oxígeno y la demanda de éste, necesaria para ritmos rápidos de crecimiento y eficiencias altas de alimentación, es la causa de la ascitis en pollos de engorde. Por su relación con la demanda de oxígeno, la ascitis se ve afectada y/o desencadenada por factores como crecimiento rápido, altitud (hipoxia) y temperatura ambiental. Teniendo en cuenta que los ritmos metabólicos altos (crecimiento rápido) son un factor que contribuye a la susceptibilidad de los broilers a la ascitis, piensos de primera edad o restricciones nutritivas (cualitativas o cuantitativas) o restricciones de luz que reduzcan el ritmo de crecimiento parecen ser métodos viables ya que el peso corporal final no se ve afectado. La manipulación de la composición de la dieta y/o el sistema de distribución del pienso pueden tener un efecto importante en la incidencia de la ascitis. La optimización de la temperatura y ventilación de las naves en climas fríos parecen ser prácticas útiles para reducir la incidencia de ascitis.
Introduction
The modern chicken (Gallus gallus domesticus) has been intensely selected for higher growth rates and so indirectly for a high rate of protein synthesis, which requires more oxygen (Decuypere et al., Citation2005), increased feed conversion (Decuypere et al., Citation2000; Pakdel et al., Citation2002), egg production (Decuypere et al., Citation2000) or meat yield and breast percentage (Hoving-Bolink et al., Citation2000). Modern strains of broilers are able to achieve market weight in 60% less time than broilers of 40 years ago. Nevertheless, the pulmonary and cardiac capacity of modern broilers is very similar to the old broiler strains, which forces their cardiopulmonary system to work very close to its physiological limit (Lorenzoni et al., Citation2006). The lung capacity does not always meet the oxygen demands necessary for rapid growth. This results in impaired ability to regulate the energy balance under extreme conditions, such as low ambient temperature or high altitude (Luger et al., Citation2003). If the lung of the chicken grows less rapidly than the rest of the body, hypoxia and ascites could result (Julian, Citation2000). Recent data suggest that ascites is not caused by an increased oxygen requirement of fast growth rate per se at low altitude, but by an impaired oxygen supply to sustain fast growth rate (Decuypere et al., Citation2005). Ascites (pulmonary hypertension syndrome, or “water belly”) is a metabolic disorder, characterized by hypoxaemia, increased workload of the cardiopulmonary system, central venous congestion (Luger et al., Citation2003), an excessive accumulation of fluid in body coelomic cavities (Olkowski et al., Citation1999), hypertrophy of the right ventricle and a flaccid heart (Riddell, Citation1991), and finally death (Luger et al., Citation2003). A high incidence of ascites can occur when broilers are reared at altitudes high enough to substantially reduce the partial pressure of oxygen (Owen et al., Citation1990; Wideman et al., Citation2003). Studies showed a high incidence of subclinical heart disease in fast-growing broilers, with many developing clinical signs of chronic heart failure and ascites. Recently, a novel observation was reported where sudden death in broilers was associated with rupture of the right atrium (Olkowski et al., Citation2007). Although the incidence of this metabolic disorder in well-managed flocks is very low, it causes important economic losses to the poultry breeding industry—an estimated 4.7% of the broilers worldwide have the disease (Maxwell & Robertson, Citation1997). It is estimated that 5% of broilers and 20% of roaster birds die of ascites (Balog, Citation2003); considering that an estimated 40 billion broilers are produced annually around the world, it is evident that the economic losses due to ascites are significant. Genetic, physiological, environmental, and management factors all seem to interact to produce a cascade of events that culminate in ascites syndrome.
Aetiology of the Ascites Syndrome
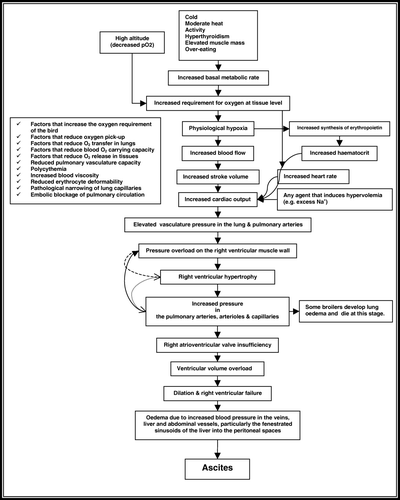
Despite the intensive investigation of the syndrome for many years, the primary cause of ascites is unclear (Crespo & Shivaprasad, Citation2003). The physiology of this syndrome (see ) has been extensively studied (Julian, Citation1993, Citation2000; Scheele, Citation1996; Hassanzadeh et al., Citation1997; Decuypere et al., Citation2000; Wideman, Citation2000). Ascites syndrome, first reported in flocks of broiler chickens reared at high altitudes in Bolivia (Hall & Machicao, Citation1968), may result from one or more of four physiological changes that cause an increased production and/or decreased removal of peritoneal lymph (Balog, Citation2003). Obstruction of lymph drainage, decreased plasma oncotic pressure, fluid leakage secondary to increased vascular permeability, and, last but not least, increased portal pressure secondary to right ventricular failure or liver damage may all result in ascites (Julian, Citation2005). Neoplasia, most frequently, oviduct carcinomas and, alternatively, right ventricular failure with valvular insufficiency, in which high venous pressure in the vena cava interferes with lymph return, may bring about blockage of lymph and, subsequently, ascites (Balog, Citation2003). Since plasma proteins, especially albumin, are mainly responsible for blood oncotic pressure, some researchers (Wise & Evans, Citation1975; Bowes et al., Citation1989) explained decreased plasma proteins in ascites-sensitive broilers or those with ascites. Decreased plasma protein could be a result of loss of high-protein lymph from the liver or a stop-eating process due to right ventricular hypertrophy. Vascular damage and subsequent leakage of fluid and proteins through the vascular epithelium can be caused by viral and/or bacterial infections, chemical toxins, chlorinated hydrocarbons and some phenolic compounds, coal-tar derivatives, dioxin, and pentacholorophenol (Balog, Citation2003). Increased vascular hydraulic pressure could be a result of hepatic pathologies, right atrioventricular valve pathologies, pulmonary hypertension and miscellaneous cardiac pathologies (Currie, Citation1999).
Pulmonary Hypertension
Pulmonary hypertension is the result of processes taking place in both respiratory and cardiovascular systems. The architecture of the modern broilers—small stature, the large, heavy breast mass, the pressure from abdominal contents on air sacs, and the small lung volume—may all be involved in the increased incidence of ascites syndrome (Julian, Citation1998; Balog, Citation2003). Theoretically, the unique anatomy of the avian respiratory system results in a model of gas exchange that is more efficient than the mammalian model (Piiper & Scheid, Citation1975), and for a given level of ventilation to the gas-exchange surfaces, cardiac output, and lung diffusing capacity, arterial O2 loading and CO2 elimination are predicted to be better in a parabronchial lung, compared with an alveolar lung with the same inspired gases and metabolic demands (Powell, Citation2000). But the rigid (parabronchial) lungs of birds conform closely to the contour of the body cavity and do not expand like the (alveolar) lungs of mammals (Balog, Citation2003). In other words, the avian respiratory system possesses rigid lungs of fixed volume (Fedde, Citation1984), which do not expand or contract with each breath as mammalian lungs do. The blood and air capillaries form a network that allows the small blood capillaries of the lung to dilate only very little to accommodate increased blood flow (Julian, Citation1998).
Pressure, generated by cardiac contraction, drives blood flow around the circulation. Poiseuille's law relates volume flow (Q) to the pressure drop (P 1 – P 2) along a tube of radius (r) and length (L) during steady flow as follows:
Vascular resistance (R) will be calculated as:
It is obvious that a slight change in a vessel radius has major effects on flow and, consequently, on resistance characteristics.
On the other hand, the Frank–Starling law of the heart describes an increase in contractility of the myocardium due to an elevated preload. In terms of the right ventricle, preload is determined by cardiac venous return. This mechanism increases cardiac output by elevating the stroke volume. If pulmonary vascular resistance increases, the afterload increases, leading to a pressure overload in the right ventricle. The classical response to this stimulus is ventricular hypertrophy, one of the common lesions in ascitic birds. One important consequence of ventricular hypertrophy is distortion of atrio-ventricular valves, which also become thicker, leaky and less efficient (see below, Avian Heart Valves Anatomy) and the production of regurgitant flow from the ventricle to the atrium during ventricular systole. The ventricle is then subjected to volume overload and the resultant haemodynamic pressure imbalance generates ascites (Currie, Citation1999).
Avian Heart Valves Anatomy
Blood entering the left ventricle from the left atrium on atrial systole passes through an orifice guarded by a membranous atrioventricular valve, similar in general structure to a mammalian atrioventricular valve, except that it is tricuspid. Blood passing from the right atrium to the right ventricle enters through an orifice guarded by an atrioventricular valve that is structurally unique to birds. In pronounced contrast to the fibrous structure characteristic of the mammalian tricuspid valve, in birds the right atrioventricular valve consists of a single spiral flap of myocardium attached obliquely to the free wall of the right ventricle, so it is capable of becoming thicker and leaky, as a consequence of ventricular hypertrophy. The outflow valves from the right and left ventricles are, at first glance, more conventional (mammalian) in nature (Smith et al., Citation2000).
Ascites Symptoms
Ascites symptoms in broiler chickens include generalized oedema, fluid accumulation in the pericardium, hydropericardium (pericardial effusion) (Olkowski et al., Citation2003), in the abdominal cavity (Balog et al., Citation2003), epicardial fibrosis, lung oedema, enlarged, flaccid heart (Balog et al., Citation2003), hypertrophy and dilation of the heart, especially the right ventricle (Decuypere et al., Citation2000), variable liver changes, hypoxaemia, pale comb and higher blood haematocrit (Luger et al., Citation2003). These symptoms indicate that a large number of organs (including the heart, lung, liver, etc.) are involved in the disease.
Erythropoiesis and Ascites
Chickens have a thicker respiratory membrane than other birds, and broilers have a thicker respiratory membrane than Leghorn-type fowl, so the ability of broilers to move oxygen into haemoglobin may not be as good as in other birds. Research on oxygen haemoglobin saturation in meat-type chickens indicates that fast-growing broilers have a lower oxygen saturation than slow-growing broilers. These results suggest that some meat-type chickens are not fully oxygenating their haemoglobin even at low altitude. This may be the result of increased blood flow rate through the lung capillary bed of the lung, which does not allow time for haemoglobin to pick up a full load of oxygen (Julian, Citation2000). In ascites, where metabolic burdening imposes on the broilers difficulties in fulfilling tissue demands for oxygen, birds exhibit a decrease in blood oxygen saturation and high haematocrit values. Elevation in haematocrit can be caused by diminished plasma volume, as a result of fluid exudation out of the blood system to the abdominal cavity, or enhanced erythropoiesis. The development of mature peripheral red blood cells from pluripotent stem cells in the bone marrow is a complex process, regulated by erythropoietin, corticosterone, triiodothyronine and growth factors. Recent studies have revealed an increase in haematocrit, with no significant change in plasma volume. It has also been concluded that continually increased corticosterone concentrations, as an inducer of erthropoiesis proliferation and differentiation arrest, in ascitic chickens, resulted in increased production of red blood cells (partially immature) with decreased haemoglobin content, which might have contributed to enhanced development of hypoxaemia and to aggravation of the syndrome (Luger et al., Citation2003). Although increased haematocrit values should be a positive aid to alleviate hypoxaemia, birds dying of ascites all show high haematocrit levels that increase blood viscosity and augment the inability of the failing right ventricle to pump blood through the vasoconstricted pulmonary blood vessels (Shlosberg et al., Citation1998)
Genetics and Ascites
Poultry breeders have been selecting very successfully on growth-related traits of broilers over the past few decades. Recently, the breeding industry has taken up new challenges and efforts are being directed to produce stock adaptable to a wide range of environments and to decrease the incidence of metabolic and physiological disorders, of which ascites syndrome is an example (Pakdel et al., Citation2004).
Genetically, the modern broiler, especially male broilers, seems to be more prone to develop ascites. This is probably due to extreme selection for either the growth rate or the feed conversion ratio, which puts high demands on the metabolic processes and on the oxygen demand (Decuypere et al., Citation2000), and oxygen requirement is affected by genetic factors other than growth rate. Birds selected both for low food conversion ratio (FCR) with low rates of heat production that were stimulated to a higher heat production by a low ambient temperature had difficulties in adapting to environmental changes. It has also been shown that the highest incidence of ascites occurs in broilers that combined low FCR with fast growth rate, whereas in broilers with either slower growth or higher FCR, the incidence of ascites was much lower. A low FCR in fast-growing birds was attributed to low values of heat production. Moreover, birds selected for a combination of both fast growth and low FCR had low pO2 and high pCO2 in venous blood at low ambient temperature compared with the slower growing birds (Decuypere et al., Citation2005). There are a few reports about genetic parameters for ascites-related traits. Lubritz et al. (Citation1995) demonstrated favourable heritabilities for fluid accumulation in the abdominal cavity and the ratio of right ventricular weight to the total ventricle weight. They suggested that selection based on these traits, which were measured under cold conditions, would be effective to reduce the incidence of ascites. Shlosberg et al. (Citation1996) evaluated the suitability of haematocrit value to select against ascites. Maxwell et al. (Citation1998) indicated that, in the presence of ascites, troponin T, an indicator of heart muscle damage, was heritable. Moghadam et al. (Citation2001) showed that the heart defect (i.e. including pulmonary hypertension, right ventricular failure and fluid accumulation in the peritoneal cavity) as a trait related to ascites was heritable and had a positive genetic correlation with body weight. de Greef et al. (Citation2001) demonstrated that the genetic parameters of ascites related traits within the same physical environment varied considerably with the severity of the disease. Therefore, the choice of selection criteria is complicated. Ascites traits, including the haematocrit value and the ratio of the right ventricular weight to the total ventricular weight, have been used as indirect criteria in selection for reduced incidence of ascites in broilers (Shlosberg et al., Citation1996; Wideman et al., Citation1997; Scheele et al., Citation2003; Pakdel et al., Citation2005). The current methodology for estimating parameters for ascites indicator traits does not account for the differences between healthy and diseased birds. However, methods are available to distinguish between healthy and diseased animals. For example, in the case of mastitis in dairy cows, the parameter estimations were improved by dividing the heterogeneous populations into two more homogeneous distributions, healthy and diseased. With these improved estimates, a better alternative for selection against susceptibility to mastitis was provided (Detilleux & Leroy, Citation2000). Mixture models have been widely used to separate heterogeneous populations into more homogeneous distributions (McLachlan & Khrishnan, Citation1997). The mixture model can be used for situations in which it is unknown how to classify individuals between distributions. This is the case when analysing ascites traits, because there are no clear criteria to distinguish between healthy and diseased birds (de Greef et al., Citation2001). Using mixture models, birds can be assigned to different distributions via probabilities estimated from trait observations (Detilleux & Leroy, Citation2000). For ascites traits, a mixture model in its simplest form may be used to assign observations to two components; that is, ascitic and non-ascitic. The identification and culling of birds could then be based on the probability of putative ascites, given ascites indicator traits, rather than on crude ascites traits (Zerehdaran et al., Citation2006).
Nutrition and Ascites
The broiler growth rate has been found to have a direct relationship with susceptibility to ascites (Camacho et al., Citation2004). Manipulation of the diet composition and/or feed allocation system can have a major effect on the incidence of ascites. In most instances, such changes to the feeding programme influence ascites via their effect on growth rate. Major nutritional factors including high nutrient density rations, high feed intake and feed form are known to influence the occurrence of ascites in broilers (Balog et al., Citation2000; Coello et al., Citation2000; Bölükbasi et al., Citation2004; Ozkan et al., Citation2006).
Managing the Growth Rate
Although the growth rate can influence metabolic diseases during the entire period of broiler production, research has shown that the early period is particularly important (Camacho et al., Citation2004). A reduced growth rate from 3 to 14 days of age not only benefits bird health during that period but also later when the growth rate is as fast or faster than birds that have not experienced slower early growth. These suggest that early days of production represents an important developmental period in poultry meat stocks. Recently, management strategies have been investigated to alter the growth curve of meat stocks with the objective of reducing the incidence of metabolic diseases while maintaining competitive production traits. Methods have included quantitative and qualitative feed restriction, altered feed form, and environmental management.
Feed restriction
Feed restriction techniques have ranged from very severe (maintenance only) to milder forms, which involve daily feed restriction or skipping feeding 1 or 2 days a week. Feed restriction has proven successful in reducing metabolic disease such as ascites, but the degree of restriction required to control health problems needs to be balanced with the time required to reach market weight and other effects on bird productivity. Feed restriction reduces growth at a critical time in a broiler chick's lifecycle when it is the most susceptible to metabolic disease due to its high oxygen demands (Balog et al., Citation2000; Coello et al., Citation2000; Ozkan et al., Citation2006). Despite some of the advantages of feed restriction as a treatment to reduce ascites, adverse secondary effects might result due to lower consumption of anticoccidial products fed to control coccidiosis. Another important problem resulting from feed restriction programmes is poor pigmentation, which is directly related to the quantity of xanthophylls consumed. Pigmentation is very important because it is perceived as a measure of quality in the marketplace (Camacho-Fernandez et al., Citation2002). Feed restriction can reduce the availability of nutrients and pigmentation precursors, which may have a direct effect on weight gain, muscle mass, and the profit–cost relationship. These effects could be more pronounced if the restriction programme was not correct (Camacho-Fernandez et al., Citation2002).
Nutrient density
Reducing the concentration of nutrients in a diet can reduce the growth rate, with the effects most pronounced from 0 to 21 days of age, during the time when birds cannot totally adapt intake to lower feed nutrient content. If diets remained balanced to energy content, the effect of nutrient density on growth rate is relatively small unless the decrease in density is very large. However, even moderately lower nutrient density reduces mortality due to ascites (Camacho-Fernandez et al., Citation2002).
Diet form
Most meat birds are fed crumbled or pelleted diets to achieve maximum growth and feed efficiency. Feeding mash reduces growth rate (1 to 2 days to market) and reduces mortality and condemnations due to metabolic disease. However, this type of programme may not be economically acceptable in all areas and has been demonstrated to increase the incidence of pendulous crops. Broilers that consume pellet feed have frequently been shown to have higher incidences of ascites than broilers that consume the same diet in mash form (Bölükbasi et al., Citation2005).
Omega-3 fatty acid sources (flax and fish oils)
It has been found that decreased deformity of the erythrocytes, as measured by the filtration index, can increase the amount of ascites in broiler chickens (Mirsalimi & Julian, Citation1991; Mirsalimi et al., Citation1993). The deformity of erythrocytes is reduced by hypoxaemia. Studies with humans have demonstrated that erythrocyte deformity can be increased by dietary supplementation of omega-3 (n-3) fatty acids from fish oils (Berlin et al., Citation1992). Archer et al. (Citation1989) found that supplementation with fish oil reduces blood viscosity and right ventricular hypertrophy in rats. However, other studies (Hulan et al., Citation1989) reported a reduction in the growth rate of birds fed red fish meal as a source of omega-3 fatty acids. This is important, because the incidence of ascites can be reduced by slowing the growth rate of broilers (Julian, Citation1993). The increased content of unsaturated fatty acids probably increases the fluidity of the erythrocyte membrane and alters membrane function to increase the deformability of the erythrocytes and potentially help reduce the incidence of ascites. This could explain the reduction in whole blood viscosity under hypobaric conditions with feeding of flax oil. These factors together would decrease the resistance to blood flow and improve the movement of the erythrocytes through the capillaries, thus improving oxygen transport and decreasing ascites (Walton et al., Citation1999).
l-Carnitine
It is generally accepted that endogenous l-Carnitine synthesis together with its dietary intake should be sufficient for normal function. However, in cases of increased metabolic rate (in fasting-growth broilers), when energy demands are elevated, the availability of l-Carnitine may become a limiting factor for fat oxidation. In these circumstances, additional exogenous l-Carnitine might prove beneficial. It is hypothesized that l-Carnitine-supplemented chickens are more resistant to the development of ascites due to an improved cardiac output. Furthermore, there is evidence that free radicals may be involved in the development of ascites (Bottje & Wideman, Citation1995). As l-Carnitine (ester) is known to have free radical scavenging properties (Packer et al., Citation1991), this might also contribute to a beneficial effect of l-Carnitine on ascites incidence. This hypothesis is currently under investigation (Buyse et al., Citation2001).
Antioxidants
The elevated production of reactive oxygen in broilers prone to ascites may potentiate the development of the disease or aggravate the disease as it occurs (Enkvetchakul et al., Citation1993). For chickens, the first line of defence against reactive oxygen is endogenous antioxidants such as tocopherols, glutathione, uric acid, and ascorbic acid. The levels of glutathione and α-tocopherol and γ-tocopherol are decreased in the mitochondria of an ascitic broiler, suggesting reactive oxygen is produced at the primary site of energy transduction (Cawthorn et al., Citation2001). Ascorbic acid and glutathione concentrations are reduced in both the liver and lung of broilers that have been reared in ascites-promoting conditions, signifying their utilization against reactive oxygen production in these tissues. In contrast to other endogenous antioxidants, uric acid is increased in the liver and lung (Enkvetchakul et al., Citation1993). This probably results from an increased net catabolism of adenosine triphosphate that occurs with the stimulation of anaerobic glycolysis, increasing the flow of substrates through the purine degradation pathway (Stinefelt, Citation2003). Therefore, the change in the antioxidant status of the broiler during ascites progression is observed in conjunction with increased markers of reactive oxygen-mediated tissue injury, indicating a state of oxidative stress during ascites. Researchers have attempted to alleviate the onset of ascites by increasing the antioxidant status of the broiler before exposure to ascites-promoting conditions. Broilers that received a vitamin E implant that released a total of 15 mg α-tocopherol from 0 to 3 weeks of age immediately before exposure to ascites had significantly reduced ascites-induced mortality than placebo-treated broilers (Bottje et al., Citation1995). Liver and lung concentrations of α-tocopherol in healthy vitamin-E-treated birds were increased, providing the bird with additional protection from reactive oxygen. Healthy vitamin-E-treated birds had plasma lipid peroxide values lower than placebo-treated birds in the same conditions, indicating the enhanced protection that vitamin E provides against lipid peroxidation. The vitamin E implant reduced ascites-induced mortality, probably by providing an enhanced antioxidant defence against the reactive species production that otherwise causes tissue damage and promotes ascites progression. In contrast to the results obtained with vitamin E implants, supplementing broiler diets with vitamin E did not reduce ascites-induced mortality (Bottje et al., Citation1997; Villar-Patino et al., Citation2002). Vitamin C supplemented in broiler feed at 400 mg/kg feed reduces lipid peroxidation in cardiac tissue as well but does not affect ascites-induced mortality. The effect of manipulating other important antioxidants, such as uric acid, flavenoids, or carotenoids, has not been investigated (Stinefelt, 2003).
Hatchery and Ascites
Oxygen requirement is the most critical trigger of ascites in broilers (Julian, Citation2000). High metabolic demands together with decreased availability of oxygen may lead to hypoxaemia and ascites (Wideman, Citation2001). Ascites susceptibility is particularly pronounced during the period of rapid juvenile growth when the metabolic rate is very high (Decuypere et al., Citation2000). Although the peak incidence of ascites occurs in the fifth or sixth week of the growing period, the aetiology of the disease may be initiated much earlier, even during the embryonic stage (Coleman & Coleman, Citation1991). Embryonic growth can be estimated by egg weight and oxygen consumption at certain stages of development. Rapid growth increases the oxygen requirement, cardiac output, and blood flow, and may result in increased pulmonary arterial pressure primarily by increasing the metabolic demand for oxygen (Julian, Citation2000; Wideman & Tackett, Citation2000). Chicken embryos grow rapidly over the last 7 days of incubation, resulting in a 60% increase in the oxygen consumption during the interval between the start of pulmonary breathing and hatching (Decuypere et al., Citation2000; Sahan et al., Citation2006). Therefore hypoxia, known to be involved in the occurrence of the ascites syndrome, could arise in the chick embryo during the interval between internal pipping and hatching (Dewil et al., Citation1996). In fact, Dewil et al., (Citation1996) reported hypoxic conditions in the late embryonic phase. Oxygen supplementation from 18 to 21 days of incubation could be used as an effective means of improving hatchability of broiler eggs. Oxygen supplementation during incubation could also increase the embryonic growth rate and 1-day-old chick weight (Sahan et al., Citation2006). The findings of Chineme et al. (Citation1995) indicated that the length and/or severity of prenatal hypoxia may influence postnatal characteristics related to ascites. Eggs incubated in an environment with a relatively high concentration of carbon dioxide hatched earlier than in an environment with normal amounts (Buys et al., Citation1998a; Hassanzadeh et al., Citation2002). The chickens incubated in the environment with increased concentration of carbon dioxide showed a lower incidence of ascites during the growing period because high concentrations of carbon dioxide in the incubation environment might decrease the length of time the embryo experiences hypoxia (Buys et al., Citation1998a; Hassanzadeh et al., Citation2002). Rouwet et al. (Citation2002) demonstrated that chronic hypoxia during embryonic development induces structural and functional cardiovascular abnormalities (e.g. left ventricular dysfunction) in the near-term chick embryos. These abnormalities may be responsible for the increased mortality of embryos incubated under high altitude. It is hypothesized that developmental changes induced by environmental or incubation conditions may play a role in the genotype and environment interaction in ascites susceptibility (Decuypere, Citation2002). Eggs incubated in an environment with a high concentration of carbon dioxide hatched earlier than those in an environment with normal carbon dioxide levels (Buys et al., Citation1998a; Hassanzadeh et al., Citation2002). Moreover, the chickens incubated in the environment with increased concentrations of carbon dioxide showed a lower incidence of ascites during the growing period. Different degrees of ventilation during incubation may therefore interact with genotype and egg shell characteristics (which determine gaseous exchange) to affect the total incubation time and, thereby, influence the susceptibility to ascites and related physiological responses in later postnatal life (Chineme et al., Citation1995).
pH and Ascites
Ascites is ultimately caused by an imbalance between the oxygen supply to the body tissues and the oxygen requirement of the tissues (Julian, Citation1993). Poor tissue oxygenation can be caused by an increased oxygen requirement of the tissues due to increased metabolism from rapid growth (Peacock et al., Citation1989) or in response to cold temperature; by a decreased availability of oxygen in the environment due to high altitude (hypobaric conditions) or poor ventilation; or by low oxygen content of the blood because of low haemoglobin–oxygen affinity or decreased blood oxygen capacity of haemoglobins or low oxygen exchange in the lung (Julian, Citation1993). Ascites can be reduced by decreasing the oxygen requirements of the bird (such as by reducing growth rate and avoiding cold) or increasing oxygen delivery to the tissues.
In mammals, acidosis causes vasoconstriction, while alkalosis causes vasodilation, which affects pulmonary arterial pressure and pulmonary hypertension. The blood pH also affects the affinity of haemoglobin for oxygen in the lung and release of oxygen to the tissues (the Bohr effect). A decrease in blood pH lowers the oxygen affinity of haemoglobin, which encourages release in the tissues, while increased blood pH increases oxygen affinity to increase haemoglobin saturation in the lung (Issacks et al., Citation1986). The feeding of excess chloride or sulphate has been shown to depress blood pH and bicarbonate levels in chickens (Ruiz-Lopez & Austic, Citation1993), while feeding bicarbonate would be expected to increase blood pH. It has been suggested that broiler chickens that have a high metabolic rate may be in a state of metabolic acidosis when they are on full feed (Julian, Citation1993). Several workers have shown that fast-growing birds have lower blood oxygen concentration than slow-growing birds; likewise, birds on full feed have lower blood oxygen than food-deprived birds (Fedde et al., Citation1998; Julian & Mirsalimi, Citation1992; Reeves et al., Citation1991). Feeding low chloride/high bicarbonate diets results in a decrease in pulmonary hypertension. Conversely, feeding diets with high chloride content tends to increase the incidence of ascites. Increased blood pH would increase oxygen haemoglobin affinity, which is low in fully fed broilers, probably because of metabolic acidosis. It has been demonstrated that decreased blood pH results in increased pulmonary arterial pressure in mammals and this may also be true in birds. Increased blood pH can improve the loading of oxygen by haemoglobin in the lung due to the Bohr effect. It therefore appears that supplementing broilers with bicarbonate may be beneficial in fast-growing birds with very high oxygen requirements and high production of carbon dioxide, as long as the decreased pH normally present in the muscles that facilitates oxygen unloading is not affected. Further work is needed to establish the mode of action of bicarbonate and increasing the cation/anion ratio in the diet on arterial blood oxygen saturation (Squires & Julian, Citation2001).
Environmental/Management Factors and Ascites
Altitude
The most obvious environmental factor to play a role in ascites development in broilers is high altitude. The effect of high altitude (either natural or simulated) is a decrease in the partial pressure of oxygen. When birds are exposed to low atmospheric oxygen levels (high altitude), pulmonary blood vessels constrict and pulmonary vascular resistance increases (Wideman, Citation1997). This immediate increase in pulmonary arterial pressure can, over time, cause right ventricular hypertrophy and eventually result in ascites syndrome (Wideman et al., Citation1998).
Cold temperature
The second most studied environmental cause of pulmonary hypertension and ascites is temperature. The strong correlation between cold temperature and cardiac hypertrophy/ascites has been recognized for several decades. Cold temperatures increase ascites by increasing both metabolic oxygen requirements and by increasing pulmonary hypertension (Julian et al., Citation1989; Stolz et al., Citation1992). Wideman & Tackett (Citation2000) attributed this increase in pulmonary arterial pressure to a cold-induced increase in cardiac output, as opposed to being caused by hypoxaemic pulmonary vasoconstriction. The effect of the timing of a cold stress on ascites development in broilers indicates that exposure to cold temperatures during brooding has a lasting effect on ascites incidence (Julian, Citation2000; Groves, Citation2002). The consensus appears to be that cold stress during the first 2 weeks of life affects the bird's metabolic rate for several weeks and increases their susceptibility to ascites. Groves (Citation2002) further reported that the duration of the cold stress is more critical than the minimum temperature reached. He also indicated that exposure to suboptimal temperatures lead to ascites mortalities about 2 weeks later and that, after 3 weeks of age, temperature stress becomes less critical. Interestingly, ascites was reported to develop 2 weeks after placement of young birds in a hypobaric chamber and, conversely, birds stopped developing ascites 2 weeks after removal from the simulated high altitude (Balog et al., Citation2001).
Lighting
Broilers are usually grown on a near-continuous lighting schedule so that feed consumption and growth rate can be maximized. Early studies in photoperiod manipulation reported a decreased growth rate for broilers raised with a step-down lighting programme (Classen et al., Citation1991). It was hypothesized that limiting the number of hours of light will slow growth slightly and will reduce activity that requires additional oxygen, and may actually improve feed efficiency (Julian, Citation1990a,Citationb). Subsequent studies on the effect of longer dark periods or intermittent lighting indicated that, similar to feed restriction, photoperiod manipulations can decrease the incidence of ascites syndrome (Julian, Citation1990b, Citation2000; Hassanzadeh et al., Citation2000).
Air quality and ventilation
It has been suggested that poor ventilation could cause low environmental oxygen or high toxic fumes (carbon monoxide, carbon dioxide or ammonia), which may have detrimental effects on the respiratory or cardiovascular systems of birds and promote ascites development (Wideman, Citation1998). It also has been suggested that environmental dust could affect oxygen transfer in the lung and increase the ascites incidence. The hypothetical effects of air quality and ventilation have been difficult to prove. Although birds exposed to poorly ventilated conditions have been reported to develop greater numbers of cartilaginous and osseous nodules in their lungs and birds with ascites syndrome have higher numbers of these nodules (Balog, Citation2003), the causal effect of low ventilation on ascites is still unproven. In addition, there are a number of reports of air quality and ventilation not affecting ascites development (Julian & Wilson, Citation1992; Julian, Citation1993, Citation1995, Citation2000; McGovern et al., Citation1999, Citation2000).
Conclusions
Ascites, like several other metabolic disorders, is a multifactorial syndrome, caused by interactions among environmental, physiological and genetic factors. Forced selection to achieve faster growing chickens has made the farmers enjoy the better phenotypical traits resulting from improved genetic potential; but, due to some anatomical and physiological limitations, the same improved potential could have adverse effects on bird health. Impaired oxygen supply to sustain a continuous fast growth rate causes may increase the risk for a higher incidence of ascites syndrome. Selection for breast meat yield, due to market demand, may impose more threat to the bird since chickens with a higher percentage of breast muscle have a lower capillary density (Hoving-Bolink et al., Citation2000). The impaired oxygen supply will stimulate the development of many compensatory mechanisms in cardiopulmonary systems which, in turn, brings about ascites syndrome such as hypertension, ventricular hypertrophy, erythropoietic responses, and so on.
Management practices to limit growth rate, such as feed restriction, nutrient density and diet form have been applied. These practices indirectly reduce the need for oxygen to partly compensate the physiological limitations; on the other hand, efforts have been made to normalize red blood cell structure and function, and free radicals scavenged.
In recent years special attention has also been paid to the factors inside the incubator that may influence normal epigenesis, predisposing the chicks hatched to ascites syndrome.
References
- Archer , S.L. , Johnson , G.J. , Gebhard , R.L. , Castlemen , W.L. , Levine , A.S. , Westcott , J.Y. , Voelkel , N.F. , Nelson , D.P. and Weir , E.K. 1989 . Effect of dietary fish oil on lung lipid profile and hypoxic pulmonary hypertension . Journal of Applied Physiology , 66 : 1662 – 1673 .
- Balog , J.M. 2003 . Ascites syndrome (pulmonary hypertension syndrome) in broiler chickens: are we seeing the light at the end of the tunnel? . Avian and Poultry Biology Reviews , 14 ( 3 ) : 99 – 126 .
- Balog , M.J. , Anthony , N.B. , Cooper , M.A. , Kidd , B.D. , Huff , G.R. , Huff , W.E. and Rath , N.C. 2000 . Ascites syndrome and related pathologies in feed restricted broilers raised in a hypobaric chamber . Poultry Science , 79 : 318 – 323 .
- Balog , M.J. , Anthony , N.B. , Cooper , M.A. , Kidd , B.D. , Huff , G.R. , Huff , W.E. , Rath , N.C. and Kirby , Y.K. 2001 . Effect of timing of hypobaric exposure on the incidence of ascites syndrome in broilers . Poultry Science , 80 ( suppl 1 ) : 262
- Balog , J.M. , Kidd , B.D. , Huff , G.R. , Huff , W.E. , Rath , N.C. and Anthony , N.B. 2003 . Effect of cold stress on broilers selected for resistance or susceptibility to ascites syndrome . Poultry Science , 82 : 1383 – 1387 .
- Berlin , E. , Bhathena , S.J. , Judd , J.T. , Nair , P.P. , Peters , R.C. , Bhagavan , H.N. , Ballard-barbash , R. and Taylor , P.R. 1992 . Effects of omega-3 fatty acid and vitamin E supplementation on erythrocyte membrane fluidity, tocopherols, insulin binding and lipid composition in men . Journal of Nutrition and Biochemistry , 3 : 392 – 400 .
- Bölükbasi , C. , Güzel , M. and Aktas , M.S. 2004 . The Effect of early feed restriction on ascites induced by cold temperatures and growth performance in broilers . Journal of Applied Animal Research , 26 : 89 – 92 .
- Bölükbasi , S.C. , Aktas , M.S. and Güzel , M. 2005 . The effect of feed regimen on ascites induced by cold temperatures and growth performance in male broilers . International Journal of Poultry Science , 4 ( 5 ) : 326 – 329 .
- Bottje , W.G. and Wideman , R.F. 1995 . Potential role of free radicals in the etiology of pulmonary hypertension syndrome . Poultry and Avian Biological Reviews , 6 : 211 – 231 .
- Bottje , W. , Enkvetchakul , B. , Moore , R. and McNew , R. 1955 . Effect of α-tocopherol on antioxidants, lipid peroxidation, and the incidence of pulmonary hypertension syndrome (ascites) in broilers . Poultry Science , 74 : 1356 – 1369 .
- Bottje , W , Erf , G. , Bersi , T. , Wang , S. , Barnes , D. and Beers , K. 1997 . Effect of dietary dl-α-tocopherol on tissue and α- and γ-tocopherol and pulmonary hypertension syndrome (ascites) in broilers . Poultry Science , 75 : 1507 – 1512 .
- Bowes , V.A. , Julian , R.J. and Stirtzinger , T. 1989 . Comparison of serum biochemical profiles of male broilers with female broilers and white leghorn chickens . Canadian Journal of Veterinary Research , 53 : 7 – 11 .
- Buys , N. , Buyse , J. , Hassanzadeh-Ladmakhi , M. and Decuypere , E. 1998a . Intermittent lighting reduces the incidence of ascites in broilers: An interaction with protein content of feed on performance and the endocrine system . Poultry Science , 77 : 54 – 61 .
- Buys , N. , Dewil , E. , Gonzales , E. and Decuypere , E. 1998b . Different CO2 levels during incubation interact with hatching time and ascites susceptibility in two broiler lines selected for different growth rate . Avian Pathology , 27 : 605 – 612 .
- Buyse , J. , Janssens , G.P.J. and Decuypere , E. 2001 . The effects of dietary l-carnitine supplementation on the performance, organ weights and circulating hormone and metabolite concentrations of broiler chickens reared under a normal or low temperature schedule . British Poultry Science , 42 : 230 – 241 .
- Camacho , M.A. , Suarez , M.E. , Herrera , J.G. , Cuca , J.M. and Garcia-Bojalil , C.M. 2004 . Effect of age of feed restriction and microelement supplementation to control ascites on production and carcass characteristics of broilers . Poultry Science , 83 : 526 – 532 .
- Camacho-Fernandez , D. , Lopez , C. , Avila , E. and Arce , J. 2002 . Evaluation of different dietary treatments to reduce the ascites syndrome and their effect on corporal characteristics in broiler chickens . Journal of Applied Poultry Research , 11 : 164 – 174 .
- Cawthorn , D. , Beers , K. and Bottje , W.G. 2001 . Electron transport chain defect and inefficient respiration may underlie pulmonary hypertension syndrome (ascites)-associated mitochondrial dysfunction in broilers . Poultry Science , 80 : 474 – 484 .
- Chineme , C.N. , Buyse , J. , Hassanzadeh-Ladmakhi , M. , Albers , G.A.A. and Decuypere , E. 1995 . Interaction of genotype, egg-shell conductance and dietary T3 supplementation in the development of heart-failure syndrome and ascites in broiler-chicken . Archiv fur Geflügelkunde , 59 : 129 – 134 .
- Classen , H.L. , Riddell , C. and Robinson , F. E. 1991 . Effects of increasing photoperiod length on performance and health of broiler chickens . British Poultry Science , 32 : 21 – 29 .
- Coello , C.L. , Arce , M.J. & Avila , G.E. 2000 . Management techniques to reduce incidence of ascites and SDS . Proceeding of the XXI World's Poultry Science Association Congress, WPSA, Montreal, Quebec, Canada .
- Coleman , M.A. and Coleman , G. E. 1991 . Ascites control through proper hatchery management . Misset World Poultry , 7 : 33 – 35 .
- Crespo , R. and Shivaprasad , H.L. 2003 . Diseases of Poultry , 11th edn , 1072 – 1075 . Ames : Iowa State Press .
- Currie , R.J.W. 1999 . Ascites in poultry: recent investigations . Avian Pathology , 28 : 313 – 326 .
- Decuypere , E. 2002 . Ascites as a multifactorial syndrome of broiler chickens: considerations from a developmental and selection view-point . ‘Proceedings of the 2nd Symposium of the World Poultry Science Association of the Iran Branch (pp. 119 – 136 ). Tehran, Iran .
- Decuypere , E. , Buyse , J. and Buys , N. 2000 . Ascites in broiler chickens: exogenous and endogenous structural and functional causal factors . Worlds Poultry Science Journal , 56 : 367 – 376 .
- Decuypere , E. , Hassanzadeh , M. and Buys , N. 2005 . Further insights into the susceptibility of broilers to ascites . Veterinary Journal , 169 : 319 – 320 .
- de Greef , K.H. , Janss , L.L.G. , Vereiken , A.L.J. , Pit , R. and Gerritsen , C.L.M. 2001 . Disease-induced variability of genetic correlations: ascites in broilers as a case study . Journal of Animal Science , 79 : 1723 – 1733 .
- Detilleux , J. and Leroy , P.L. 2000 . Application of a mixed normal mixture model for the estimation of mastitis-related parameters . Journal of Dairy Science. , 83 : 2341 – 2349 .
- Dewil , E. , Buys , N. , Albers , G.A.A. and Decuypere , E. 1996 . Different characteristics in chick embryos of two broiler lines differing in susceptibility to ascites . British Poultry Science , 37 : 1003 – 1013 .
- Enkvetchakul , B. , Bottje , W. , Anthony , N. , Moore , R. and Huff , W. 1993 . Compromised antioxidant status associated with ascites in broilers . Poultry Science , 72 : 2272 – 2280 .
- Fedde , M.R. 1984 . Respiration in birds . In M. Swenson Dukes Physiology of Domestic Animals 10th edn . (pp. 255 – 261 ), Cornell University Press San Diego , CA, USA .
- Fedde , M.R. , Weigle , G. E. and Wideman , R. F. 1998 . Influence of feed deprivation on ventilation and gas exchange in broilers: relationship to pulmonary hypertension syndrome . Poultry Science , 77 : 1704 – 1710 .
- Groves , P.J. 2002 . Environmental determinants of broiler ascites syndrome . Proceedings of the Australian Poultry Science Symposium Vol. 14 (pp. 83 – 88 ). Sydney, Australia .
- Hall , S.A. and Machicao , N. 1968 . Myocarditis in broiler chickens reared at high altitude . Avian Diseases , 12 : 75 – 84 .
- Hassanzadeh , M. , Buys , N. , Dewil , E. , Rahimi , G. and Decuypere , E. 1997 . The prophylactic effect of vitamin C supplementation on broiler ascites incidence and plasma thyroid hormone concentration . Avian Pathology , 26 : 33 – 44 .
- Hassanzadeh , M. , Bozorgmerifard , M.H. , Akbari , A.R. , Buyse , J. and Decuypere , E. 2000 . Effect of intermittent lighting schedules during the natural scotoperiod on T3-induced ascites in broiler chickens . Avian Pathology , 29 : 433 – 439 .
- Hassanzadeh , M. , Buyse , J. and Decuypere , E. 2002 . Further evidence for the involvement of cardiac α-adrenergic receptors in right ventricle hypertrophy and ascites in broiler chickens . Avian Pathology , 31 : 177 – 181 .
- Hoving-Bolink , A.H. , Kranen , R.W. , Klont , R.E. , Gerritsen , C.L.M. and de Greef , K.H. 2000 . Fibre area and capillary supply in broiler breast muscle in relation to productivity and ascites . Meat Science , 56 : 397 – 402 .
- Hulan , H.W. , Ackman , R.G. , Ratnayake , W.M.N. and Proudfoot , F.G. 1989 . Omega-3 fatty acid levels and general performance of commercial broilers fed practical levels of red fish meal . Poultry Science , 68 : 153 – 162 .
- Issacks , R. , Goldman , P. and Kim , C. 1986 . Studies on avian erythrocyte metabolism XIV. Effect of CO2 and pH on P50 in the chicken . American Journal of Physiology, R260 , 250 : R266
- Julian , R.J. 1988 . Pulmonary hypertension as a cause of right ventricular failure and ascites in broilers . Zootechnica International , 11 : 58 – 62 .
- Julian , R.J. 1989 . Lung volume of meat-type chickens . Avian Disease , 33 : 174 – 176 .
- Julian , R.J. 1998 . Rapid growth problems: ascites and skeletal deformities in broilers . Poultry Science , 77 : 1773 – 1780 .
- Julian , R.J. 1990a . “ Cardiovascular diseases ” . In Poultry Diseases , 3rd edn , Edited by: Jordan , F.T.W. 330 – 353 . London : Bailliere Tindall .
- Julian , R.J. 1990b . Pulmonary hypertension: a cause of right heart failure, ascites in meat-type chickens . Feedstuffs , 78 : 19 – 22 .
- Julian , R.J. 1993 . Ascites in poultry . Avian Pathology , 22 : 419 – 454 .
- Julian , R.J. 1995 . Suggestions for reducing ascites in meat-type chickens . Proceedings of the 44th Western Poultry Disease Conference (pp. 19 – 20 ), Sacramento, CA, USA .
- Julian , R.J. 2000 . Physiological management and environmental triggers of the ascites syndrome: a review . Avian Pathology , 29 : 519 – 527 .
- Julian , R.J. 2005 . Production and growth related disorders and other metabolic diseases of poultry—A review . The Veterinary Journal , 169 : 350 – 369 .
- Julian , R.J. and Mirsalimi , S.M. 1992 . Blood oxygen concentration of fast growing and slow growing broiler chickens, and chickens with ascites from right ventricular failure . Avian Diseases , 36 : 730 – 732 .
- Julian , R.J. and Wilson , B. 1992 . Pen oxygen concentration and pulmonary hypertension-induced right ventricular failure and ascites in meat-type chickens at low altitude . Avian Disease , 36 : 733 – 735 .
- Lorenzoni , A.G. and Ruiz-Feria , C.A. 2006 . Effects of vitamin E and l-arginine on cardiopulmonary function and acites parameters in broiler chickens reared under subnormal temperatures . Poultry Science , 85 : 2241 – 2250 .
- Lubritz , D. , Smith , J. and McPherson , B. 1995 . Heritability of ascites and the ratio of right to total ventricle weight in broiler breeder male lines . Poultry Science , 74 : 1237 – 1241 .
- Luger , D. , Shinder , D. , Wolfenson , D. and Yahav , S. 2003 . Erythropoiesis regulation during the development of ascites syndrome in broiler chickens: A possible role of corticosterone on egg production . Journal of Animal Science , 81 : 784 – 790 .
- McGovern , R.H. , Feddes , J.J.R , Robinson , F.E. and Hanson , J.A. 1999 . Growth performance, carcass characteristics, and the incidence of ascites in broilers in response to feed restriction and litter oiling . Poultry Science , 78 : 522 – 528 .
- McGovern , R.H. , Feddes , J.J.R. , Robinson , F.E. and Hanson , J.A. 2000 . Growth, carcass characteristics, and incidence of ascites in broilers exposed to environmental fluctuations and oiled litter . Poultry Science , 79 : 324 – 330 .
- McLachlan , G.J. & Krishnan , T. 1997 . The EM algorithm and extensions . Wiley , New York , NY, USA .
- Maxwell , M.H. and Robertson , G.W. 1997 . World broiler ascites survey 1996 . Poultry International , 36 : 16 – 30 .
- Maxwell , M.H. , Robertson , G.W. , Bautista Ortega , J. and Hocking , P.M. 1998 . A preliminary estimate of the heritability of plasma troponin T in broiler chickens . British Poultry Science , 39 : 16 – 19 .
- Mirsalimi , S.M. and Julian , R.J. 1991 . Reduced erythrocyte deformability as a possible contributing factor to pulmonary hypertension and ascites in broiler chickens . Avian Diseases , 35 : 374 – 379 .
- Mirsalimi , S.M. , Julian , R.J. and Squires , E.J. 1993 . Effect of hypobaric hypoxia on slow and fast growing chickens fed diets with high and low protein levels . Avian Diseases , 37 : 660 – 667 .
- Moghadam , H.K. , McMillan , I. , Chambers , J.R. and Julian , R.J. 2001 . Estimation of genetic parameters for ascites syndrome in broiler chickens . Poultry Science , 80 : 844 – 848 .
- Olkowski , A.A. , Krover , D. , Rathgeber , B. and Classen , H. L. 1999 . Cardiac index, oxygen delivery, and tissue oxygen extraction in slow and fast growing chickens, and in chickens with heart failure and ascites: a comparative study . Avian Pathology , 28 : 137 – 146 .
- Olkowski , A.A. , Wajnarowicz , C. , Rathgeber , B.M. , Abbott , J.A. and Classen , H.L. 2003 . Lesions of pericardium and their significance in the aetiology of heart failure in broiler chickens . Research in Veterinary Science , 74 : 203 – 211 .
- Olkowski , A.A. , Nain , S. , Wajnarowicz , C. , Laarveld , B. , Alcorn , J. and Ling , B.B. 2007 . Comparative study of myocardial high energy phosphate substrate content in slow and fast growing chicken and in chickens with heart failure and ascites . Comparative Biochemistry and Physiology, Part A: Molecular & Integrative Physiology , (148) 1 : 230 – 238 .
- Owen , R.L. , Wideman , R.F. , Hattel , A.L. and Cowen , B.S. 1990 . Use of a hypobaric chamber as a model system for investigating ascites in broilers . Avian Diseases , 34 : 754 – 758 .
- Ozkan , S. , Plavnik , I. and Yahav , S. 2006 . Effects of early feed restriction on performance and ascites development in broiler chickens subsequently raised at low ambient temperature . Journal of Applied Poultry Research , 15 : 9 – 19 .
- Packer , L. , Valenza , M. , Serbinosa , E. , Starke-Reed , P. , Frost , K. and Kagan , V. 1991 . Free radical scavenging is involved in the protective effects of L-propionyl-Carnitine against ischemia reperfusion injury of the heart . Archive Biochemistry Biophysics , 288 : 533 – 537 .
- Pakdel , A. , Van-Arendonk , J.A.M. , Vereijken , A.L.J. and Bovenhuis , H. 2002 . Direct and maternal genetic effects for ascites-related traits in broilers . Poultry Science , 81 : 1273 – 1279 .
- Pakdel , A. , Rabie , T. , Veenendaal , T. , Crooijmans , R.P.M.A. , Groenen , M.A.M. , Vereijken , A.L.J. , Van Arendonk , J.A.M. & Bovenhuis , H. 2004 . Genetic analysis of ascites-related traits in broilers . Doctoral thesis, Animal Breeding and Genetics Group, Department of Animal Sciences, Wageningen University, The Netherlands , p. 144 .
- Pakdel , A. , Bijma , P. , Ducro , B.J. and Bovenhuis , H. 2005 . Selection strategies for body weight and reduced ascites susceptibility in broilers . Poultry Science , 84 : 528 – 535 .
- Peacock , A.J. , Pickett , I.C. , Morris , K. and Reeves , J.T. 1989 . The relationship between rapid growth and pulmonary hemodynamics in the fast-growing broiler chicken . American Review of Respiratory Diseases , 139 : 1524 – 1530 .
- Piiper , J. and Scheid , P. 1975 . Gas transport efficacy of gills, lungs and skin: theory and experimental data . Respiratory Physiology , 23 : 209 – 221 .
- Powell , F.L. 2000 . Respiration . In: G.C. Whittow Sturkie's Avian Physiology 5th edn (pp. 233 – 264 ) Academic Press San Diego , CA, USA .
- Reeves , J.T. , Ballam , G. , Hofmeister , S. , Picket , C. , Morris , K. and Peacock , A. 1991 . Improved arterial oxygenation with feed restriction in rapidly growing broiler chickens . Comparative Biochemistry and Physiology, Part A, Physiology , 99 ( 3 ) : 481 – 485 .
- Riddell , C. 1991 . Developmental, metabolic, and miscellaneous disorders . In B.W. Calnek , H.J. Barnes , C.W. Beard , W.M. Reid & H.W. Yoder Jr Diseases of Poultry 9th edn (pp. 839 – 841 ). Ames : Iowa State University .
- Rouwet , E.V. , Tintu , A.N. , Schellings , M.V.M. , Van Bilsen , M. , Lutgens , E. , Hofstra , L. , Slaaf , D.W. , Ramsay , G. and Le Noble , F.A.C. 2002 . Hypoxia induces aortic hypertrophic growth, left ventricular dysfunction and sympathetic hyper innervation of peripheral arteries in the chick embryo . Circulation , 105 : 2791 – 2796 .
- Ruiz-Lopez , B. and Austic , R.E. 1993 . The effect of selected minerals on the acid–base balance of growing chicks . Poultry Science , 72 : 1054 – 1062 .
- Sahan , U. , Ipek , A. , Altan , O. and Yilmaz-Dikmen , B. 2006 . Effects of oxygen supplementation during the last stage of incubation on broiler performance, ascites susceptibility and some physiological traits . Animal Research , 55 : 145 – 152 .
- Scheele , C.W. 1996 . Ascites in chickens , Thesis, Landbouwuniversiteit, Wageningen, The Netherlands .
- Scheele , C.W. , Van Der Klis , J.D. , Kwakernaak , C. , Buys , N. and Decuypere , E. 2003 . Hematological characteristics predicting susceptibility for ascites. 2. High haematocrit values in juvenile chickens . British Poultry Science , 44 : 484 – 489 .
- Shlosberg , A. , Bellaiche , M. , Zeitlin , G. , Yaacobi , M. and Cahaner , A. 1996 . Hematocrit values and mortality from ascites in cold stressed broilers from parents selected by hematocrit . Poultry Science , 75 : 1 – 5 .
- Shlosberg , A. , Belaiche , M. , Berman , E. , Perk , S. , Deeb , N. , Neumark , E. and Cahaner , A. 1998 . Relationship between broiler chicken hematocrit-selected parents and their progeny with regard to hematocrit, mortality from ascites and body weight . Research in Veterinary Science , 64 : 105 – 109 .
- Smith , F.M. , West , N.H. & Jones , D.R. 2000 . The cardiovascular system . In G.C. Whittow Sturkie's Avian Physiology 5th edn (pp. 141 – 231 ). Academic Press .
- Squires , E.J. and Julian , R.J. 2001 . The effect of dietary chloride and bicarbonate on blood pH, haematological variables, pulmonary hypertension and ascites in broiler chickens . British Poultry Science , 42 : 207 – 212 .
- Stinefelt , B.M. 2003 . Uric acid as an antioxidant and the effect of changes in plasma uric acid concentrations on broiler susceptibility to ascites , MSc Thesis, Davis College of Agriculture, Forestry, and Consumer Sciences At West Virginia University, WV, USA .
- Stolz , J.L. , Rosenbaum , L.M. , Jeong , D. and Odom , T.W. 1992 . Ascites syndrome, mortality and cardiological responses of broiler chickens subjected to cold exposure . Poultry Science , 71 ( Suppl 1 ) : 4
- Villar-Patino , G. , Diaz-Cruz , A. , Avila-Gonzalez , E. , Guinzberg , R. , Pablos , J.L. and Pina , E. 2002 . Effects of dietary supplementation with vitamin C or vitamin E on cardiac lipid peroxidation and growth performance in broilers at risk of developing ascites syndrome . American Journal of Veterinary Research. , 63 ( 5 ) : 673 – 676 .
- Walton , J-P. , Bond , J.M. , Julian , R.J. and Squires , E.J. 1999 . Effect of dietary flax oil and hypobaric hypoxia on pulmonary hypertension and haematological variables in broiler chickens . British Poultry Science , 40 : 385 – 391 .
- Wideman , R.F. 1997 . Understanding pulmonary hypertension syndrome (ascites) . Hubbard Farms Technical Report (pp. 1 – 6 ), Walpole, NH, USA .
- Wideman , R.F. 1998 . Causes and control of ascites in broilers . National Meeting on Poultry Proceeding , 33 : 56 – 85 .
- Wideman , R.F. 2000 . Cardio-pulmonary hemodynamics and ascites in broiler chickens . Avian and Poultry Biology Review , 11 : 21 – 43 .
- Wideman , R.F. 2001 . Pathophysiology of heart/lung disorders: pulmonary hypertension syndrome in broiler chickens . World Poultry Science Journal , 57 : 289 – 307 .
- Wideman , R.F. and Tackett , C. 2000 . Cardiopulmonary function in broilers reared at warm or cold temperatures: effect of acute inhalation of 100% oxygen . Poultry Science , 79 : 257 – 264 .
- Wideman , R.F. , Kirby , Y.K. , Owen , R.L. and French , H. 1997 . Chronic unilateral occlusion of an extrapulmonary primary bronchus induces pulmonary hypertension syndrome (ascites) in male and female broilers . Poultry Science , 76 : 400 – 404 .
- Wideman , R.F. , Wing , T. , Kirby , Y.K. , Forman , M.F. , Marson , N. , Tackett , C.D. and Ruiz-Feria , C.A. 1998 . Evaluation of minimally invasive indices for predicting ascites susceptibility in three successive hatches of broilers exposed to cool temperatures . Poultry Science , 77 : 1565 – 1573 .
- Wideman , R.F. , Hooge , D.M. and Cummings , K.R. 2003 . Dietary sodium bicarbonate, cool temperatures, and feed withdrawal: impact on arterial and venous blood-gas values in broilers . Poultry Science , 82 : 560 – 570 .
- Wise , D.R. and Evans , E.T.R. 1975 . Turkey syndrome 65, oedema syndrome and mycoplasma meleagridis . Research in Veterinary Science , 18 : 190 – 192 .
- Zerehdaran , S. , Van Grevehof , E.M. , Van Der Waaij , E.H. and Bovenhuis , H. 2006 . A Bivariate mixture model analysis of body weight and ascites traits in broilers . Poultry Science , 85 : 32 – 38 .