Abstract
The objective of this work was to examine the impact of subclinical coccidial infection on commercial performance, expressed as a modified European Production Index, in broilers. Performance data, and litter and faecal samples, were collected from two independent observational surveys of Norwegian broilers receiving in-feed narasin during 2000 to 2004. Numbers of oocysts per gram (OPG) of litter collected during rearing (Study 1) or faecal samples collected at slaughter (both studies), and relative frequencies of Eimeria species categories (both studies) were calculated. Polymerase chain reaction-based identification of Eimeria species was performed in Study 2. A definition of flocks at risk of impaired performance associated with coccidia (“risk flock”), using the predominant species and OPG level as criteria, was tested. Coccidia had a significant effect on performance in the first, but not the second study. In Study 1 the following coccidia variables were found to be associated with impaired performance in multivariate models: OPG at slaughter (ordinal), mean OPG during rearing (ordinal) and “risk flock” (binomial). The European Production Index was ∼9% lower in flocks with infection levels >50 000 OPG at slaughter in Study 1. The composition of coccidial populations shifted between Study 1 and Study 2, from a dominance of medium and large oocysts to a dominance of small oocysts. There was a substantial increase in prevalence of coccidial infection from Study 1 to Study 2, but mean infection levels were similar in the two surveys. The “risk flock” definition was useful as an indicator of coccidia-associated performance loss in Study 1, where subclinical coccidiosis was an important factor. The results suggest that the economic importance of subclinical coccidiosis may vary substantially with time, and they emphasize the need for population studies on the importance and dynamics of specific coccidial infections under different field conditions.
Enquête sur l'impact économique des infections sub-cliniques à Eimeria chez les poulets de chair en Norvège
L'objectif de ce travail a été d'examiner l'impact des infections coccidiennes sub-cliniques sur les performances commerciales, exprimées en Index de Production Européen modifié (EPI), chez les poulets de chair. Les données des performances ont été enregistrées, et des échantillons fécaux et de litière ont été prélevés lors de deux enquêtes indépendantes réalisées chez les poulets de chair norvégiens recevant du narasin dans l'aliment au cours de la période 2000–2004. Le nombre d'oocytes par gramme (OPG) de litière prélevée durant la période d’élevage (étude 1) ou les échantillons de fèces prélevés à l'abattage (pour les deux études), et les fréquences relatives d'espèces d’Eimeria (pour les deux études) ont été calculés. L'identification des espèces d’Eimeria basée sur la PCR à été réalisée dans l’étude 2. Une définition des troupeaux à risque avec des performances dégradées associées à des coccidies (“troupeau à risque”), prenant comme critères les espèces prédominantes et le niveau d'OPG a été testée. Les coccidies ont eu un effet significatif sur les performances dans la première étude, mais pas dans la seconde. Dans l’étude 1, les variables relatives aux coccidies associées aux performances dégradées dans des modèles multivariables ont été les suivantes: les OPG à l'abattage (ordinal), la moyenne des OPG durant la période d’élevage (ordinal) et le troupeau à risque (binomial). L'EPI a été de ∼9% inférieur dans les troupeaux avec des niveaux d'infection dont l'OPG était >50.000 à l'abattage dans l’étude 1. La composition des populations coccidiennes a varié entre l’étude 1 et l’étude 2, d'une dominance d'oocytes grands et moyens à une dominance de petits oocytes. Il y a eu une augmentation substantielle de la prévalence des niveaux d'infection coccidienne de l’étude 1 à l’étude 2, mais les niveaux moyens d'infection ont été similaires dans les deux enquêtes. La définition du “troupeau à risque” a été utile comme indicateur de la perte de performance associée aux coccidies dans l’étude 1, où la coccidiose sub-clinique a été un facteur important. Les résultats suggèrent que l'importance économique de la coccidiose sub-clinique peut varier substantiellement avec le temps, et soulignent le besoin pour les études de population de l'importance et des dynamiques des infections coccidiennes spécifiques dans les différentes conditions du terrain.
Übersichtsstudie zur ökonomischen Bedeutung von subklinischen Eimeria-Infektionen in Broilerherden in Norwegen
Ziel dieser Studie war es, die Bedeutung subklinischer Kokzidieninfektionen auf die Wirtschaftlichkeit der Broilerproduktion ausgedrückt als modifizierter europäischer Produktionsindex (EPI) zu untersuchen. Dazu wurden zwischen 2000 und 2004 bei norwegischen Broilern, die Narasin-haltiges Futter erhielten, die Leistungsdaten erfasst sowie Einstreu und Kotprobenuntersuchungen in zwei unabhängigen Reihenuntersuchungen durchgeführt. Die Zahl der Oozysten pro Gramm (OPG) während der Mastperiode eingesammelter Einstreu (Studie 1) oder zum Zeitpunkt der Schlachtung entnommener Kotproben (beide Studien) wurde berechnet. In Studie 2 wurde die Identifikation von Eimeriaspezies mittels PCR vorgenommen. Die Befunde aus den Herden wurden hinsichtlich des Risikos einer durch Kokzidien beeinträchtigten Leistung (,,Risikoherden“) mit vorherrschender Eimeriaspezies und OPG als Kriterien statistisch bewertet. Kokzidien hatten in der ersten, aber nicht in der zweiten Studie einen signifikanten Effekt auf die Mastleistung. In Studie 1 wurden in einem multivariablen Modell folgende Kokzidien-Variable im Zusammenhang mit beeinträchtigter Leistung gefunden: OPG bei der Schlachtung (ordinal), mittlere OPG während der Aufzucht (ordinal) und ,,Risikoherde“ (binominal). In Studie 1 war der EPI in Herden mit einem Infektionsgrad von >50000 OPG bei der Schlachtung etwa 9 % geringer. Die Zusammensetzung der Kokzidienpopulationen veränderte sich zwischen Studie 1 und 2, und zwar von einer Dominanz der mittleren und großen Oozysten zu einer Dominanz von kleinen Oozysten. Es gab einen erheblichen Anstieg im Vorkommen von Kokzidieninfektionen von Studie 1 zu Studie 2, aber der mittlere Infektionsgrad war in beiden Studien gleich. Die Begriff ,,Risikoherde“ war als Indikator für Kokzidien-bedingte Leistungseinbußen in Studie 1 nützlich, wobei subklinische Kokzidiose ein wichtiger Faktor war. Die Ergebnisse legen nahe, dass die ökonomische Bedeutung der subklinischen Kokzidiose zeitlich gesehen beträchtlich variieren kann, und sie unterstreicht die Notwendigkeit von Populationsstudien zur Bedeutung und Dynamik von spezifischen Kokzidieninfektionen unter verschiedenen Feldbedingungen.
Estudio del impacto económico de las infecciones subclínicas de Eimeria en pollos broiler en Noruega
El objetivo de este estudio fue determinar el impacto de la coccidiosis subclínica en el rendimiento comercial de los broilers, expresado según el Índice de Producción Europeo (EPI) modificado. Se obtuvieron datos productivos y muestras de cama y de heces en dos estudios observacionales independientes en broilers noruegos que recibían narasina en pienso durante el 2000–2004. Se calculó el número de ooquistes por gramo (OPG) de cama obtenida durante la cría (estudio 1) o en muestras de heces obtenidas en el matadero (ambos estudios) y la frecuencia relativa de las especies de Eimeria (ambos estudios). En el estudio 2 se realizó una identificación de las especies de Eimeria mediante PCR. Se testó una definición de lotes en riesgo de rendimiento desequilibrado asociado a coccidia (“lote en riesgo”) a través del uso de especies predominantes y niveles de OPG como criterios. La coccidiosis tuvo un efecto significativo en el rendimiento en el primer estudio pero no en el segundo. En el estudio 1 se observó una asociación entre las siguientes variables de coccidiosis y un rendimiento alterado en modelos multivariables: OPG al matadero (ordinal), media de OPG durante la cría (ordinal) y el “lote en riesgo” (binomial). El EPI fue ∼9% menor en los lotes con niveles de infección >50,000 OPG en el matadero en el estudio 1. La composición de las poblaciones de coccidia varió entre el estudio 1 y el estudio 2, de una dominancia de ooquistes medianos y grandes a una dominancia de ooquistes pequeños. Se observó un incremento sustancial en la prevalencia de coccidiosis entre el estudio 1 y el estudio 2, pero los niveles medios de infección fueron similares en ambos estudios. La definición de “lote en riesgo” fue útil como un indicador de pérdidas productivas asociadas a coccidiosis en el estudio 1, en el cual la coccidiosis subclínica fue un factor importante. Los resultados sugieren que la importancia económica de las coccidiosis subclínica puede variar sustancialmente con el tiempo, y señalan la necesidad de estudios de población de la importancia y dinámica de las infecciones específicas por coccidia bajo distintas condiciones de campo.
Introduction
Coccidiosis in chickens is a complex disease caused by one or more of several Eimeria species. The Eimeria species are obligate microscopic intracellular parasites (phylum Apicomplexa) with site-specific habitats in the gut of chickens. Relative terms used both in the field and in the laboratory comprise clinical coccidiosis (clinically sick birds), subclinical coccidiosis (apparently disease-free birds that nevertheless have impaired commercial performance), and coccidiasis (lightly infected birds exhibiting normal health and performance). As a result of the development of effective prophylactic drugs, clinical outbreaks are considered rare in modern poultry production today; however, subclinical coccidiosis is still considered one of the most significant problems in the industry.
Accurate estimations of the effects of coccidial infections are difficult to achieve because of substantial differences in clinical impact due to the different Eimeria species (Braunius, Citation1980; Graat et al., Citation1996; Williams, Citation1999, Citation2006a). The occurrence of different species combinations and the intensity of infection vary considerably, both globally and locally (Oikawa et al., Citation1979; Braunius, Citation1986; McDougald et al., Citation1986, Citation1997; Williams et al., Citation1996; Graat et al., Citation1998; Al Natour et al., Citation2002; Haug et al., Citation2008) and with time (Braunius, Citation1986; Hamet, Citation1986; Peeters et al., Citation1994; Haug et al., Citation2008). Furthermore, husbandry and prophylactic regimes also vary. Therefore, finding a threshold level in infection intensity from which a negative impact on broiler chicken performance might be predicted, has proven difficult (Braunius, Citation1980; Hamet et al., Citation1985; Graat et al., Citation1996; Williams, Citation2006a).
The objective of this study was to study the impact of subclinical coccidial infection on commercial performance, expressed as European Production Index (EPI), in commercial broilers in Norway. We wanted to investigate the relationships between the EPI and the following aspects of coccidial infection; the infection intensity measured as total numbers of oocysts per gram (OPG) of litter or faeces, the relative frequency of oocyst length categories, and the presence of different Eimeria species and their combinations. In addition, a method of predicting flocks at risk of production impairment associated with coccidial infection intensity was proposed and was tested against commercial performance. The data were collected from two independent observational studies of broiler chickens in Norway covering separate time spans during 2000 to 2004.
Materials and Methods
Norwegian broiler industry
The Norwegian broiler industry is concentrated in three regions in the southern half of the country (Haug et al., Citation2008, ). The industry is small scaled compared with that of other industrial countries, with approximately 550 broiler chicken farms, rearing about 45 million chickens annually. A nationwide farm-owned cooperative has 85% of the market share of broiler meat production. This cooperative manages a central database (“productivity control”) that records the commercial performances of all broiler flocks.
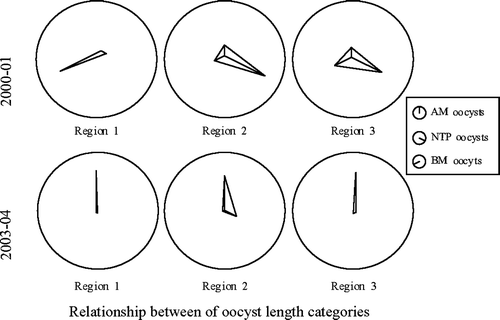
Figure 1. Distribution of oocysts by length categories in Study 1 (2000/01) and Study 2 (2003/04). The length of the pointer indicates mean relative frequency of respective oocyst category.
The chickens in the present study were reared on wood shavings on concrete floors, in insulated broiler houses made of wood, metal or concrete. The indoor climate was regulated by heating systems (in-floor heating, electric heaters or hot air systems), and a mechanical ventilation system (positive-pressure or negative-pressure systems) controlled by a climate computer. Automatic cup feeders and nipple drinkers were standard.
Most broiler flocks were raised to approximately 31 days. The most common commercial hybrid reared during the study period was Ross 208, but some rearing of Cobb 500 also occurred. According to Norwegian legislation, the maximum bird density allowable is 34 kg live weight/m2, corresponding to about 23 birds/m2 on the day of slaughter. Average performance in a typical broiler flock slaughtered in 2004 was a feed consumption of 2.27 kg/kg carcass weight (corresponding to 1.35 kg feed/kg live weight, based on an expected mean carcass yield of 62%) at a carcass weight of 1.04 kg, and 2.30% accumulated mortality during rearing (data from Centre for Poultry Science, Oslo, Norway).
The feed consisted of pellets (starter ∼day 0 to 10, grower ∼day 11 to 26 and finisher ∼day 26 to slaughter) with main ingredients of wheat, oat and soy flour, sometimes combined with whole wheat. In-feed anticoccidial drugs were used prophylactically until 5 days prior to slaughter. Narasin was used almost exclusively in this study, and polyether ionophores have dominated since 1988. Anticoccidial vaccines were not used in flocks included in this study, and antibacterial growth promoters have not been used in Norway since 1995.
Between successive grow-outs, used litter was removed and the broiler houses were cleaned and usually chemically disinfected (type of disinfectant not recorded).
Study population and sample collection
Two observational field studies were conducted during April 2000 to December 2001 (Study 1) and during December 2003 to November 2004 (Study 2).
In Study 1, litter and faecal samples were collected from 85 commercial broiler farms (one flock per house and farm), selected by stratified random sampling from the total Norwegian broiler population. Litter samples were collected when birds were approximately 20 and 26 days of age, and faecal samples on the day of slaughter. In Study 2, faecal samples were collected on the day of slaughter from 98 commercial broiler flocks (one flock per house and farm), selected by simple random sampling. Broilers flocks slaughtered after 36 days of age and at private abattoirs, and flocks with incomplete performance data, were excluded from the study. Hence, 58 and 86 flocks were finally included in Studies 1 and 2, respectively.
The litter samples in Study 1 were collected from five evenly distributed areas of the house after dividing the house into 10 sectors, and the faecal samples were collected from 10 different places in the transport containers at the slaughterhouse, as previously described by Haug et al. (Citation2008).
Determination of infection level and classification of oocysts
The infection level, measured as OPG of a sample, was determined using a standard McMaster technique as previously described by Haug et al. (Citation2008). Using a calibrated ocular micrometer at 400x magnification (Long & Reid, Citation1982), 50 random oocysts from each sample were measured and categorized into three groups: an AM group (small oocysts, ≤18.8 µm long; presumptively Eimeria acervulina and/or Eimeria mitis), an NTP group (medium-sized oocysts, 18.9 to 23.8 µm long; presumptively Eimeria necatrix, Eimeria tenella and/or Eimeria praecox) or a BM group (large oocysts, ≥23.9 µm long; presumptively Eimeria brunetti and/or Eimeria maxima).
Identification of Eimeria species by polymerase chain reaction
A total of 51 faecal samples from Study 2 were tested by polymerase chain reaction (PCR). To be able to identify any Eimeria species present in only very small numbers in mixed infections, only samples containing approximately 100 000 oocysts/50 µl test sample were selected for testing. The DNA preparation and PCR were performed as previously described by Haug et al. (Citation2007, Citation2008). Oocysts were ruptured by pestle grinding, DNA was extracted using a modified Gene-Releaser protocol and the Eimeria species were identified by PCR using species-specific primers targeting ITS-1. The numbers of species detected and the species combinations were recorded.
Definition of flocks at risk
A theoretical threshold for flocks at risk was defined based on two properties; predominant species category and OPG level at slaughter. The following criteria were used to define flocks at risk; the infection levels exceeded 50 000 OPG of faeces, and >50% of the oocysts measured belonged to the NTP and/or BM group. Additionally, in Study 1 an alternative definition of flocks at risk, based on the average OPG of the three samples (litter and faeces) taken during rearing, was also tested. Flocks at risk were termed “risk flocks”, and flocks not at risk were termed “non-risk flocks”.
Commercial performance
Performance data were collected from on-farm records and from reports from the productivity control database. Data on the following variables were collected: flock size, weight on day 0, slaughter age, carcass weight, feed conversion ratio (FCR), mortality, hatchery, hybrid and percentage carcass condemnations due to liver lesions. Carcass condemnations due to liver lesions were used as an indicator of necrotic enteritis as previously described by Løvland & Kaldhusdal (Citation1999).
As a means of evaluating commercial performance, a modification of the EPI previously described by Teilen (Citation1970) was calculated as follows:
Statistical analysis
Initial descriptive statistics was performed using the statistical package JMP 5.0.1 (SAS Institute Inc.). These analyses consisted of tabular and graphical examination of data and univariate analyses (Student's t test, Fisher's exact test, or Wilcoxon or Kruskal–Wallis tests, and linear regression) to identify possible associations between the EPI and different explanatory variables. The following explanatory variables were tested; coccidial infection (binomial), OPG at slaughter (continuous), ordinal OPG at slaughter and ordinal mean OPG during rearing (0 = below detection limit; 1 = 100 to 999; 2 = 1000 to 9999; 3 = 10 000 to 49 999; and 4 = > 50 000), mean OPG during rearing, number of Eimeria species present, Eimeria species combinations encountered in this study ordered after potential pathogenicity (0 = coccidia-negative flocks; 1 = E. acervulina; 2 = E. acervulina and E. maxima or E. acervulina, E. maxima and E. praecox; 3 = E. acervulina and E. tenella or E. acervulina, E. tenella and E. praecox; 4 = E. acervulina, E. maxima and E. tenella or E. acervulina, E. maxima, E. tenella and E. praecox), flock size (continuous), weight on day 0 (continuous), level of liver condemnations (continuous), slaughter age (continuous), region (nominal) and season of sample collection (binomial). In all of the introductory analyses, the two studies were assessed separately. Differences between the studies were also tested using Student's t test, Fisher's exact test, or corresponding non-parametric tests (Wilcoxon or Kruskal–Wallis tests).
Data from Study 1 were used to assess the relationship between the EPI and the study variables in multivariate models by linear regression using Stata SE/10 for Windows (Stata Corp LP, College Station, Texas, USA). Three models (impact of coccidial infection based on one or three sampling occasions, and impact of “risk flock”) were constructed using a backward stepwise regression approach from a full model containing all explanatory variables listed above, and confirmed by a forward stepwise regression procedure as described by Dohoo et al. (Citation2003). The models were compared using the adjusted R 2 value and each variable by the Wald test. Model fit was assessed using standard graphical techniques (plotting residuals versus explanatory variables, leverage plots and the normal quantile plot of residuals).
Finally, a model constructed on the basis of data from Study 1 was tested with data from Study 2.
Results
Comparison of data from Studies 1 and 2
All trends in the epidemiology of the coccidial infection found in the flocks included in this study are in agreement with the trends found in the original study populations (compare Haug et al., Citation2008 and ).
Table 1. Studies 1 and 2: basic data and significance of the differences between studies in univariate analysis
There were substantial differences between the studies in this survey (). Performance, expressed as the EPI, showed an approximately 25% increase within the time frame of the two studies. There was a considerable overlap in EPI ranges between the studies; however, only 25% of the flocks in Study 2 contributed to this overlap. The relative frequency of oocysts of the different length categories also varied significantly between the two study periods ( and ). In Study 1, only two (9.5%) of the infected flocks were dominated by small oocysts, compared with about 75% of the flocks in Study 2 (data not shown). Because of these differences, the two surveys were analysed separately.
There were substantial regional differences in distribution of oocyst length categories within and between studies (), but only insignificant differences were observed at different infection levels (data not shown).
There was a significant difference between the surveys in the seasonal distribution of flocks. Whereas >90% of the flocks in Study 2 were slaughtered during the summer, the corresponding figure for Study 1 was 36%. The mean weight at day 0 was higher in 2003/04 compared with 2000/01, and the carcass condemnations due to liver lesions were decreased.
Impact of coccidial infection on commercial performance
Univariate analysis showed a considerable decrease in EPI in coccidia-infected broiler flocks compared with the non-infected in Study 1 (). Using ordinal infection levels as explanatory variables, two multivariate regression models were obtained with P(F) ≤ 0.0001 for both models ( and ). In one model (), the explanatory variable was based on one sample collected at slaughter; in the other (), on the average of three samples collected during rearing. Both models showed a significant decrease in EPI in flocks with infection levels above 50 000 OPG ( and , and ). Based on sampling at slaughter only, the EPI was ∼8.9% lower in flocks with infection levels above 50 000 compared with coccidia-negative flocks. The corresponding estimate based on the average OPG level from three samples collected during rearing was ∼7.7%. Region and slaughter age were also shown to have significant effects on the EPI. Approximately 36% and 35% of the variation in the outcome variable (i.e. adjusted R 2 value) could be explained by the explanatory variables, respectively ( and ).
Figure 2. Association between the EPI and coccidial infection levels in Study 1 (2000/01) and Study 2 (2003/04), respectively. The infection level categories were 0 = below detection limit; 1 = 100 to 999; 2 = 1000 to 9999; 3 = 10 000 to 49 999; and 4 = > 50 000. The box indicates the 25th, 50th and 75th percentiles, and the whiskers the 2.5th and 97.5th percentiles.
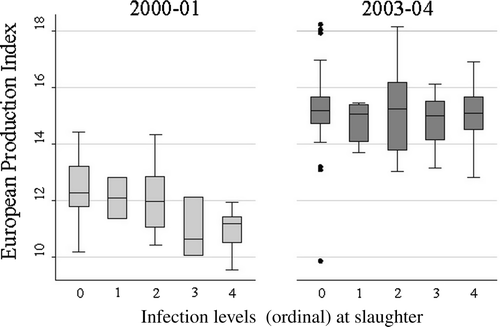
Table 2. Studies 1 and 2: test statistics for univariate analysis with EPI as the test outcome
Table 3. Study 1: test statistics for multivariate analysis of the impact of coccidial infection, based on a single faecal sample collected at slaughter, on the EPI
Table 4. Study 1: test statistics for multivariate analysis of the impact of coccidial infection, based on the average of three samples taken during rearing, on the EPI
The overall trend in the univariate analysis in Study 2 showed a consistent but modest decline in EPI in coccidia-infected flocks compared with non-infected flocks (). However, the multivariate model based on data from Study 1, using ordinal infection levels at slaughter as well as region and slaughter age as explanatory variables, showed no significant effect of coccidial infection on the EPI in Study 2.
Evaluation of the definition of risk flocks
There were 8.6% risk flocks at slaughter in Study 1, compared with 2.3% in Study 2 (). Defining risk flocks based on the average of three samples collected during rearing in Study 1 increased the frequency to 22.4% (). The EPI of risk flocks in Study 1 was significantly lower than that for non-risk flocks (). This was regardless of whether the risk flocks were identified based on one or three samples per flock (data not shown). Based on data from three sampling occasions per flock, the EPI was ∼7.2% lower in risk flocks compared with non-risk flocks. The fitted regression model explained approximately 37% (i.e. adjusted R 2 value) of the variability in the EPI (P(F) > 0.0001) (). The adjusted R 2 value was approximately 32% when defining risk flocks based on one sample (P(F) > 0.0001) (results not shown).
Table 5. Study 1: test statistics for multivariate analysis of the impact of being classified as a risk flock, based on three samples collected during rearing, on the EPI
The number of risk flocks in Study 2 was too low for statistical analysis.
Discussion
This study was performed to gain further knowledge about the impact of subclinical coccidiosis in broilers on commercial performance. We report the results of two independent observational studies conducted in 2000/01 and in 2003/04, respectively, in the same population of broiler chickens in Norway.
The data revealed a considerable difference between the studies with regard to the association between coccidial infection and commercial flock performance. Subclinical coccidiosis had a statistically significant effect on the performance of Norwegian broilers in 2000/01, but no apparent effect was found in the study conducted a few years later.
Another distinct difference between the two studies was the change in Eimeria species composition, from a majority of medium and large-sized oocysts in 2000/01 to small-sized oocysts in 2003/04 (). Similar shifts towards small oocysts were previously reported (for example, Braunius, Citation1986; Hamet, Citation1986; Peeters et al., Citation1994; Stayer et al., Citation1995). Ionophores have been used exclusively as in-feed anticoccidials in this broiler population since 1988, and narasin has completely dominated since the beginning of 1996, Braunius (Citation1986), Hamet (Citation1986) and Peeters et al. (Citation1994) observed increases in the frequency of small oocysts after prolonged use of a single anticoccidial drug. Their observations support an assumption that the prophylactic regime could be a factor in the apparent shift in Eimeria species predominance in this study.
The shift towards small oocysts might explain the rapidly changing role of coccidia from a factor causing up to 9% reduction of the EPI, to a factor apparently without significant effect on productivity. Small oocysts originate from less pathogenic species than those associated with medium-sized and large oocysts (Conway et al., Citation1993). Based on data from Study 2, E. acervulina was the only “small oocyst” species likely to be found in this broiler population. E. acervulina possesses a high reproductive potential (Brackett & Bliznick, Citation1952; Williams, Citation2001) and the ability to suppress the fecundity of other Eimeria species (Williams, Citation1973). E. tenella and E. maxima were the most prevalent species associated with medium-sized and large oocysts, respectively. Our data therefore suggest that a shift from the more pathogenic E. tenella and E. maxima to the less harmful E. acervulina caused the apparent reduction of the adverse effect exerted by coccidia on commercial performance.
A third major difference between the two studies was the flock prevalence of coccidial infection (). The low levels of coccidial infection in Study 1 had a minor influence on performance, whereas infection levels above 50 000 OPG (and possibly levels above 10 000 OPG; ) reduced the EPI substantially. These results suggest that the increasing prevalence of coccidia in Study 2 may have been of minor importance because it was largely caused by low-level infections with a predominance of E. acervulina.
A possible contribution factor to the difference in effect found in the two surveys might be the difference in occurrence of liver condemnations, suggesting a decreasing level of Clostridium perfringens-associated subclinical necrotic enteritis from Study 1 to Study 2. Løvland & Kaldhusdal (Citation1999) found that the incidence of liver condemnation varied between approximately 0.02% and approximately 0.20%. The lowest levels coincided with the lowest levels of necrotic enteritis in the same population, and the highest levels coincided with peak levels of necrotic enteritis epidemics. Subclinical necrotic enteritis has been shown to impair the FCR and growth (Kaldhusdal & Hofshagen, Citation1992). It is therefore conceivable that a drop in mean liver condemnations from 0.11% to 0.07% contributed to the improved performance found in Study 2. However, we were not able to statistically confirm such an impact of the percentage liver condemnation on the EPI in the multivariate statistical model.
Some of the observed differences between Study 1 and Study 2 may be due to biased sampling rather than to true changes in the population. Bias may be introduced if a population is sampled twice, owing to chance alone. The slight difference in sampling technique could also introduce a bias. Also, the non-random way of selecting flocks from the original study populations could have added to the bias. The remarkably large difference in EPI between the studies also raises the question of farm selection bias. However, data from the central productivity control data base showed almost identical commercial performances in the study sample and the background population during the time period 2000/01 and only a 2.3% higher mean EPI in the study sample compared with the background population during 2003/04.
To meet the need of a tool to evaluate the impact of coccidiosis and to plan the course of measures against the disease, we defined flocks at risk using the predominant species (NTP + BM > 50%) and the OPG level (>50 000) as criteria. The OPG level criterion for defining risk flocks was based on practical experience gathered at the laboratory at the National Veterinary Institute in Sweden (Per Thebo, personal communication). Hamet et al. (Citation1985) also found that, on average, an infection level of 50 000 OPG (faeces collected from 4-week-old broiler chickens) constituted a threshold for influence of oocyst output on performance. However, in another detailed study, no association between OPG > 50 000 and performance was found (Hamet et al., Citation1985).
Our findings from Study 1 indicate that risk flock may be used as an indicator of likely production loss in a broiler population. However, an ordinal OPG level variable was equally useful as an indicator of production loss, and both models explained the EPI variation about equally well ( and ). In Study 1, there were rather few flocks with predominance of small oocysts. This could possibly preclude the full exploitation of including oocyst length information in the indicator.
Based on repeated sampling during the grow-out, the status of a risk flock often did not last throughout the rearing period. Although it is more inconvenient to collect and process multiple samples, this definition probably gives a better measure of risk than a snap-shot picture based only on one sample. The indicator based on three sampling occasions was able to identify substantially more risk flocks (22%) in Study 1 than the indicator based on sampling at slaughter only (9%) (). In this way, the former indicator could increase the likelihood of detecting the effect of coccidiosis on performance.
The risk flock indicator could not be evaluated in Study 2, due to the low frequency of risk flocks (3% of coccidia-positive flocks). This low frequency agrees well with the finding that coccidial infection was not significantly associated with performance loss in Study 2. However, full validation of the application of the risk flock indicator is needed.
Numerous reports have been published on the increasing incidence of ionophore tolerance and cross-resistance between ionophores (for example, Hamet, Citation1986; Stallbaumer & Daisy, Citation1988; Bedrník et al., Citation1989; Raether & Paeffgen, Citation1989; Peeters et al., Citation1994; Stephan et al. Citation1997). With the exclusive use of ionophores as a means of anticoccidial control for almost two decades in the Norwegian broiler population, one might expect a decline in commercial performance rather than an improvement due to development of ionophore-tolerant coccidia in the latter study. On the other hand, the change observed in this work may represent only a transitory phase of the coccidial dynamics in the broiler population. The results showed a shift to less pathogenic species and an increase in flock prevalence, but no increase in infection levels. Anticoccidial ionophores do not prevent parasite replication completely (referred to as oocyst leakage) (Ryley & Wilson, Citation1975; Chapman & Johnson, Citation1992). An increased oocyst leakage due to drug tolerance could possibly induce a stronger immunity, and thus prevent not only adverse effects on commercial performance, but also elevated oocyst production and increased contamination of the environment (Williams, Citation2006b) . Repeated follow-up studies of broiler populations treated continuously with one type of anticoccidial would be most useful in order to gain more insight into the population dynamics of coccidial infections and their impact on commercial performance.
Acknowledgements
The authors would like to thank PRIOR Norway for providing the flock data and performance data for this study, and Youssef Rohoma, Reidun Bolstad and Per Thebo for technical assistance in the laboratory. Scientific discussions and assistance in language by Ray Williams is gratefully acknowledged. This work has been supported by grants from The Research Council of Norway.
References
- Al Natour , M.Q. , Suleiman , M.M. and Abo-Shehada , M.N. 2002 . Flock-level prevalence of Eimeria species among broiler chicks in northern Jordan . Preventive Veterinary Medicine , 53 : 305 – 310 .
- Bedrník , P. , Jurkovic , P. , Kucera , J. and Firmanova , A. 1989 . Cross resistance to the ionophorous polyether anticoccidial drugs in Eimeria tenella isolates from Czechoslovakia . Poultry Science , 68 : 89 – 93 .
- Brackett , S. and Bliznick , A. 1952 . The reproductive potential of five species of coccidia of the chicken as demonstrated by oocyst production . Journal of Parasitology , 38 : 133 – 139 .
- Braunius , W.W. 1980 . Clinical aspects, detection methods and the damage caused by coccidiosis in broilers . Archiv für Geflügelkunde , 44 : 99 – 104 .
- Braunius , W.W. 1986 . “ Incidence of Eimeria species in broilers in relation to the use of anticoccidial drugs ” . In Research in Avian Coccidiosis, Proceedings of the Georgia Coccidiosis Conference , Edited by: McDougald , L.R. , Joyner , L.P. and Long , P.L. 409 – 414 . Athens, GA, USA .
- Chapman , H.D. and Johnson , Z.B. 1992 . Oocysts of Eimeria in the litter of broilers reared to eight weeks of age before and after withdrawal of lasalocid or salinomycin . Poultry Science , 71 : 1342 – 1347 .
- Conway , D.P. , Sasai , K. , Gaafar , S.M. and Smothers , C.D. 1993 . Effects of different levels of oocyst inocula of Eimeria acervulina, E. tenella, and E. maxima on plasma constituents, packed cell volume, lesion scores, and performance in chickens . Avian Diseases , 37 : 118 – 123 .
- Dohoo , I. , Martin , W. and Stryhn , H. 2003 . Veterinary Epidemiologic Research , Charlottetown : University of Prince Edward Island .
- Graat , E.A. , Ploeger , H.W. , Henken , A.M. , De Vries , R.G. , Noordhuizen , J.P. and Van Beek , P.N. 1996 . Effects of initial litter contamination level with Eimeria acervulina on population dynamics and production characteristics in broilers . Veterinary Parasitology , 65 : 223 – 232 .
- Graat , E.A. , van der Kooij , E. , Frankena , K. , Henken , A.M. , Smeets , J.F. and Hekerman , M.T. 1998 . Quantifying risk factors of coccidiosis in broilers using on-farm data based on a veterinary practice . Preventive Veterinary Medicine , 33 : 297 – 308 .
- Hamet , N. 1986 . “ Resistance to anticoccidial drugs in poultry farms in France from 1975 to 1984 ” . In Research in Avian Coccidiosis, Proceedings of the Georgia Coccidiosis Conference , Edited by: McDougald , L.R. , Joyner , L.P. and Long , P.L. 415 – 420 . Athens, GA, USA .
- Hamet , N. , Josse , J. , Robin , B. and Toucas , L. 1985 . An epidemiological investigation into coccidiosis and drug resistance in broiler chickens . World's Poultry Science Journal , 41 : 210 – 225 .
- Haug , A. , Thebo , P. and Mattsson , J. G. 2007 . A simplified protocol for molecular identification of Eimeria species in field samples . Veterinary Parasitology , 146 : 35 – 45 .
- Haug , A. , Gjevre , A. , Thebo , P. , Mattsson , J.G. and Kaldhusdal , M. 2008 . Coccidial infections in commercial broilers; epidemiological aspects and comparison of Eimeria species identification by morphometric and PCR techniques . Avian Pathology , 37 : 161 – 170 .
- Kaldhusdal , M. and Hofshagen , M. 1992 . Barley inclusion and Avoparcin supplementation in broiler diets. 2. Clinical, pathological, and bacteriological findings in a mild form of necrotic enteritis . Poultry Science , 71 : 1145 – 1153 .
- Long , P.L. and Reid , W.M. 1982 . A guide for the diagnosis of coccidiosis in chickens Research Report 404, University of Georgia, College of Agriculture, Experiment Stations, GA, USA
- Løvland , A. and Kaldhusdal , M. 1999 . Liver lesions seen at slaughter as an indicator of necrotic enteritis in broiler flocks . FEMS Immunology & Medical Microbiology , 24 : 345 – 351 .
- McDougald , L.R. , Fuller , L. and Solis , J. 1986 . Drug-sensitivity of 99 isolates of coccidia from broiler farms . Avian Diseases , 30 : 690 – 694 .
- McDougald , L.R. , Fuller , L. and Mattiello , R. 1997 . A survey of Coccidia on 43 poultry farms in Argentina . Avian Diseases , 41 : 923 – 929 .
- Oikawa , H. , Kawaguchi , H. , Katagiri , K. and Nakamoto , K. 1979 . Incidence of chicken coccidia from broiler houses in Japan, 1973–1977. Zentralblatt für Bakteriologie, Mikrobiologie und Hygiene . Originale A] , 244 : 339 – 344 .
- Peeters , J.E. , Derijcke , J. , Verlinden , M. and Wyffels , R. 1994 . Sensitivity of avian Eimeria spp. to seven chemical and five ionophore anticoccidials in five Belgian integrated broiler operations . Avian Diseases , 38 : 483 – 493 .
- Raether , W. and Paeffgen , D. 1989 . “ Drug sensitivity of coccidia recently selected from broiler farms in Europe ” . In Coccidia and Intestinal Coccidiomorphs, Proceedings of the Vth International Coccidiosis Conference , Edited by: Yvoré , P. 309 – 312 . Tours, France .
- Ryley , J.F. and Wilson , R.G. 1975 . Laboratory studies with some recent anticoccidials . Parasitology , 70 : 203 – 222 .
- Stallbaumer , M. and Daisy , J.K. 1988 . The effect of monensin, narasin, salinomycin and nicarbazin, on field strains of chicken coccidia . Avian Pathology , 17 : 793 – 801 .
- Stayer , P.A. , Pote , L.M. and Keirs , R.W. 1995 . A comparison of Eimeria oocysts isolated from litter and fecal samples from broiler houses at two farms with different management schemes during one growout . Poultry Science , 74 : 26 – 32 .
- Stephan , B. , Rommel , M. , Daugschies , A. and Haberkorn , A. 1997 . Studies of resistance to anticoccidials in Eimeria field isolates and pure Eimeria strains . Veterinary Parasitology , 69 : 19 – 29 .
- Teilen , M.J.M. 1970 . A production score for broiler operations . World's Poultry Science Journal , 26 : 569 – 570 .
- Williams , R.B. 1973 . The effect of Eimeria acervulina on the reproductive potentials of four other species of chicken coccidia during concurrent infections . British Veterinary Journal , 129 , xxix – xxxi .
- Williams , R.B. 1999 . A compartmentalised model for the estimation of the cost of coccidiosis to the world's chicken production industry . International Journal for Parasitology , 29 : 1209 – 1229 .
- Williams , R.B. 2001 . Quantification of the crowding effect during infections with the seven Eimeria species of the domesticated fowl: its importance for experimental designs and the production of oocyst stocks . International Journal for Parasitology , 31 : 1056 – 1069 .
- Williams , R.B. 2006a . The impact of drug resistant parasites on the epizootiology of coccidiosis . World Poultry , 22 : 32 – 34 .
- Williams , R.B. 2006b . Tracing the emergence of drug-resistance in coccidia (Eimeria spp.) of commercial broiler flocks medicated with decoquinate for the first time in the United Kingdom . Veterinary Parasitology , 135 : 1 – 14 .
- Williams , R.B. , Bushell , A.C. , Répérant , J.M. , Doy , T.G. , Morgan , J.H. , Shirley , M.W. , Yvoré , P. , Carr , M.M. and Frémont , Y. 1996 . A survey of Eimeria species in commercially-reared chickens in France during 1994 . Avian Pathology , 25 : 113 – 130 .