Abstract
In a recent study (Oldoni & García, 2007), some field strains of infectious laryngotracheitis viruses (ILTV) were characterized as genotypically different (group VI) from ILT vaccine strains. The objective of this study was to evaluate the protection elicited by one chicken embryo origin (CEO) and one tissue culture origin (TCO) vaccine against a field isolate from group VI after direct and indirect exposure to ILTV live attenuated vaccines. In phase 1 of the experiment, non-vaccinated chickens were placed into contact with the eye drop vaccinates for a period of four weeks after vaccination. Transmission of the vaccine virus to these in-contact birds was demonstrated by real time PCR and antibody production, although the in-contact birds did not become protected against disease when subsequently challenged in phase 2 of the experiment. This emphasized the importance of uniform vaccination to obtain adequate protection, both to avoid the occurrence of susceptible chickens, and to minimize the potential for reversion to virulence of live-attenuated vaccines. In phase 2, protection against challenge with a group VI field virus was assessed four weeks after vaccination by scoring clinical signs and mortality, and quantifying weight gain. Sentinel birds were added to the groups one day after challenge to assess shedding of challenge virus, using real time PCR and virus isolation, during the period 2 to 12 days post challenge. The results showed that the CEO and TCO eye drop-vaccinated chickens were protected against challenge with the group VI virus, even though it was genetically different from the vaccine strains, and that challenge virus was not transmitted from these protected birds to the sentinels.
Evaluation de la protection conférée par une exposition directe et indirecte aux vaccins à vivant virus atténué de la laryngotrachéite infectieuse aviaire vis-à-vis d'une souche d'épreuve récente isolée aux Etats-Unis
Dans une étude récente, (Oldoni & García, 2007), quelques souches de virus de la larygotrachéite infectieuse aviaire (ILTV) ont été caractérisées comme étant génotypiquement différentes (goupe VI) de celles des souches vaccinales d'ILT. L'objectif de cette étude a été d’évaluer la protection conférée par un vaccin préparé sur embryon de poulet (CEO) et un autre sur culture cellulaire (TCO) vis-à-vis d'un isolat du terrain appartenant au groupe VI après une exposition directe et indirecte aux vaccins à virus vivant atténué d'ILT. Dans la première phase de l'expérimentation, des poulets non vaccinés ont été placés au contact de sujets vaccinés par instillation oculaire durant une période de quatre semaines après la vaccination. La transmission du virus vaccinal à ces sujets contacts a été mise en évidence par PCR en temps réel et par la production d'anticorps. Bien que ces sujets contacts n'aient pas été protégés contre la maladie quand ils ont été éprouvés dans la deuxième phase de l'expérimentation. Ceci souligne l'importance d'une vaccination uniforme pour obtenir une protection adéquate, à la fois pour éviter l'apparition de poulets sensibles et pour minimiser la potentialité du retour à la virulence des vaccins atténués vivants. Dans la seconde phase de l'expérimentation, la protection contre l'épreuve avec un virus du terrain du groupe VI a été évaluée 4 semaines après la vaccination en enregistrant les symptômes et la mortalité, ainsi qu'en quantifiant le gain de poids. Des sujets sentinelles ont été ajoutés aux groupes, un jour après l’épreuve, pour évaluer la diffusion du virus d’épreuve en utilisant la PCR en temps réel et l'essai d'isolement du virus du 2ème au 12ème jour après l’épreuve. Les résultats ont montré que les poulets vaccinés par instillation oculaire avec les vaccins CEO et TCO ont été protégés contre l'épreuve avec le virus du groupe VI, alors même qu'il était génétiquement différent des souches vaccinales et que le virus d'épreuve n'a pas été transmis par ces sujets protégés aux sujets sentinelles.
Bestimmung der durch direkte und indirekte Aufnahme von attenuierten Lebendvakzinen des infektiöse Laryngotracheitis-Virus (ILTV) hervorgerufene Schutzwirkung gegen einen neuen Belastungsstamm aus den USA
Kürzlich (Oldoni & Garcia, 2007) wurden einige Feldstämme des Virus der infektiösen Laryngotracheitis als genotypisch unterschiedlich (Gruppe VI) zu den ILT-Vakzinestämmen charakterisiert. Ziel der vorliegenden Studie war es nun, die Schutzwirkung von ILTV-Lebendvirusvakzinen induziert durch direkte und indirekte Aufnahme einer Hühnerembryo-adaptierten (CEO) und einer Gewebekultur-adaptierten Vakzine (TCO) gegenüber einem Feldisolat aus der Gruppe VI zu ermitteln. In der Phase 1 des Experiments wurden nicht vakzinierte Hühner mit via Augentropfen vakzinierten für einen Zeitraum von vier Wochen nach der Vakzination in Kontakt gebracht. Mittels Real Time-PCR und Antikörpernachweis wurde die Übertragung des Vakzinevirus auf die Kontakttiere nachgewiesen. Trotzdem waren sie gegen eine Belastungsinfektion, die in der zweiten Versuchsphase durchgeführt wurde, nicht geschützt. Dies unterstreicht die Bedeutung einer gleichmäßigen Vakzination für einen adäquaten Impfschutz, um einerseits das Vorkommen empfänglicher Hühner in einer Herde zu vermeiden und andererseits das Potential der Virulenzrückkehr von attenuierten Lebendvakzineviren zu minimieren. In der zweiten Phase des Experiments wurde 4 Wochen nach der Vakzination die Schutzwirkung gegen eine Belastungsinfektion mit einem Gruppe VI-Feldvirus durch graduelle Erfassung der klinischen Symptome und der Mortalitätsrate sowie durch Bestimmung der Gewichtszunahmen ermittelt. Außerdem wurde vom 2. bis 12. Tag nach der Belastungsinfektion mittels Real Time-PCR und Virusisolierung überprüft, ob die vakzinierten Hühner das Feldvirus ausscheiden und auf Sentineltiere übertragen. Die festgestellten Parameter erbrachten den wichtigen Nachweis, dass die mit CEO und TCO via Augentropf vakzinierten Hühner gegen eine Belastungsinfektion mit dem Virus der Gruppe VI geschützt waren, obwohl es sich genetisch vom Vakzinevirus unterschied, und dass das Belastungsinfektionsvirus nicht von den geschützten Hühnern auf die Sentineltiere übertragen wurde.
Introduction
Infectious laryngotracheitis (ILT) is a highly contagious disease of chickens that may cause severe production losses due to morbidity, mortality, decreased egg production, weight loss and/or predisposition to other respiratory avian pathogens (Guy & Bagust, Citation2003). Infectious laryngotracheitis virus (ILTV) belongs to the family herpesviridae, subfamily alphaherpesvirinae, and it is taxonomically classified as Gallid herpesvirus 1 (Davison et al., Citation2006).
The two main types of ILTV vaccines commercially available in the US are those attenuated by sequential passages in chicken embryos (CEO), and those attenuated by sequential passages in tissue culture (TCO). These attenuated vaccines induce protection, preventing clinical signs and mortality (Gelenczei & Marty, Citation1964; Fulton et al., Citation2000; Han & Kim, Citation2003). Both types can persist in apparently healthy birds (Andreasen et al., Citation1989; Hughes et al., Citation1989) and can spread from vaccinated to unvaccinated birds in close contact (Gelenczei & Marty, Citation1964; Hilbink et al., Citation1987; Andreasen et al., Citation1989; Rodríguez-Avila et al., Citation2007). The route of vaccination is extremely important since some of the available live attenuated vaccines provide different grades of protection, particularly when applied by coarse spray or in the drinking water (Hilbink et al., Citation1987; Fulton et al., Citation2000). Eye-drop vaccination has been demonstrated to provide a more uniform protection (Fulton et al., Citation2000), and less severe reactions as compared with spray vaccination (Hilbink et al., Citation1987).
Outbreaks of mild to moderate forms of ILT are common in commercial layer flocks worldwide, while sporadic outbreaks of ILT in broiler flocks have also been recognized as an emerging problem in several countries including USA (Davison, Citation2005). Molecular epidemiology studies suggest that the majority of the strains associated with disease outbreaks in broilers in the USA are closely related to the CEO vaccines, while outbreaks associated with TCO type isolates are rare (Guy et al., Citation1989; Keller et al., Citation1992; Keeler et al., Citation1993). Recently, commercial poultry isolates were genotyped into four groups (groups III, IV, V, VI). Groups III, IV and V were closely related to the live attenuated vaccine strains, while group VI isolates were characterized as different from the vaccine strains (Oldoni & García, Citation2007).
The protection efficacy of live attenuated ILT vaccines has been evaluated against a variety of ILTV strains by clinical signs, mortality, viral recovery and spread (Gelenczei & Marty, Citation1964; Hilbink et al., Citation1987; Fulton et al., Citation2000; Han & Kim Citation2003). The objective of the present study was to evaluate the protection induced by direct and indirect exposure to these vaccines against a current group VI ILTV isolate.
Materials and Methods
Virus strains and titration
The live attenuated ILTV vaccines used in this study were the Schering Plough (Omaha, Nebraska, USA) ILT-Vax® (TCO) (serial number LX06/07; expiry date 24 January 2009) and the Schering Plough (Millsboro, Delaware, USA) Trachivax® (CEO) (serial number LT51/07; expiry date 23 August 2008). The challenge virus used in this study was identified as 2/A/04/BR and was originally isolated from broilers, and classified by multiple polymerase chain reaction (PCR)-restriction fragment length polymorphism (RFLP) as a member of group VI genotype (Oldoni & García, Citation2007). For this study, the virus was plaque purified in the chicken liver tumour cell line LMH (Kawaguchi et al., Citation1987), and passed three times on chicken kidney (CK) cells. Vaccine and challenge virus titrations were performed in 96-well plates of CK cells prepared from 3-week-old to 4-week-old chickens, using a final concentration of 8×105 cells/ml in five replicates from 10−1 to 10−10 dilutions. The median tissue culture infective dose (TCID50) titre was estimated by the method of Reed & Muench (Citation1938).
Experimental design
In this challenge system, the experimental procedures were performed in two phases. Phase 1. Contact-exposed chickens were used to evaluate indirect exposure and transmission of live attenuated vaccines for 4 weeks after vaccination. Phase 2. Fresh sentinel chickens were used to assess the shedding of the challenge virus (group VI strain) up to 12 days post challenge.
A total of 160 leghorn specific pathogen free chickens were obtained from Merial (Gainesville, Georgia, USA). The chickens were distributed between eight polycarbonate Plexiglas isolation units with filtered air and positive pressure at the Poultry Diagnostic and Research Centre (Athens, Georgia, USA), and fed a standard diet and water ad libitum.
Phase 1
At 4 weeks of age, four groups of 10 chickens were vaccinated, two groups with TCO (TCOVx) and the other two groups with CEO (CEOVx). The day after vaccination, 10 non-vaccinated chickens were placed together with the 10 vaccinated chickens in each unit for a total of four units each containing 20 chickens, 10 vaccinated and 10 non-vaccinated. The non-vaccinated chickens were identified with wing bands as contact-exposed chickens (CT-TCOVx and CT-CEOVx). Chickens were vaccinated by eye drop according to the recommendations of the manufacturer using 0.033 ml vaccine per chicken. Four weeks after vaccination, the 40 contact-exposed chickens (20 CT-TCOVx and 20 CT-CEOVx) were removed from the isolation units holding vaccinated chickens (TCOVx and CEOVx) and were placed in two different units. The same day, 20 TCOVx, 20 CEOVx, 20 CT-TCOVx, 20 CT-CEOVx, and 10 non-vaccinated (NVx) chickens were challenged (Ch). Chickens were challenged with Isolate 2/A/04/BR at a final dose of 103.02 TCID50 per chicken inoculated in a total volume of 200 µl, 50 µl onto each eye and 100 µl in the trachea.
Phase 2
One day post-challenge, five groups of 10 chickens were placed into the four units holding vaccinated challenged chickens and into the unit holding non-vaccinated challenged chickens (NVx-Ch). These freshly placed chickens were used as sentinels for the challenged groups (SE-TCOVx-Ch, SE-CEOVx-Ch, and SE-NVx-Ch). Twenty chickens were used as a non-vaccinated, non-challenged group (NVx-NCh) or negative control. Chickens were then observed daily from days 2 to 12 post challenge (d.p.c.).
Sample collection
Tracheal swabs were collected at day 9 after vaccination from five chickens in each of the CT-TCOVx and CT-CEOVx groups for real-time PCR. Trachea and eye-conjunctiva swabs were collected from two chickens in the SE-TCOVx-Ch and SE-CEOVx-Ch groups and from two chickens in the SE-NVx-Ch and NVx-NCh groups every day from 2 to 12 d.p.c. Swabs were placed in 1 ml sterile phosphate-buffered saline solution containing 2% antibiotic–antimycotic 100X (Gibco, Grand Island, New York, USA) and 2% newborn calf serum (Gibco, Grand Island, New York, USA). All samples were stored at −80°C until processing for virus isolation and DNA extraction.
Clinical signs and body weight
Clinical signs were scored every day from 2 to 12 d.p.c. in TCOVx-Ch, CEOVx-Ch, CT-TCOVx-Ch, CT-CEOVx-Ch, NVx-Ch, and NVx-NCh groups by examining five chickens per group. To score clinical signs from the same bird every day, chickens were identified using vegetable colours sprayed on the chicken wings feathers. Breathing patterns, conjunctivitis, and the level of depression were evaluated and scored daily for all groups. Breathing patterns were scored on a scale of zero (normal breathing), one (open-mouth breathing) and two (gasping with an extended neck). Conjunctivitis was scored on a scale of zero (normal), one (swollen and partial closure of the eyes) and two (complete closure of the eyes). The level of depression was scored on a scale of zero (normal behaviour), one (mildly depressed) and two (severely depressed). Mortality was given a score of three. All chickens were weighed the day before vaccination (4 weeks of age), one day pre-challenge (8 weeks of age), and at 12 d.p.c. The average weight gain and clinical sign scores were calculated for each group.
Virus isolation
Virus isolation was performed in adult CK cells as previously described (Rodríguez-Avila et al., Citation2007). Briefly, cells were seeded at 100 µl/well into 96-well plates. After 24 h, cells were inoculated in triplicate with 70 µl sample/well. All samples were passed three consecutive times in CK cells. Samples were considered positive by virus isolation when the cytopathic effect characteristic of ILTV was observed, and they were considered negative after three passages without observation of ILTV cytopathic effect. Before inoculation, samples were frozen and thawed three times. Samples were thawed at 37°C, vortexed, and frozen at −80°C. After thawing, samples were vortexed and centrifuged for 3 min at 1024 g, and the supernatant was used to inoculate CK cells.
DNA extraction
The DNA extraction was executed using the MagaZorb DNA Mini-prep 96-well kit (CORTEX BIOCHEM™, San Leandro, California, USA) according to the manufacturer's instructions.
Real-time PCR Taqman assay
The real-time PCR Taqman assay (ReTi-PCR) was performed as previously described (Callison et al., Citation2007). The primers and probe utilized in the assay were located in the viral glycoprotein C gene, and were synthesized by IDT (Coralville, Iowa, USA) and BioSearch Technologies (Novato, California, USA). The genome copy number (GCN) log10 per amplification reaction was estimated using the standard curve equation (y= − 0.289x+12.487) generated from the gC plasmid and expressed as log10. The GCN log10 value reported was the average of two samples.
Serology
Ten blood samples were collected from each group before vaccination (4 weeks of age), pre-challenge (8 weeks of age), and 12 d.p.c. Sera were analysed with a commercial LT ELISA kit (ProFLOCK® LT ELISA Kit; Synbiotics Corp., San Diego, California, USA).
Statistical analysis
The Kruskal–Wallis test was used to compare the total clinical scores of chickens in the different treatment groups. Dunn's procedure was used to make post-hoc comparisons between the non-vaccinated, non-challenge group and challenge groups while maintaining an overall type I error rate of 5%. One-way analysis of variance was used to compare the percentage body weight gain for the different groups with post-hoc comparisons made with the non-vaccinated non-challenge control group using the Bonferroni procedure to control the type I error rate at 5%. The normality assumption was assessed by examining normal probability plots and equality of variance was assessed using Levene's test. Fisher's exact test was used to compare mortality proportions between the challenge and negative control groups.
Results
Virus titration
The CEO and TCO vaccines were titrated in CK cells before and after vaccination. The CEO vaccine titre was 104.70 and 104.59 TCID50/ml, respectively. The TCO vaccine titre was 104.92 and 104.79 TCID50/ml, respectively. A final dose of 103.21 and 103.39 per chicken of CEO and TCO vaccines, respectively, was applied. The titre of the group VI challenge virus in CK cells was 103.77 and 103.69 TCID50/ml pre-challenge and post-challenge. A final viral dose of 103.02 TCID50/200 µl was applied per chicken.
Real-time PCR Taqman assay and virus isolation
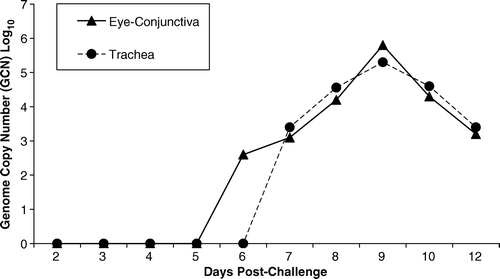
Viral DNA was detected in all five tracheal swabs collected at day 9 after vaccination from each chicken in the contact-exposed groups with an average of 104.1 and 104.4 GCN log10, and identified by PCR-RFLP as TCO and CEO vaccine viruses, respectively (data not shown). From all samples collected post-challenge, viral DNA was detected and the challenge virus was isolated in samples from the SE-NVx-Ch and contact-exposed groups (data not shown). Viral DNA was detected from day 6 to 12 d.p.c. in the eye conjunctiva and from day 7 to 12 d.p.c. in the trachea (). The peak of viral DNA was observed for eye conjunctiva and trachea at 9 d.p.c., with 105.8 and 105.3 GCN log10, respectively. The challenge virus was isolated from eye conjunctiva and trachea at 8, 9, and 10 d.p.c. from all samples collected from the SE-NVx-Ch group and identified by PCR-RFLP as group VI viral genotype (data not shown). Samples with GCN equal to or higher than 104.27 were positive for virus isolation. Samples from SE-TCOVx-Ch, SE-CEOVx-Ch and NVx-NCh groups were all negative by both ReTi-PCR and virus isolation.
Clinical signs
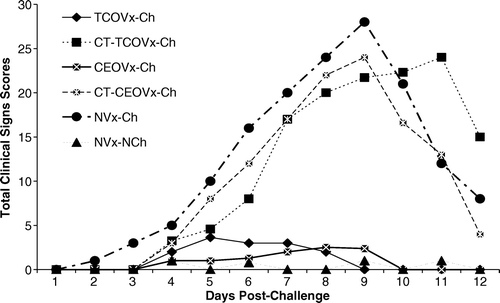
Total clinical sign scores per day for CEOVx-Ch, CT-CEOVx-Ch, TCOVx-Ch, CT-TCOVx-Ch, NVx-Ch, and NVx-NCh groups are summarized in . Chickens in the CEOVx-Ch, TCOVx-Ch and NVx-NCh groups showed mild clinical signs characterized by mild breathing and depression with no mortality. The total clinical sign scores among these groups were not significantly different. On the other hand, chickens in the CT-CEOVx-Ch, CT-TCOVx-Ch and NVx-Ch groups showed open-mouth breathing, gasping with an extended neck, mild and severe conjunctivitis with closed and watery eyes, different levels of depression and mortality. Clinical signs were observed in all five chickens from day 2 to 12 d.p.c. in the NVx-Ch group, and from day 4 to 12 d.p.c. in the CT-CEOVx-Ch and CT-TCOVx-Ch groups (). The total clinical sign scores for CT-CEOVx-Ch, CT-TCOVx-Ch, and NVx-Ch groups were significantly (P < 0.05) greater than the scores of the NVx-NCh group, while those of the CEOVx-Ch and TCOVx-Ch groups were not significantly different from the NVx-NCh group.
Figure 2. Total clinical sign scores recorded per day in 8-week-old chickens from day 2 to 12 d.p.c. with ILT virus. Contact-exposed to tissue culture origin vaccinated challenge (CT-TCOVx-Ch), contact-exposed to chicken embryo origin (CT-CEOVx-Ch), and non-vaccinated challenge (NVx-Ch) groups were significantly different (P < 0.05) from the non-vaccinated-non-challenged (NVx-NCh) group, while the tissue culture origin vaccinated challenged (TCOVx-Ch) and chicken embryo origin vaccinated challenged (CEOVx-Ch) groups were not significantly different from the non-vaccinated-non-challenged (NVx-NCh) group.
Mortality
The percentage mortality per group up to 12 d.p.c. is presented in . No mortality occurred in the CEOVx-Ch, SE-CEOVx-Ch, TCOVx-Ch, SE-TCOVx-Ch, and NVx-NCh groups. However, mortality was observed in the CT-CEOVx-Ch, CT-TCOVx-Ch, NVx-Ch and SE-NVx.Ch groups between 8 and 12 d.p.c., with mortality ranging from 25% to 40%. Mortality recorded for the CT-CEOVx-Ch (P≤0.021), CT-TCOVx-Ch (P≤0.012), NVx-Ch (P≤0.014, and SE-NVx-Ch (P≤0.005) groups were significantly different from that in the NVx-NCh group.
Table 1. Percentage mortality 12 d.p.c. with a group VI ILT virus
Body weight gain
The body weight gain for each group pre-challenge (from 4 to 8 weeks of age), and 12 d.p.c. is presented in . The percentage of body weight gain pre-challenge did not differ significantly between groups (P=0.234), but there was a significant difference between groups during the 12 d.p.c. (P < 0.001). Compared with the negative control group, the mean weight gain of the NVx-Ch, SE-NVX-Ch, CT-CEOVx-Ch and CT-TCOVx-Ch groups were all significantly lower during the post-challenge period ().
Table 2. Mean (standard error) percentage change in body weight for groups of 20 chickens at 4 to 8 weeks of age (pre ILT challenge) and 12 days post ILT challenge
Serology
ELISA results for seven samples collected before vaccination (4 weeks of age), pre-challenge (8 weeks of age), and 12 d.p.c. are presented in . Under the experimental conditions used in this study, any titre detected by the ILT ELISA test was considered a specific antibody response to the vaccines and challenge viruses. Samples collected before vaccination were negative. More than 40% of the samples collected pre-challenge from the CEOVx, CT-CEOVx, TCOVx and CT-TCOVx groups showed antibodies against ILTV, while samples collected from the NVx-NCh group were negative. Samples collected 12 d.p.c. from the CEOVx-Ch, CT-CEOVx-Ch, TCOVx-Ch, CT-TCOVx-Ch, NVx-Ch and SE-NVx-Ch groups showed ILTV antibodies, while samples collected from the SE-CEOVx-Ch, SE-TCO-Ch and NVx-NCh groups were negative.
Table 3. ILT ELISA results for sera samples collected before vaccination (4 weeks of age), pre-challenge (8 weeks of age), and 12 d.p.c.
Discussion
It is believed that most of the ILT outbreaks in the USA are caused by vaccine-related isolates that persist in the field. However, in a recent study, some ILTV isolates from the US were characterized as group VI, genetically different from the vaccines (Oldoni & García, Citation2007). The objective of the present study was to evaluate the protection elicited against challenge with a field isolate from group VI after direct and indirect exposure to ILTV live attenuated CEO and TCO vaccines.
In contrast to other challenge studies (Hilbink et al., Citation1987; Fulton et al., Citation2000; Han & Kim Citation2003), clinical signs and mortality were scored every day from day 2 to 12 d.p.c., in order to monitor the length of infection. The protection induced by CEO and TCO vaccines in vaccinated and challenged chickens was demonstrated by assessing clinical signs, mortality, body weight and shedding of the challenge virus to sentinel chickens. No significant differences were observed between vaccinated challenged groups and the non-vaccinated-non-challenged group for clinical signs, mortality, or body weight gain. However, a significant difference was found between non-vaccinated challenged groups and the non vaccinated-non challenged group for the same parameters. No viral DNA was detected, or virus isolated, or antibody response detected in samples collected from sentinel chickens in the CEO or TCO vaccinated challenged groups, indicating significant reduction of the challenge virus shedding. Nevertheless, shedding of the challenge virus was confirmed in samples collected from sentinel chickens from the non-vaccinated challenged group as demonstrated by viral DNA detection, virus isolation, and the presence of ILTV antibodies as early as 12 d.p.c.Therefore CEO and TCO eye drop vaccinations significantly reduced shedding of challenge virus in immunized chickens during the period tested.
There was a correlation between genome copy number equal to or higher than 104.3, successful virus isolation (Rodríguez-Avila et al., Citation2007) and the peak of clinical signs observed post challenge in the non-vaccinated challenged and sentinel chickens.
As reported by Kirkpatrick et al. (Citation2006), body weight was a significant parameter to determine protection in vaccinated chickens. No significant differences were found among groups on the day of vaccination and pre-challenge, indicating that neither bird husbandry nor eye-drop vaccination influenced body weight gain. On the other hand, significant differences were observed up to 12 d.p.c. between the non-vaccinated challenged sentinel chickens and non-vaccinated non-challenged groups. Vaccinated chickens were protected against group VI challenge as demonstrated by clinical signs, mortality and body weight gain, and neither did they shed the challenge virus up to 12 d.p.c.
In previous studies (Gelenczei & Marty, Citation1964; Hilbink et al., Citation1987), chickens exposed contact to vaccinates have been used to assess vaccine spread and protection by demonstrating seroconversion and the presence of neutralizing antibodies. Even though in this study seroconversion and viral DNA detection pre-challenge (4 weeks after vaccination) demonstrated that vaccine viruses were shed to contact-exposed chickens, significant evidence was obtained 12 d.p.c. (clinical signs, mortality and body weight gain) from both contact-exposed groups to indicate that chickens indirectly exposed to vaccines were not protected against group VI challenge virus and, as previously shown (Fahey et al., Citation1983; Fahey & York, Citation1990), despite the detection of antibodies, these chickens were not protected. The lack of protection the is probably the outcome of variation of exposure to airborne transmission and to the quantity and frequency of exposure to the vaccine viruses.
In conclusion, protection induced by CEO and TCO eye-drop vaccination against ILTV group VI genotype virus was demonstrated. Even though this group of viruses is genetically different to the live attenuated vaccines (Oldoni & García, Citation2007), antigenically they appear to be closely related.
In this study, the transmission of the vaccine viruses was confirmed and, based on the parameters utilized to define protection, neither group of contact-exposed chickens was protected against challenge. In particular, this result emphasizes the importance of a uniform vaccination to obtain adequate protection, both to avoid the occurrence of susceptible chickens and to minimize the potential for reversion to virulence of live-attenuated vaccines. Overall, the use of contact-exposed and sentinel chickens was useful to assess transmission and shedding of the vaccine and challenge viruses. Together with clinical signs, mortality and body weight gain this challenge model was a reliable tool to evaluate the protection induced by these live attenuated ILT vaccines. In addition, this challenge model could be applied to evaluate the safety and efficacy of newly developed ILTV vaccines.
Acknowledgements
The authors gratefully acknowledge Dr Roy Berghaus for his collaboration in the statistical analysis and Dr John Glisson for thorough review of this manuscript. The present study was supported by University of Georgia Veterinary Medical Agricultural Research funds.
References
- Andreasen , J.R. Jr , Glisson , J.R. , Goodwin , M.A. , Resurreccion , R.S. , Villegas , P. and Brown , J. 1989 . Studies of infectious laryngotracheitis vaccines: immunity in layers . Avian Diseases , 33 : 524 – 530 .
- Callison , S.A. , Riblet , S.M. , Sun , S. , Jones , K. , Jaramillo , M. , Zavala , G. , Williams , S. , Resurreccion , R. , Spackman , E. and García , M. 2007 . Development and validation of a Real-Time Taqman® PCR assay for the detection of infectious laryngotracheitis virus in poultry . Journal of Virological Methods , 139 : 31 – 38 .
- Davison , A.J. , Eberle , R. , Hayward , G.S. , McGeoch , D.J. , Minson , A.C. , Pellett , P.E. , Roizman , B. , Studdert , M.J. and Thiry , E. 2006 . “ Herpesviridae ” . In Virus Taxonomy: Eighth Report of the International Committee on Taxonomy of Viruses , Edited by: Fauquet , C.M. , Mayo , M.A. , Maniloff , J. , Desselberger , U. and Ball , L.A. 193 – 212 . San Diego , CA : Elsevier Academic Press .
- Davison , S. 2005 . Vaccinal laryngotracheitis—overview in the United States . In Proceedings of the 109th Annual Meeting of the United States Animal Health Association (pp. 580 – 618 ). Hershey, PA, USA .
- Fahey , K.J. and York , J.J. 1990 . The role of mucosal antibody in immunity to infectious laryngotracheitis virus in chickens . Journal of General Virology , 71 : 2401 – 2405 .
- Fahey , K.J. , Bagust , T.J. and York , J.J. 1983 . Laryngotracheitis herpesvirus infection in the chicken: the role of humoral antibody in immunity to a graded challenge infection . Avian Pathology , 12 : 505 – 514 .
- Fulton , R.M. , Schrader , D.L. and Will , M. 2000 . Effect of route of vaccination on the prevention of infectious laryngotracheitis in commercial egg-laying chickens . Avian Diseases , 44 : 8 – 16 .
- Gelenczei , E.F. and Marty , E.W. 1964 . Studies on a tissue-culture modified infectious laryngotracheitis virus . Avian Diseases , 8 : 105 – 122 .
- Guy , J.S. and Bagust , T.J. 2003 . “ Laryngotracheitis ” . In Diseases of Poultry , 11th edn , Edited by: Saif , Y.M. , Barnes , H.J. , Glisson , J.R. , Fadly , A.M. , McDougald , L.R. and Swayne , D. E. 121 – 134 . Ames : Iowa State Press .
- Guy , J.S. , Barnes , H.J. , Munger , L.L. and Rose , L. 1989 . Restriction endonuclease analysis of infectious laryngotracheitis viruses: comparison of modified-live vaccine viruses and North Carolina field isolates . Avian Diseases , 33 : 316 – 323 .
- Han , M.G. and Kim , S.J. 2003 . Efficacy of live virus vaccines against infectious laryngotracheitis assessed by polymerase chain reaction-restriction fragment length polymorphism . Avian Diseases , 47 : 261 – 271 .
- Hilbink , F.W. , Oei , H.L. and Van Roozelaar , D.J. 1987 . Virulence of five live virus vaccines against infectious laryngotracheitis and their immunogenicity and spread after eyedrop or spray application . Veterinary Quarterly , 9 : 215 – 225 .
- Hughes , C.S. , Gaskell , R.M. , Jones , R.C. , Bradbury , J.M. and Jordan , F.T.W. 1989 . Effects of certain stress factors on the re-excretion of infectious laryngotracheitis virus from latently infected carrier birds . Research in Veterinary Science , 46 : 247 – 276 .
- Kawaguchi , T. , Nombra , K. , Hirayama , Y. and Kitagawa , T. 1987 . Establishment and characterization of a chicken hepatocellular carcinoma cell line, LMH . Cancer Research , 47 : 4460 – 1164 .
- Keeler , C.L. , Hazel , J.W. , Hastings , J.E. and Rosenberger , J.K. 1993 . Restriction endonuclease analysis of Delmarva field isolates of infectious laryngotracheitis virus . Avian Diseases , 37 : 418 – 426 .
- Keller , L.H. , Benson , C.E. , Davison , S. and Eckroade , R.J. 1992 . Differences among restriction endonuclease DNA fingerprints of Pennsylvania field isolates, vaccine strains and challenge strains of infectious laryngotracheitis virus . Avian Diseases , 36 : 575 – 581 .
- Kirkpatrick , N.C. , Mahmoundian , A. , Colson , C.A. , Devlin , J.M. and Noormohammadi , A.H. 2006 . Relationship between mortality, clinical signs and tracheal pathology in infection laryngotracheitis . Avian Pathology , 35 : 449 – 453 .
- Oldoni , I. and García , M. 2007 . Characterization of infectious laryngotracheitis virus (ILTV) isolates from United States by polymerase chain reaction and restriction fragment length polymorphism (PCR-RFLP) of multiple genome regions . Avian Pathology , 36 : 167 – 176 .
- Reed , L.J. and Muench , H. 1938 . A simple method for estimating fifty percent endpoints . American Journal of Hygiene , 27 : 493 – 497 .
- Rodríguez-Avila , A. , Oldoni , I. , Riblet , S.M. and García , M. 2007 . Replication and transmission of live-attenuated Infectious laryngotracheitis virus (ILTV) vaccines . Avian Diseases , 51 : 905 – 991 .