Abstract
A survey of infectious bronchitis virus (IBV) genotypes in poultry flocks in selected countries in Western Europe was carried out between March 2002 and December 2006. Identification of IBV was by reverse transcriptase-polymerase chain reaction of RNA extracted from oropharyngeal swabs taken from poultry flocks exhibiting signs of clinical disease thought to be attributable to IBV. Part of the hypervariable S1 gene of IBV was sequenced to differentiate between the various genotypes. During the survey, 4103 samples were processed, of which 2419 (59%) were positive for IBV. The predominant IBV genotypes detected were 793B and Massachusetts. The third and fourth most common genotypes were two new economically important field types: Italy02, and a virus similar to genotypes originally detected in China called QX. Analysis of the partial S1 sequences of the genotypes detected suggested that approximately 50% of all 793B, Massachusetts types and D274 IBVs were identical to the homologous commercially available live vaccines. Since 2004 the prevalence of Italy02 (present in all countries from which samples were received) has been declining in all countries except Spain, where it appeared to be the predominant genotype. Since 2004 an IBV genotype has been detected in Holland, Germany, Belgium and France similar to QX and the incidence has increased. QX was not detected in the United Kingdom or Spain. When detections thought to be attributable to vaccines were removed, the dominant genotype in France and Europe overall was 793B; in Germany, Holland and Belgium, it was QX-like IBV; and in the United Kingdom and Spain the dominant genotype was Italy02. The present study is the first to identify the prevalence of both Italy02 and QX field-type variants in poultry flocks in Western Europe. Several novel genotypes have also been detected.
Une étude RT-PCR des génotypes des virus de la bronchite infectieuse en Europe de l'Ouest de 2002 à 2006
Une étude des génotypes des virus de la bronchite infectieuse (IBV) chez les troupeaux de volailles dans certains pays de l'Europe de l'Ouest a été réalisée entre mars 2002 et décembre 2006. L'identification des IBV a été réalisée par les réactions de transcription inverse et de polymérisation en chaîne de l'ARN extrait d'écouvillons oropharyngiens, prélevés dans des troupeaux de volailles présentant des signes de maladie pouvant être attribués à l'IBV. La partie hypervariable du gène S1 de l'IBV a été séquencée pour différencier les divers génotypes. Durant l'étude, 4103 échantillons ont été analysés et 2071 (59%) se sont révélés positifs pour l'IBV. Les génotypes prédominants détectés ont été le 793B et le Massachusetts. Les troisième et quatrième génotypes les plus communs ont été deux nouveaux types importants économiquement sur le terrain, l'Italy02 et un virus similaire aux génotypes originellement détectés en Chine appelés QX. L'analyse des séquences partielles de S1 des génotypes détectés a suggéré qu'approximativement 50% de tous les IBVs, 793B, types Mass et D274 étaient été identiques aux vaccins vivants homologues disponibles dans le commerce. Depuis 2004, la prévalence de la souche Italy02 (présente dans tous les pays à partir desquels des échantillons ont été prélevés) a été en déclin dans tous les pays excepté en Espagne, où elle est apparue être le génotype prédominant. Depuis 2004 un génotype d'IBV a été détecté en Hollande, Allemagne Belgique et en France similaire au QX et l'incidence a augmenté. Le génotype QX n'a pas été détecté au Royaume-uni ni en Espagne. Quand les détections, susceptibles d’être attribuées aux vaccins, étaient supprimées, le génotype dominant en France et dans toute l'Europe a été le 793B; en Allemagne, Hollande et Belgique c'était des IBV ressemblant au QX, et au Royaume-uni et en Espagne c'était l'Italy02. Cette étude est la première qui a identifié la prévalence des deux types variants du terrain l'Italy02 et le QX dans les troupeaux de volailles en Europe de l'Ouest. Plusieurs nouveaux génotypes ont également été détectés.
Eine RT-PCR-Untersuchung zur Verbreitung von Genotypen des Virus der infektiösen Bronchitis in Westeuropa in den Jahren 2002–2006
Zwischen März 2002 und Dezember 2006 wurde in ausgewählten Ländern Westeuropas eine Studie zur Verbreitung von Genotypen des infektiöse Bronchitis-Virus (IBV) in Hühnerherden durchgeführt. Der Nachweis von IBV erfolgte mittels Reverse Transkriptase-Polymerasekettenreaktion (RT-PCR) der RNS, die aus oropharyngealen Abstrichen, die in Hühnerherden mit IBV-verdächtigen klinischen Symptomen entnommen worden waren, extrahiert wurde. Zur Differenzierung zwischen den verschiedenen Genotypen wurde ein Teil des hypervariablen S1-Gens sequenziert. Während der Studie wurden 4103 Proben getestet, von denen 2071 (59 %) IBV-positiv waren. Die vorherrschenden Genotypen waren 793B und Massachusetts. An dritter und vierter Stelle standen zwei neue ökonomisch bedeutsame Feldtypen, Italy02 und ein Virus, das dem ursprünglich in China entdeckten QX-Genotyp ähnelte. Die Analyse der S1-Teilsequenzen der nachgewiesenen Genotypen ließ darauf schließen, dass ungefähr 50 % aller 793B-, Masstypen- und D 274-IBV-Stämme mit den homologen kommerziell erhältlichen Lebendvakzinen identisch waren. Seit 2004 nimmt das Vorkommen des in allen untersuchten Ländern präsenten Italy02- Genotyps außer in Spanien, wo es der verherrschende Genotyp zu sein scheint, kontinuierlich ab. Seit 2004 wird ein QX- ähnlicher Genotyp mit zunehmender Häufigkeit in Holland, Deutschland, Belgien und Frankreich nachgewiesen, während er in Großbritannien und Spanien bislang nicht festgestellt worden ist. Nach Ausschluss der den Vakzinestämmen zurechenbaren Isolate, erwies sich 793B als der dominante Genotyp in Frankreich und Europa insgesamt; während es in Deutschland, Holland und Belgien das QX-ähnliche IBV und in Großbritannien und Spanien der Italy02-Genotyp war. Diese Studie beschreibt erstmals das Vorkommen von sowohl Italy02- als auch QX-Feldtypvarianten in Hühnerherden in Westeuropa. Außerdem wurden weitere neue Genotypen nachgewiesen.
Estudio mediante RT-PCR de los genotipos víricos del virus de la bronquitis infecciosa aviar de Europa Occidental entre 2002 y 2006
Se llevó a cabo un estudio de los genotipos del virus de la bronquitis infecciosa (IBV) presentes en explotaciones avícolas de algunos países de Europa Occidental entre Marzo del 2002 y Diciembre del 2006. La identificación de IBV se realizó mediante transcripción reversa y reacción en cadena de la polimerasa del RNA aislado de hisopos orofaríngeos obtenidos de lotes de aves que mostraban signos clínicos compatibles con una infección por IBV. Se secuenció parcialmente el gen S1 de IBV para diferenciar entre diferentes genotipos. Durante el estudio se procesaron 4103 muestras de las cuales 2071 (59%) fueron positivas para IBV. Ls genotipos predominantes de IBV detectados fueron el 793B y el Massachussets. El tercer y cuarto genotipos más comunes fueron dos genotipos de campo nuevos y económicamente importantes, Italy02 y un virus similar a genotipos detectados originalmente en China denominados QX. El análisis parcial de las secuencias del gen S1 de los genotipos detectados sugirió que aproximadamente el 50% de todas las cepas 793B, Mass y D274 detectadas eran idénticas a las cepas vacunales vivas homólogas disponibles comercialmente. Desde el 2004 la prevalencia de Italy 02 (presente en todos los países de los cuales se recibieron muestras) ha ido disminuyendo en todos los países excepto España, donde parece ser el genotipo predominante. Desde el 2004 se ha detectado un genotipo similar a la cepa QX en Holanda, Alemania, Bélgica y Francia, la incidencia del cual ha aumentado. El genotipo QX no se ha detectado en UK ni en España. Al eliminar del estudio las cepas detectadas de posible origen vacunal, el genotipo dominante en Francia y en toda Europa fue el 793B; en Alemania, Holanda y Bélgica el genotipo QX; y en UK España el Italy02. Este es el primer estudio que analiza la prevalencia de los dos genotipos variantes Italy02 y QX en explotaciones avícolas de Europa Occidental. También se han detectado otros genotipos nuevos.
Introduction
Infectious bronchitis virus (IBV) is a coronavirus belonging to Group 3 of the Coronaviridae (Cavanagh, Citation2005), causing respiratory disease in chickens of all ages and loss of production and egg quality in mature hens. Some strains cause nephritis in young birds and infectious bronchitis is occasionally reported to be associated with enteritis. It is an economically important disease for the poultry industry, and vaccination strategies are essential. It is well established that the main problem in the control of infectious bronchitis is the ability of the virus to generate antigenic variants, due to mutation or sometimes recombination of the S1 spike gene (Gelb et al., Citation2005). The S1 spike protein is responsible for cell attachment and for a large component of immunity and is important in virus neutralization, which has been used traditionally to determine serotyping of IBVs (Cavanagh et al., Citation1997). Small changes in the amino acid sequences of the spike protein can result in the generation of new antigenic types, which may be quite different from existing vaccine types (Adzhar et al., Citation1997) and may require a homologous vaccine.
There have been reports of studies in Belgium (Meulemans et al., Citation2001), the United Kingdom (Cavanagh et al., Citation1999), and Italy and Poland (Capua et al., Citation1999) on the epidemiology, circulation and spread of various specific IBV genotypes. However, no systematic survey of the genotypes of IBV in Western Europe by reverse transcriptase-polymerase chain reaction (RT-PCR) using primers thought to detect most IBV variants has previously been reported. In view of the regular but unpredictable emergence of new IBV genotypes, constant surveillance is essential to monitor the prevalence of strains and the emergence of new and potentially important viruses that could necessitate adjustments to existing vaccination strategies.
Between 2002 and 2006 we undertook an RT-PCR-based survey to detect IBV in commercial poultry flocks in Western Europe with disease problems that could be associated with this virus. The main aim of this study was to detect the major IBV variants circulating in poultry flocks in Western Europe and to monitor the possible emergence of any new IBV genotypes. This could provide a guide to the optimal use of existing live vaccines, and could alert the industry to the need for development of new vaccines or vaccine strategies.
This report describes the outcome of the study from 2002 to the end of 2006, and includes the emergence of two economically important IBV field genotypes that have proved to be widespread in Western Europe.
Materials and Methods
Reverse transcriptase-polymerase chain reaction survey
Source of samples. Sets of 10 dry oropharyngeal swabs were submitted by veterinarians or farm managers from selected farms in the United Kingdom, France, the Netherlands, Germany, Belgium and Spain. They were from chicken flocks experiencing problems such as respiratory disease, reduced egg quality or production, nephritis and enteric disease, thought possibly to be attributable to IBV. Written information concerning the flock(s) in question was requested with each submission. Details asked for included type of flock, age, disease status at sampling, vaccination history, particularly relating to IBV vaccines, and any other information of relevance. Samples were submitted from a range of types of flocks, including broilers, broiler breeders, layers and, occasionally, rare breeds. Sometimes samples were received from countries outside the European Union, and these had been previously sterilized by microwave to render any virus or possible contaminants in them non-infectious (Elhafi et al., Citation2004).
Reverse transcriptase-polymerase chain method
On receipt, each set of 10 swabs was pooled for examination. RNA was extracted using the guanidinium isothiocyanate phenol–chloroform method (Chomczynski & Sacchi, Citation1987; Li et al., Citation1993) and a RT-PCR was carried out according to the method of Cavanagh et al. (Citation1999) with a few modifications (Jones et al., Citation2005a). The IBV oligonucleotide primers used were common for most of the known strains of IBV virus, spanning a region of the S1 gene, have been described previously (Jones et al., Citation2005a) and are presented in . These had been previously validated using reference IBV genotypes. The region of the gene within the universal primers is variable for each individual genotype of IBV. For the RT stage, SX2– was used; for the first PCR amplification (PCR1), SX2– and SX1+ were used; and for the second nested PCR (PCR2), SX3+ and SX4– were used—generating a copy DNA of 393 base pairs. The amplified DNA products from positive IBV samples were then treated with 1 u exonuclease 1 and 0.66 u shrimp alkaline phosphatase (USB) at 37°C for 30 min followed by 80°C for 10 min to remove any extraneous material in preparation for sequencing. The treated DNA samples together with either or both of the negative or positive sense primers were sent to be sequenced by the Advanced Biotechnology Centre, Division of Biomedical Sciences, Imperial College London. Genotype identity was by comparison with those on the NCBI Genbank nucleotide database.
Table 1. Oligonucleotide primers used for the universal IBV RT-PCR.
The genotype D1466 has been shown to differ significantly from others in the nucleotide sequence of the S1 spike gene (Kusters et al., Citation1989) and for this reason is not detected when using the universal primers described above. Since 2005 we have been performing a separate RT-PCR for D1466 using the type-specific primer D2–, D1+, D4– and D3+ as described by Cavanagh et al. (Citation1999).
Virus isolation and full S1 gene sequencing
In order to perform full-length IBV S1 gene sequencing of certain IBV field strains, viable virus was required to obtain good quality RNA. We obtained samples of the trachea, lung, duodenum, kidney and caecal tonsils from chickens in poultry flocks from which the QX type and Italy02 IBV variants had already been detected by us. Each tissue was homogenized in sterile nutrient broth (1:10, w/v), frozen, thawed and centrifuged at 1500 × g for 15 min to remove cell debris. The supernatant was used to inoculate 10-day-old fertile specific pathogen free eggs via the allantoic route. Each sample was passaged twice and the presence of virus was confirmed by typical IBV embryo effects such as dwarfing, curling or embryo death. RNA was extracted from the harvested allantoic fluid using a Roche® High Pure RNA Isolation Kit and the presence of IBV was confirmed by RT-PCR as described above. Full S1 spike sequencing was carried out according to the method of Adzhar et al. (Citation1996). Alignment of the sequences was performed utilizing the computer programs Clustal W, Pileup and Pretty available from Seqweb. The sequences were submitted to the NCBI Genbank nucleotide database.
Accession numbers
The complete and partial S1 gene nucleotide sequences of the various IBV genotypes detected in this survey have been deposited with the Genbank. The accession numbers and details of country, date and tissue of origin are presented in .
Table 2. NCBI Genbank accession numbers for the S1 gene nucleotide sequences of the Italy02, QX-like and novel IBVs detected in this survey.
Results
Overview of IBV genotypes detected
In the period from March 2002 to December 2006, 4103 samples were examined; of these, 2419 (59%) were found to be positive for IBV (). The distribution of the various IBV genotypes, expressed as a percentage of the total IBV detected, is shown in . The most common type was the major variant 793B (otherwise called 4/91 or CR88), followed by the Massachusetts type viruses including different vaccine forms such as M41, H120 and IBMM. Both of these genotypes are extensively used in live vaccines The third most frequently detected genotype was Italy02, described in 2004 (Worthington et al., Citation2004), in 2005 (Jones et al., Citation2005a, Citationb) and in 2006 (Dolz et al., Citation2006). It was detected in all countries covered by this survey. It was originally isolated in Italy (Bochkov et al., Citation2007) and it has also been reported to be present in Russia (Bochkov et al., Citation2006).
Figure 1. Percentage distribution of different IBV genotypes detected by RT-PCR in Western Europe between 2002 and 2006. *Results for D1466 are from 2005 only.
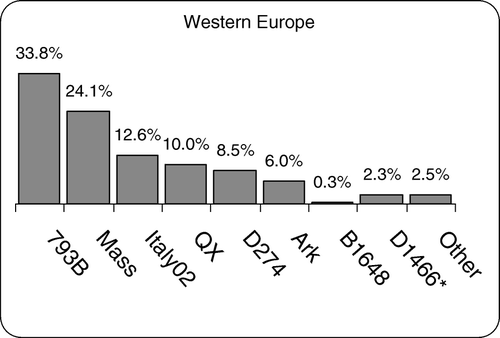
Table 3. Numbers of IBV genotypes detected in the countries of Western Europe by RT-PCR between 2002 and 2006.
The fourth most common virus was a field strain similar to several Chinese IBVs, many of which were nephropathogenic and originally isolated from the kidney, but three were isolated from the proventriculus of birds with proventriculitis (). When this virus was first detected in Europe in this survey in 2004, the closest nucleotide match on the NCBI GenBank database was with a proventricular isolate from the Qindago province in China called QX, and the name QX type IBV was adopted. However, subsequent submissions to the database have shown closer matches and this is discussed later. Although this survey was started in 2002 we did not detect this virus in Western Europe until the beginning of 2004, and by 2006 it constituted 10% of the total IBVs. It was first detected in samples from Holland in January, Germany in May, France in September and Belgium in December 2004. Up to December 2006 we had not detected it in the United Kingdom or Spain. The European QX-type IBV has been detected in flocks of broilers, broiler breeders and commercial layers between the ages of 18 days and 52 weeks. Of these flocks, 86% had respiratory signs, 22% had wet litter or enteric problems, 14% had increased mortality, 2% had swollen kidneys and 2% had arthritis. In 60% of the layers there was a reduction of egg production and quality.
Table 4. IBV genotypes closely related to the European QX-type virus.
The next most frequently identified viruses were D274 and Arkansas, both of which are used in Europe as live vaccines. The Arkansas genotype is not indigenous to Europe and was only detected in flocks that had received the commercial IBMM + Ark vaccine combination. The information regarding the use of vaccines other than Arkansas in the flocks tested was often incomplete.
B1648, a virus associated with nephritis (Meulemans et al., Citation1987), was detected infrequently and only in France and Germany.
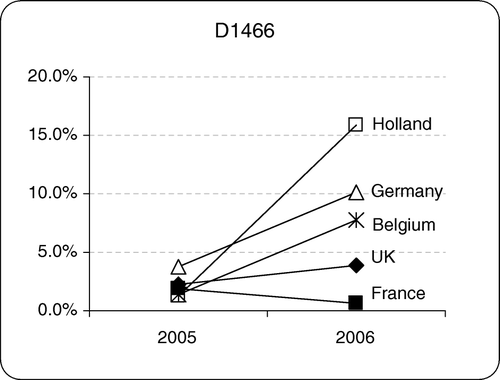
D1466, which was only tested for since the beginning of 2005, was found in all countries except Spain (). The majority of the findings were from flocks of layers, and breeders. In the United Kingdom and France only low levels were detected, but in Belgium, Germany and Holland the levels in 2006 had increased since 2005. In Holland in 2006, 15% of all IBV-positive samples were D1466.
Field and vaccine viruses
Because our methodology could not conclusively distinguish between vaccine and field viruses of the same genotype, we compared the sequences of the viruses detected with those of the standard vaccine types. presents the percentage of detections of each vaccine genotype that had 100%, between 99 and 100% or less than 99% sequence identity with commercially available vaccines. It is probable that the findings that were identical to the vaccine types were in fact vaccine. Less than 99% may have been a field challenge, with the intermediate range being questionable. For 793B, Massachusetts types and D274, approximately 50% of our findings in Western Europe had 100% sequence identity to the vaccine types presented in . The findings for individual countries are also shown and are dealt with in the following sections.
Table 5. Proportion of the IBV genotypes showing between 100% and 99% identity with commercially available vaccines.
Distribution of IBV genotypes over time
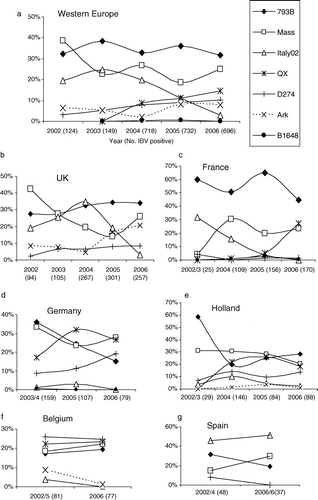
In every year of the study except for 2003, the two predominant IBV genotypes were 793B and Massachusetts types. In 2003 Italy02 was the second most common type detected, being slightly more prevalent than Massachusetts type (a). Of the known field types (i.e. those for which live vaccines are not available), the frequency of detection of Italy02 fell after 2003, while that of the QX type virus increased after 2004. In 2006, the QX-type virus was the third most common genotype detected, accounting for 14.2% of the total, whereas Italy02 was the second least common, accounting for only 3% of the total. However, the distribution of the IBV genotypes with time shows distinct differences for each country.
IBV genotypes in different countries
United Kingdom. The distribution of IBVs detected in the United Kingdom is presented in . Overall, the predominant genotype since 2002 was 793B (32.4% of the total detections), of which 51% were identical to vaccine types (). Massachusetts types were in second place (22.6%), of which 35% shared 100% identity with vaccines. In third place was Italy02 (19.8%). However, b shows that the prevalence of Italy02 had been decreasing since 2004, when it was the predominant virus. The proportion of 793B remained relatively stable, being the predominant type in 2005 and 2006. Massachusetts types fell in frequency until 2005, after which they increased to become the second most common in 2006. Arkansas became more common in 2005 and 2006, reflecting an increase in the use of Arkansas vaccine. The level of D274 remained relatively low (7%) throughout the testing period, and 58% of samples were identical to vaccine virus. No QX was detected in the United Kingdom up to the end of 2006.
France
The predominant genotype in France was 793B (), followed by Massachusetts types, with QX and Italy02 IBV in third and fourth places. In France, 63% of 793B, 75% of the Massachusetts types and all of the D274 detections were identical to vaccine viruses (). Only low levels (less than 2%) of the other genotypes were detected. Since 2003 Italy02 declined in France (c), while in contrast, during 2006, QX increased dramatically in prevalence. The Chinese QX strain was first detected in September 2004 in a flock of broilers in the North East, close to the Belgium border and was thought to be related to use of layer litter compost coming from the Netherlands, where QX had already been detected. In 2005 it was found in three further broiler flocks in the same location. However, in December 2005 it appeared in broiler flocks in Brittany in the North West of France, and in 2006 it spread to several other departments, including the Vendee in January, Drome in the South East in June and in Poitou-Charente, to the east of the Vendee, in October. In 2006, the incidence of QX increased to become the predominant genotype detected (27% of total infectious bronchitis), being more prevalent than Massachusetts type.
Germany
Massachusetts-type IBV was the dominant IBV (29%), being slightly more prevalent than 793B (27.5%), with QX type being the third most common (23.8%) (). In Germany, 35% of 793B, 63% of Massachusetts and 46% of D274 detections were identical to vaccine types (). Italy02 was rarely found in Germany in this study. The QX type was detected in Germany after 2004 (d), and in 2005 it was the predominant genotype. In 2006, the prevalence of QX decreased slightly when it was 1.2% less prevalent than Massachusetts type but was still the second most common type detected. It has been found to cause nephritis in growing birds and false layers in laying flocks at sexual maturity (H. Block, personal communication). The proportion of 793B types appeared to decline, but both D274 (d) and D1466 () increased markedly in 2006.
Holland
The Massachusetts types (27.7%) and the 793B group (26.5%) ( and e) were the most common viruses detected in Holland, followed by QX (20.2%). In 2002 to 2003 we examined relatively few samples (n = 29) so the distribution of genotypes is unlikely to reflect the true situation. However, since 2004 it would appear that 793B levels increased whilst Italy02 and Massachusetts types fell. QX IBV reached a peak in 2005 (approximately one-third of all IBV-positives) and appeared to be declining in 2006. In Holland, 37% of 793B, 35% of Massachusetts and 63% of D274 had identical sequences to vaccine types (). In 2006 the incidence of the D1466 genotype, as in Germany, increased dramatically when it accounted for 15.9% of the total IBV detected in that year ().
Belgium
Relatively fewer samples were received from Belgium than from the countries above, but of these D274 was the most common (25.3%), followed by QX (22.8%) and Massachusetts (20.3%), with 793B in fourth place (18.4%) ( and f). The trend in Belgium in 2006 showed a slight increase in Massachusetts, QX, and 793B, and a slight decrease in D274 and Arkansas genotypes. As in Germany and Holland, D1466 detections increased in 2006 (). Italy02 was rarely detected in Belgium and not at all in 2006. Of the vaccine types 45% of 793B, 59% of Massachusetts and 48% of D274 were identical to the commercially available vaccines ().
Spain
The total number of samples received from Spain was smaller than from any of the other countries. Italy02 appears to be the predominant type (48%), followed by 793B, Massachusetts and D274. Up until December 2006, as with the United Kingdom, we did not detect any QX in Spain ( and g). Again in Spain over 50% of 793B and Massachusetts types were identical to the vaccines and 25% of D274 detections ().
Sequence analysis of the complete S1 gene of Italy02 and QX-type isolates
The Italy02 virus was isolated in this laboratory in 2004 from the trachea, lung, kidney and caecal tonsils of a 50-day-old broiler from a UK flock experiencing a rise in mortality, with respiratory and wet litter problems. The S1 spike gene of the UK tracheal isolate (designated UK/L-633T/03) has been fully sequenced together with that of another virus of the same genotype isolated in Italy in 2002, designated It/497/02 (kindly supplied by Dr I Capua, Instituto Zooprofilattico Sperimentale delle Venezie, Italy; ). This strain was used in the vaccine efficacy trials previously described (Jones et al., Citation2005a). These two viruses and the original Italy02 IBV (accession number AJ457137) share greater than 98.1% nucleotide identity.
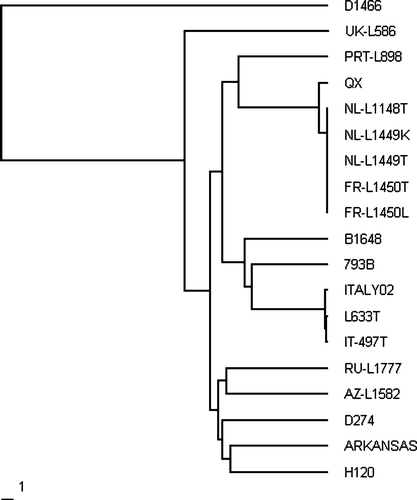
The European QX-type virus has been isolated in this laboratory from a variety of tissues. These include the trachea, lung, caecal tonsils, duodenum and kidneys of broilers, broiler breeder pullets and broiler breeder parents from Europe. The S1 spike genes of five of these isolates have been fully sequenced and submitted to the Genbank database (); three were from the Netherlands and two from France. The nucleotide identity between them was greater than 99.6%. A phylogenetic tree () shows the relationship between these isolates, the standard IBV types, and the other novel types detected in this survey. It can be seen that these five isolates cluster with QX IBV to form a distinct genotype. The isolate we used as the European QX reference is NL/L-1148/04, and this has 97% nucleotide identity with the original Chinese QX IBV ().
Variant IBV genotypes
A novel genotype (reference UK/L-586/03) was detected in Scotland on four occasions from four different sites (Worthington & Jones, Citation2006). The first detection was from a site in Fife in December 2003 from 34-week-old broiler breeders exhibiting respiratory signs and a 5% production drop. Then in November 2005 we detected an IBV that was 99.1% similar to reference L-586 from another site in Fife, again from broiler breeders with a loss in egg and chick quality and mild respiratory signs. In March and April 2006 the same IBV with 100% identity to the virus detected in 2005 was found on two different sites in adjacent Perthshire, from broiler breeders and commercial layers, both exhibiting a loss in egg quality and production and also mild respiratory signs. We sequenced a 1368 base section of the S1 spike genome of this virus (accession number DQ901375; ). It did not appear to be related to any of the other genotypes () and the sequence shares less than 80% identity with any of the reference IBVs or other variant types detected in this survey ().
Table 6. Percentage nucleotide similarity of the S1 spike gene of several reference IBV genotypes and the novel genotypes detected in this survey.
Four other novel IBV sequences have been detected. These were from Portugal, Western Siberia, Azerbaijan and the Yemen. Of these, a small section (330 nucleotide bases) of the S1 spike of the genome has been sequenced (accession numbers EF066521 to EF066524; ). Over the region of the S1 spike gene examined, these viruses appear to be unrelated genotypes () and share less than 86.5% identity with any previously detected genotypes of IBV ().
Discussion
This paper reports a survey of IBV genotypes detected in commercial chicken flocks from the United Kingdom and selected Western European countries from March 2002 to December 2006. RT-PCR technology was used for identification without culturing, followed by sequencing.
The method of sampling, using groups of 10 pooled dry oropharyngeal swabs, followed that described by Cavanagh et al. (Citation1999) and has the particular advantage that viral RNA will remain stable in samples at ambient temperature while in transit from farm to laboratory. Similar diagnostic and surveillance reports to this one for IBV have described the use of an initial short passage of material in fertile eggs before RT-PCR (Jackwood et al., Citation2005). While this protocol, with its initial enhancement stage, may increase sensitivity in detecting live virus, the same would not apply for non-viable virus. Furthermore, it requires transportation of live virus, it is more expensive and, being more time consuming, would not have allowed us to examine such a large number of samples as reported here. The use of universal primers for IBV, followed by sequencing of positive PCR products for the S1 spike gene, enabled us to compare the sequences from positive samples with all others previously submitted to the international database. An alternative approach, using primers for known genotypes only would not have identified new or unexpected genotypes as soon as they emerge and potentially cause problems.
The samples submitted to us were from poultry flocks suffering from conditions likely to be caused by IBV, being mostly from outbreaks of respiratory disease or loss of egg production and quality in layers or breeders, but sometimes from nephritis or wet litter. While every effort was made to obtain as much relevant information on each affected flock and especially the nature of IBV vaccines used, the information in many instances was less than complete. Hence a comprehensive correlation in IBV-positive flocks between vaccines administered and genotypes detected was not possible.
Of the IBV genotypes, D1466 is unusual in that it has the lowest shared identity with other known genotypes over the S1 spike genome (Kusters et al., Citation1989) and requires different primers from the others for its detection. The search for this genotype was not begun by us until 2005, so the results are not as comprehensive as for the others. It is not known whether other types of IBV exist requiring yet different sets of primers and that are hitherto undetected.
The result of our survey have shown that the most frequently detected IBV genotypes were 793B and Massachusetts types, including their vaccine formats. This was not surprising, considering that live vaccines are widely used for these viruses and our methods did not discriminate between field and vaccine strains. Furthermore, recent work (Alvarado et al., Citation2006) and work in this laboratory (K.J. Worthington and R. C. Jones, unpublished) has confirmed earlier reports (Alexander & Gough, Citation1977; Naqi et al., Citation2003) that live IBV vaccines have been found to persist in chicks for many weeks after administration. Therefore, it is likely that many of the 793B, Massachusetts and D274 type IBVs detected in this study represented vaccine virus. Furthermore, the analysis of the nucleotide sequences of these viruses indicated that approximately one-half of the detections shared 100% identity with the vaccine strains over the section of the S1 spike gene examined. However, although it is very likely that these were vaccine detections, it is not possible to categorically state that this is so. It has been shown by Callison et al. (Citation2001) in the case of 4/91 that the nucleotide sequence of the whole S1 spike gene of the vaccine strain and pathogenic virus differ only by 0.6%, and over the section of the gene that we sequenced there was no difference at all.
In an attempt to determine the contribution to the results made by field-type (non-vaccine) viruses, results for the 100% vaccine matches have been removed and revised figures are presented in . As mentioned previously, these revised figures can only be taken as a guide, since some detections not included could be vaccines and vice versa. For Western Europe as a whole, the revised figures show 793B as the dominant genotype with Italy02 slightly more prevalent than Massachusetts and QX, increasing from 10% to 17% of the total detected. In all countries, the prevalent types changes, except for France where it remained 793B. In Germany, Holland and Belgium it becomes QX, and in the United Kingdom and Spain it is Italy02 and this will be discussed further for individual countries.
Table 7. Proportion (%) of IBV per country after removing all of the 100% vaccine detections.
The Arkansas genotype is not an indigenous wild type in Europe but the vaccine is used because it has been found to be protective against 793B types (R.C. Jones & K.J. Worthington, unpublished data). It is important to note that the Arkansas genotype was detected only in flocks given the Arkansas vaccine.
The high prevalence of Italy02 IBV in Europe was not evident prior to the commencement of this survey. In this laboratory it was first detected during a routine sentinel screen of breeder birds in Germany in 2000 (C.J. Naylor, unpublished data), although at the time its significance was not realized. The same genotype was detected in Italy in 1999 and the sequence was submitted to the NCBI database in 2002 by Y.A. Bochkov and V.V. Drygin (accession number AJ457137) (Bochkov et al., Citation2007). Dolz et al. (Citation2006) detected the genotype in Spain and characterized four isolates. The S1 sequences from these viruses showed maximum amino acid identities with the 4/91 serotype (81.7% to 83.7%), the D274 group (79.8% to 81.7%) and B1648 serotype (79.3% to 80%). They showed that the genotype had been in Spain since as early as 1997 and asserted that it constituted a new serotype although its origin is unknown. We showed that protection against this wild genotype could be conferred using combinations of two different commercial live IBV vaccines (Jones et al., Citation2005a). While no live vaccine exists against Italy02, the numbers of detections has fallen markedly since 2003 and it may be that widespread use of such vaccine combinations has been instrumental in this. An interesting feature of this genotype is that it soon became widespread throughout Europe, or at least in the countries covered by this survey, including the United Kingdom. This is in contrast to the other major novel genotype, QX, which up until December 2006 had not been detected in the United Kingdom or Spain in this survey. Whether this discrepancy relates to differences in infectivity or robustness away from the host, or even to spread by wild birds, is unknown.
The genotype called QX was first reported in China in by Wang et al. (Citation1998), where it had been described since 1996 as the cause of proventriculitis in 25-day-old to 70-day-old chickens showing severe weight loss and diarrhoea. Since then there have been more than 20 S1 nucleotide sequences submitted to the NCBI Genbank, including five of our own, with between 95.9% and 99.0% identities with the original virus (). Most of the submissions have been from China, but the majority derived from cases of nephritis rather than proventriculitis. The Chinese submissions have ranged from 1997 to 2004. The genotype was detected in Russia in 2001 in Khabarovsk, only 30 km from the Chinese border, and in 2002 in Western Russia, in Volgograd (Bochkov et al., Citation2006). In addition, two similar viruses have been reported in the database from Korea in 2005 and Israel in 2004, but in these instances the nature of disease was not described. More recently the QX virus has been reported to be present in Poland (Domanska-Blicharz et al., Citation2006) in birds with a 1% increase in mortality, with the most severe lesions being in the kidneys and occasional proventriculitis, similar to the situation found in China mentioned above.
In the present survey we first detected the QX-type virus in 2003 in Germany, and since then it has been detected in Holland, Belgium, France and Italy (Beato et al., Citation2005). There have been no reports of proventriculitis associated with this virus in Europe in this study, but it has caused nephritis and false layers in mature hens due to early infection of the oviduct causing non-patency and cyst formation at maturity (S.J. De Wit, personal communication). The false layer syndrome was first described by several workers (Crinion et al., Citation1971; Jones & Jordan, Citation1972) who showed that it was due to neonatal infection of female chicks with a virulent IBV to which they had no maternal antibodies. Epithelial cells of the oviduct are especially susceptible to virulent IBV and the damage results in abnormal oviduct development at maturity, so that some hens fail to lay eggs. Why QX caused these effects when introduced into the virgin European poultry population while the other novel variant Italy02 did not is unknown, but may relate to different tissue tropisms. The apparently rare association of European QX with proventriculitis is also puzzling. IBV-associated proventriculitis has been little reported or indeed understood.
QX has spread from China to Europe in approximately 7 years, somewhat longer than taken by the H5N1 highly pathogenic avian influenza virus, by what appears to be a similar route. Migratory waterfowl are of course strongly implicated in H5N1 spread (Brown et al., Citation2006). Whether QX could have spread this way deserves discussion.
Coronaviruses have recently been detected in a range of wild bird species. Jonassen et al. (Citation2005) reported coronaviruses in greylag geese, teal and pigeons that, on the basis of their replicase gene sequences, were different from IBVs. However, other authors have described coronaviruses in peafowl (Liu et al., Citation2005; Sun et al., Citation2007), teal (Liu et al., Citation2005) and pigeons (Qian et al., Citation2006), with close or identical sequences in the S1 spike gene to IBV strains. The pigeon virus caused pancreatitis in that species and similar disease in chickens. The peafowl viruses were pathogenic for chickens although harmless in the species of isolation. The currently available evidence, therefore, suggests that some wild bird species can, after infection from chickens, become carriers of IBVs and may prove to be important, perhaps unrecognized, reservoirs of infection. However, there is no evidence yet that IBV strains can infect migratory wild birds and be transmitted over long distances in the way that avian influenza viruses can be. Until this is established, it seems that illegal movement of stock or infected chicken meat could be the most likely means of spread, since legitimate movement of new breeding stock is normally in the West to East direction. This aspect of infectious bronchitis epidemiology needs further study.
Among the genotypes detected in the United Kingdom, the 793B group has been remarkably consistent over the period of surveillance, while the incidence of Massachusetts types fell by some 40% between 2002 and 2006. The reasons for this are not apparent, since there is unlikely to have been a reduction in the uptake of the vaccinal forms of the latter. Italy02, the major European wild type, reached a peak in the United Kingdom in 2004 but has since declined, presumably due to the demonstrated efficacy of IBV vaccine combinations as previously discussed. The fact that Arkansas has shown a steady increase since 2004, has reflected the increased uptake of a commercially available vaccine in the United Kingdom. This attenuated virus, which is not indigenous to Europe, does not appear to have spread to non-vaccinated flocks. After removing detections thought to be attributable to vaccine () the dominant IBV detected in the United Kingdom over the period of surveillance becomes Italy02 although levels have been declining since 2004. Relevant to this is the longitudinal study of IBV in the UK by Cavanagh et al. (Citation1999). Their method of detection involved the use of type-specific oligonucleotide primers. However, Italy02 cross-reacts with the specific primers for 793B (BCE + ) as both genotypes have an identical sequence over the region of the S1 spike gene where the primer attaches. There is the possibility that Italy02 was present in the United Kingdom at this time but was not detected by their methods.
In France, Italy02 was never present at high frequency and fell to a negligible level, but, correspondingly, the prevalence of QX rose since 2005. This virus was found at first in north-eastern France and subsequently in Brittany and more southerly in Atlantic coastal regions. It is only in France that we have a possible explanation for the spread of QX from region to region, mainly because the information was particularly comprehensive. The strong circumstantial evidence is that it spread in infected litter from flocks in Netherlands, where it was already present. This highlights the potential dangers of this practice in IBV epidemiology. The high proportion of 793B in France may reflect the true field situation as this was still the dominant type after removing all of the 100% vaccine detections. QX-type IBV became the second most common followed by Italy02.
Germany, Holland and Belgium are three countries in which QX-type-related disease has been prevalent, especially in the form of nephritis and false layers in mature hens. When 100% vaccine detections are removed and the figures recalculated, QX-type IBV becomes the dominant field type in all these countries. Detections increased during the observation period, peaking in Holland and Germany in 20 05 and in Belgium in 2006. Interestingly, in Germany where levels of Italy02 were consistently low during the observation period, QX was high. In France, and to some extent in Holland, the proportion of Italy02 decreased coinciding with an increase in QX. It is perhaps bold to suggest that these two field genotypes may not co-exist in a poultry population.
The distribution of the IBV genotypes detected by us in Belgium between 2002 and 2006 differs from that reported by Meulemans et al. (Citation2001) for isolates collected between 1986 and 1995. Then the predominant type was Massachusetts (51%) followed by D274 (39%) and B1648 (10%), with very little 793B (1%). In the present survey we did not detect any B1648 and, after removing samples thought to be attributable to vaccines, QX became the most common type. There has also been a marked increase in the level of 793B in Belgium. During the intervening years, B1648 has declined in prevalence, 793B has increased, and the QX type has appeared.
In this survey B1648 was only detected on seven occasions, six in France and one in Germany. The study by Capua et al. (Citation1999) showed that this nephropathogenic Belgian strain was present in Italy in 1997. As we did not examine samples from Italy we were unable to directly compare our results, but during our survey B1648 did not appear to be causing significant problems in poultry flocks from the countries tested. The reason for this is unclear but may be due to certain vaccine strategies controlling the spread of different genotypes more favourably than others.
Spain was the source of the fewest samples so results may not reflect the true IBV situation, but from our results it would appear that Italy02 was causing major problems. Spain is notable for the lack of QX detected in this survey and it is puzzling as to why it is absent from this country as it is from United Kingdom. In part, it is likely to relate to the small sample sizes and may not indicate the true picture.
D1466, for which we tested only since 2005, showed an increase in 2006 not only in Germany but also in Holland and Belgium, where there was an upsurge of activity of this virus causing egg losses in layers and broiler breeders (several personal communications). To date, no live D1466 vaccines have been used; only a killed product given with other vaccines. Historically, D1466 has never presented big disease problems and appears to be an IBV of relatively low pathogenicity. However, this upsurge requires investigation, and recent isolates are being examined to see whether they differ in S1 spike sequences and are more virulent than those originally described.
Of the additional novel genotypes included in this report, the Scottish virus may be a virus of low pathogenicity, which appears to survive at a low level but not to cause explosive outbreaks of disease. However, this would appear to be a rarity among IBVs. The Portuguese genotype is of particular interest, since it differs from viruses detected in its closest neighbour, Spain. The sole Russian virus reported here cannot be compared directly with any described by Bochkov et al. (Citation2006) in their survey covering 1998 to 2002, since they sequenced a different region of the S1 spike gene. None of these novel genotypes shared S1 spike gene identity of more than 87% with the other genotypes, and D1466 appeared to be the least related to any of the others. The phylogenetic analysis of all these novel types indicates that each may be a new genotype, although in some cases only a short section of the S1 spike gene has been sequenced. Further investigation and full gene sequencing would be required to confirm this. The major field viruses, QX type and Italy02, detected and isolated in this study have been shown to be distinct genotypes, and prior to the commencement of this survey the prevalence of both of these viruses throughout Europe was not evident.
In conclusion, this is the most comprehensive study to date of IBV genotypes in Western Europe and it has illustrated the value of continued surveillance. Because of the scope of the survey, it was not possible to relate the presence of specific genotypes to precise geographic areas and to trace the movement of types from farm to farm or region to region. We have shown the variable prevalence of the main IBV vaccinal types and the emergence of two major variants, Italy02 and QX, which appeared to be the dominant field strains in several countries in Western Europe over the time period examined. However, while it may be too early to speculate, it seems unlikely that Italy02 is a major variant in the way that 793B types have been, since it appears to be controllable to some extent with combinations of existing live IBV vaccines (Jones et al., Citation2005a). On the other hand the QX-type virus now represents the major field genotype in several countries and its control with conventional vaccines has yet to be conclusively determined. This study has also highlighted an increase in the D1466 genotype particularly in older birds. For both these viruses it may be that a type-specific vaccine is required.
The present study has also shown, predictably, that novel types appear unexpectedly and randomly in any country. Their origin can occasionally be related to others reported elsewhere, may be the result of recombination between known types or, as in many cases, are of unknown origin. Some viruses, for example the Scottish genotype, may show relatively low ability to cause disease. Most of the variants are likely to be of little importance, but where they become prevalent the efficacy of existing vaccines must still be determined by vaccination/challenge animal trials.
Acknowledgements
The authors wish to thank Clive Naylor for helpful discussions and advice, Barbara Sargent, Jayne Clubbe, John Kane and Carol Savage for technical assistance. Thanks are also due to veterinarians and farmers who submitted samples. This work was funded by Fort Dodge Animal Health and special thanks are due to Herve le Galludec, Willem Wijmenga and Ian Church.
References
- Adzhar , A. , Shaw , K. , Britton , P. and Cavanagh , D. 1996 . Universal oligonucleotides for the detection of infectious bronchitis virus by the polymerase chain reaction . Avian Pathology , 25 : 817 – 836 .
- Adzhar , A. , Gough , R.E. , Haydon , D. , Shaw , K. , Britton , P. and Cavanagh , D. 1997 . Molecular analysis of the 793/B serotype of infectious bronchitis virus in Great Britain . Avian Pathology , 26 : 625 – 640 .
- Alexander , D.J. and Gough , R.E. 1977 . Isolation of avian infectious bronchitis virus from experimentally infected chickens . Research in Veterinary Science , 23 : 344 – 347 .
- Alvarado , I.R. , Villegas , P. , El-Attrache , J. and Jackwood , M.W. 2006 . Detection of Massachusetts and Arkansas Serotypes of infectious bronchitis virus in broilers . Avian Diseases , 50 : 292 – 297 .
- Beato , M.S. , De Battisti , C. , Terregino , C. , Drago , A. , Capua , I. & Ortali , G. 2005 . Evidence of circulation of a Chinese strain of infectious bronchitis virus (QXIBV) in Italy . Veterinary Record , 156 , 720 .
- Bochkov , Y.A. , Batchenko , G.V. , Shcherbakova , L.A. , Borisov , A.V. and Drygin , V.V. 2006 . Molecular epizootiology of avian infectious bronchitis in Russia . Avian Pathology , 35 : 379 – 393 .
- Bochkov , Y.A. , Tosi , G. , Massi , P. and Drygin , V.V. 2007 . Phylogenic analysis of partial S1 and N gene sequences of infectious bronchitis virus isolates from Italy revealed genetic diversity and recombination . Virus Genes , 35 : 65 – 71 .
- Brown , I.H. , Banks , J. , Manvell , R.J. , Essen , S.C. , Shell , W. , Slomka , M. , Londt , B. and Alexander , D.J. 2006 . Recent epidemiology and ecology of influenza A viruses in avian species in Europe and the Middle East . Developmental Biology , 124 : 45 – 50 .
- Callison , S.A. , Jackwood , M.W. and Hilt , D. A. 2001 . Molecular characterization of infectious bronchitis virus isolates foreign to the United States and comparison with United States isolates . Avian Diseases , 45 : 492 – 499 .
- Capua , I. , Minta , Z. , Karpinska , E. , Mawditt , K. , Britton , P. , Cavanagh , D. and Gough , R. E. 1999 . Co-circulation of four types of infectious bronchitis virus (793/B, 624/I, B1648 and Massachusetts) . Avian Pathology , 28 : 587 – 592 .
- Cavanagh , D. 2005 . Coronaviruses in poultry and other birds . Avian Pathology , 34 : 439 – 448 .
- Cavanagh , D. , Ellis , M.M. and Cook , J.K.A. 1997 . Relationship between sequence variation in the S1 spike protein of infectious bronchitis virus and the extent of cross-protection in vivo . Avian Pathology , 26 : 63 – 74 .
- Cavanagh , D. , Mawditt , K. , Britton , P. and Naylor , C.J. 1999 . Longitudinal field studies of infectious bronchitis virus and avian pneumovirus in broilers using type-specific polymerase chain reactions . Avian Pathology , 28 : 593 – 605 .
- Chomczynski , P. and Sacchi , N. 1987 . Single-step method of RNA isolation by acid guanidium thiocyanate–phenol–chloroform extraction . Analytical Biochemistry , 162 : 156 – 159 .
- Crinion , R.A.P. , Ball , R.A. and Hofstad , M.S. 1971 . Abnormalities in laying chickens following exposure to infectious bronchitis virus at one day old . Avian Diseases , 15 : 42 – 48 .
- Dolz , R. , Pujols , J. , Ordonez , G. , Porta , R. and Majo , N. 2006 . Antigenic and molecular characterization of isolates of the Italy 02 infectious bronchitis virus genotype . Avian Pathology , 35 : 77 – U2 .
- Domanska-Blicharz , Z. , Minta , Z. and Smientanka , K. 2006 . New variant of IBV in Poland . The Veterinary Record , 158 : 808
- Elhafi , G. , Naylor , C.J. , Savage , C.E. and Jones , R.C. 2004 . Microwave or autoclave treatments destroy the infectivity of infectious bronchitis virus and avian pneumovirus but allow detection by reverse transcriptase-polymerase chain reaction . Avian Pathology , 33 : 303 – 306 .
- Gelb , J. , Weisman , Y. , Ladman , B.S. and Meir , R. 2005 . Gene characteristics and efficacy of vaccination against infectious bronchitis virus field isolates from the United States and Israel (1996 to 2000) . Avian Pathology , 34 : 194 – 203 .
- Jackwood , M.W. , Hilt , D.A. , Lee , C.W. , Kwon , H.M. , Callison , S.A. , Moore , K.M. , Moscoso , H. , Sellers , H. and Thayer , S. 2005 . Data from 11 years of molecular typing infectious bronchitis virus field isolates . Avian Diseases , 49 : 614 – 618 .
- Jonassen , M.C. , Kofstad , T. , Larsen , I.-L. , Lovland , A. , Handeland , K. , Follestad , A. and Lillehaug , A. 2005 . Molecular identification of novel coronaviruses infecting greylag geese (Anser anser), feral pigeons (Columbia livia) and mallards (Anser platyrhynchos) . Journal of General Virology , 86 : 1597 – 1602 .
- Jones , R.C. and Jordan , F.T.W. 1972 . Persistence of virus in the tissues and development of the oviduct of the fowl following infection at day-old with infectious bronchitis virus . Research in Veterinary Science , 32 : 52 – 60 .
- Jones , R.C. , Worthington , K.J. , Capua , I. and Naylor , C.J. 2005a . Efficacy of live infectious bronchitis vaccines against a novel European genotype, Italy 02 . Veterinary Record , 156 : 646 – 647 .
- Jones , R.C. , Worthington , K.J. and Gough , R.E. 2005b . Detection of the Italy 02 strain of infectious bronchitis virus in the UK . Veterinary Record , 156 , 260 .
- Kusters , J.G. , Niesters , H.G.M. , Lenstra , J.A. , Horzinek , M.C. and Van Der Zuest , B.A. M. 1989 . Phylogeny of antigenic variants of avian coronavirus IBV . Virology , 169 : 217 – 221 .
- Li , J. , Cook , J.K.A. , Brown , D.K. , Shaw , K. and Cavanagh , D. 1993 . Detection of turkey rhinotracheitis virus in turkeys using the polymerase chain reaction . Avian Pathology , 22 : 771 – 783 .
- Liu , S. , Chen , J. , Chen , J. , Kong , X. , Shao , Y. , Han , Z. , Feng , L. , Cai , X. , Gu , S. and Liu , M. 2005 . Isolation of avian infectious coronavirus from domeatic peafowl (Pavo cristatus) and teal (Anas) . Journal of General Virology , 86 : 719 – 725 .
- Meulemans , G. , Carlier , M.C. , Gonze , M. , Petit , P. and Vandenbroek , M. 1987 . Incidence, characterization and prophylaxis of nephropathogenic avian infectious bronchitis viruses . Veterinary Record , 120 : 205 – 206 .
- Meulemans , G. , Boschmans , M. , Decaesstecker , M. , van den Berg , T.P. , Denis , P. and Cavanagh , D. 2001 . Epidemiology of infectious bronchitis virus in Belgian broilers: a retrospective study, 1986 to 1995 . Avian Pathology , 30 : 411 – 421 .
- Naqi , S. , Gay , K. , Patalla , P. , Mondal , S. and Liu , R. 2003 . Establishment of persistent avian infectious bronchitis virus infection in antibody-free and antibody-positive chickens . Avian Diseases , 47 : 594 – 601 .
- Qian , D.H. , Zhu , G.J. , Wa , L.Z. & Hua , G.X. 2006 . Isolation and characterization of a coronavirus from pigeons with pancreatitis . American Journal of Veterinary Research , 67 , 1575 – 1579 .
- Sun , L. , Zhang , G. , Jiang , J. , Fu , J. , Ren , T. , Cao , W. , Xin , C. , Liao , M. and Liu , W. 2007 . A Massachusetts prototype like coronavirus isolated from wild peafowls is pathogenic to chickens . Virus Research , 130 : 121 – 128 .
- Wang , Y.D. , Wang , Y.L. , Zhang , Z. , Fan , G. , Jiang , Y. , Liu , X. , Ding , J. and Wang , S. 1998 . Isolation and identification of glandular stomach type IBV (QX IBV) in chickens . Chinese Journal of Animal Quarantine , 15 : 1 – 3 .
- Worthington , K.J. and Jones , R.C. 2006 . New genotype of infectious bronchitis virus in chickens in Scotland . The Veterinary Record , 159 : 291 – 292 .
- Worthington , K.J. , Savage , C. , Naylor , C.J. , Wijmenga , W. and Jones , R.C. 2004 . “ An RT-PCR survey of infectious bronchitis genotypes in the UK and selected European countries between 2002 and 2004 and the results from a vaccine trial ” . In Proceedings of the IVth International Symposium on Avian Corona- and Pneumovirus Infections , 125 – 133 . Rauischholzhausen , , Germany : VVB Laufersweiler Verlag, Wettenberg .