Abstract
Metapneumoviruses (MPVs) were first reported in avian species (aMPVs) in the late 1970s and in humans in 2001. Although aMPVs have been reported in Europe and Asia for over 20 years, the virus first appeared in the United States in 1996, leaving many to question the origin of the virus and why it proved to be a different subtype from those found elsewhere. To examine the potential role of migratory waterfowl and other wild birds in aMPV spread, our study focused on determining whether populations of wild birds have evidence of aMPV infection. Serum samples from multiple species were initially screened using a blocking enzyme-linked immunosorbent assay. Antibodies to aMPVs were identified in five of the 15 species tested: American coots, American crows, Canada geese, cattle egrets, and rock pigeons. The presence of aMPV-specific antibodies was confirmed with virus neutralization and western blot assays. Oral swabs were collected from wild bird species with the highest percentage of aMPV-seropositive serum samples: the American coots and Canada geese. From these swabs, 17 aMPV-positive samples were identified, 11 from coots and six from geese. Sequence analysis of the matrix, attachment gene and short hydrophobic genes revealed that these viruses belong to subtype C aMPV. The detection of aMPV antibodies and the presence of virus in wild birds in Georgia, South Carolina, Arkansas and Ohio demonstrates that wild birds can serve as a reservoir of subtype C aMPV, and may provide a potential mechanism to spread aMPVs to poultry in other regions of the United States and possibly to other countries in Central and South America.
Mise en évidence d'une infection par un métapneumovirus aviaire de sous-type C chez des oiseaux sauvages en Georgie, Caroline du Sud, Arkansas et en Ohio, USA
Les métapneumovirus aviaires (aMPV) ont été décrits pour la première fois chez les espèces aviaires à la fin des années 1970 et chez l'homme en 2001. Bien que les aMPVs ont été décrits en Europe et en Asie depuis plus de 20 ans, le virus est apparu pour la première fois aux USA en 1996, laissant beaucoup d'interrogations sur l'origine du virus et sur le fait qu'il ait été prouvé être de sous type différent de ceux trouvés ailleurs. Pour examiner le rôle potentiel des palmipèdes migrateurs et des autres oiseaux sauvages dans la diffusion du aPMV, notre étude s'est focalisée à déterminer si les populations des oiseaux sauvages étaient infectées par l'aMPV. Des échantillons de sérum de différentes espèces ont été initialement sélectionnées en utilisant un test d'immunoadsorption à enzyme conjuguée bloquant. Des anticorps anti aMPV ont été identifiés chez cinq des 15 espèces testées : foulque d'Amérique, Corneille d'Amérique, bernache du Canada, héron garde-bœufs; pigeon biset. La présence des anticorps spécifiques a été confirmée par les tests de séroneutralisation et de Western blot. Des écouvillonnages oraux ont été réalisés chez les espèces d'oiseaux sauvages, foulque d'Amérique et bernache du Canada, présentant le pourcentage le plus élevé d'échantillons de sérums positifs en aMPV. A partir de ces écouvillons, 17 échantillons positifs en aMPV ont été identifiés, 11 de foulque et six de bernache. L'analyse de la séquence des gènes de la matrice, de la protéine d'attachement et de la petite protéine hydrophobe a révélé que ces virus appartiennent au sous-type C de l'aMPV. La détection des anticorps d'aMPV et la présence de virus chez les oiseaux sauvages en Georgie, Caroline du Sud, Arkansas et en Ohio démontrent que ces oiseaux sauvages peuvent servir de réservoir au sous-type C d'aMPV et peuvent servir de mécanisme potentiel à la diffusion d'aMPV aux volailles dans d'autres régions des US et éventuellement dans d'autres pays de l'Amérique Centrale et du Sud.
Nachweis von aviärer Metapneumovirus Subtyp C-Infektion in Wildvögeln in Georgia, Südcarolina, Arkansas und Ohio, USA
Metapneumoviren wurden erstmals in den späten 1970igern bei aviären Spezies (aMPV) und 2001 beim Menschen beschrieben. Erst nach 20-jährigem Vorkommen von aMPV in Europa und Asien trat das Virus erstmals 1996 in den USA auf, wobei es unklar blieb, wo das Virus herkam und warum es sich um einen anderen Subtyp handelte. Um die mögliche Bedeutung von Zugvögeln (Wassergeflügel und andere Wildvögel) für die aMPV-Verbreitung zu untersuchen, befassten wir uns mit der Überprüfung von Wildvogelpopulationen auf das Vorkommen von aMPV-Infektionen. Dazu wurden Serumproben von verschiedenen Spezies primär mittels Blocking-ELISA gescreent. In fünf der 15 getesteten Spezies wurden Antikörper gegen aMPV festgestellt: Amerikanisches Blässhuhn (Fulica americana), Amerikanische Krähe (Corvus brachyrhynchos), Kanadagans (Branta canadensis), Kuhreiher (Bubulcus ibis) und Felsentaube (Columba livia livia). Das Vorhandensein spezifischer Antikörper wurde mittels Neutralisations- und Western Blot-Test bestätigt. Rachentupfer wurden von den Wildvogelspezies mit den höchsten Prozentzahlen positiver Serumproben entnommen: Amerikanische Blässhühner und Kanadagänse. Von diesen Proben waren 17 aMPV-positiv, 11 von Blässhühnern und 6 von Gänsen. Sequenzanalysen der Matrix, des Anheftungsgens und kurzer hydrophober Gene zeigten, dass die Viren zum Subtyp C des aMPV gehörten. Der Nachweis von aMPV-Antikörpern und das Vorkommen des Virus in Wildvögeln in Georgia, Südcarolina, Arkansas und Ohio weist darauf hin, dass Wildvögel als Reservoir für Subtyp C-aMPV fungieren und damit eine potentielle Quelle für die Übertragung von aMPV auf Geflügel in anderen Regionen in den USA und möglicherweise auch anderen Ländern in Zentral- und Mittelamerika darstellen können.
Evidencias de la infección por el metapneumovirus aviar subtipo C en aves salvajes de Georgia, Carolina del Sur, Arkansas y Ohio en USA
Los Metapneumovirus (aMPV) se describieron por primera vez en especies aviares a finales de los años 70 y en humanos en 2001. A pesar de que los aMPVs se han descrito en Europa y Asia durante 20 años, los virus aparecieron por primera vez en USA en 1996, creando muchas preguntas respecto el origen del virus y por qué se trató de un subtipo distinto a los encontrados en otras regiones. Para determinar el papel potencial de las aves acuáticas migratorias y otras aves salvajes en la diseminación del aMPV, nuestro estudio se centró en comprobar si las poblaciones de aves salvajes mostraban evidencias de infección por aMPV. Inicialmente, se incluyeron muestras de suero de varias especies mediante un ensayo enzimático inmunoabsorvente de bloqueo. Se detectaron anticuerpos frente a aMPV en 5 de las 15 especies testadas: Focha Cenicienta, Cuervo Americano, Barnacla Canadiense, Garcillas Bueyeras y Palomas domésticas.La presencia de anticuerpos específicos frente a aMPV se confirmó mediante neutralización viral y Western Blot. Se obtuvieron hisopos orales de las especies de aves salvajes con mayores porcentajes de muestras de suero positivas frente a aMPV: Focha Cenicienta y Barnacla Canadiense. De estos hisopos, se identificaron 17 muestras positivas para aMPV, 11 de Fochas Cenicientas y seis de ocas. El estudio de las secuencias de los genes de la proteína de la matriz, de la proteína de unión y de la proteína hidrofóbica corta revelaron que estos virus pertenecían al subtipo C de aMPV. La detección de anticuerpos frente aMPV y la presencia de virus en aves salvajes de Georgia, Carolina del Sur, Arkansas y Ohio demuestra que las aves salvajes pueden servir como reservorio del subtipo C de aMPV y pueden actuar como un mecanismo potencial para la diseminación de aMPV a las aves de producción de otras regiones de US y posiblemente a las de otros países de América Central y del Sur.
Introduction
The viruses in the genus Metapneumovirus (MPV) belong to the family Paramyxoviridae, subfamily Pneumovirinae. These viruses contain a non-segmented, single-stranded, negative-sense RNA genome. The MPVs have a gene order of 3′-Leader-N-P-M-F-M2-SH-G-L-Trailer-5′ (Ling et al., Citation1992). This differs from the gene order and number of genes found in other members of the Pneumovirinae subfamily, 3′-NS1-NS2-N-P-M-SH-G-F-M2-L-5′ (Lamb & Kolakofsky, Citation1996). To date, only two viruses are classified as MPVs: the human metapneumovirus (hMPV) and the avian metapneumovirus (aMPV).
The hMPVs were first identified in the Netherlands associated with respiratory illness (van den Hoogen et al., Citation2001). Serological evidence suggests the hMPVs have circulated in the human population for at least the past 50 years, and are now associated with respiratory infections worldwide (van den Hoogen et al., Citation2001; Nissen et al., Citation2002; Bastien et al., Citation2003; Ebihara et al., Citation2003; Esper et al., Citation2003; Freymouth et al., Citation2003). Analysis of hMPV isolates revealed closer antigenic and sequence homology to the aMPVs than to any mammalian pneumovirus. Surprisingly, the hMPVs have the highest sequence identity with the aMPV subtype C viruses, which are currently found only in the USA, France and Korea (Seal, Citation1998; Cook, Citation2000b; van den Hoogen et al., Citation2001; Toquin et al., Citation2006; Lee et al., Citation2007). This has led many to speculate on the origin of these viruses and the possibility of a common ancestor.
Naturally occurring aMPV infections have been reported in chickens, turkeys, and ducks. Disease in poultry is characterized by catarrhal inflammation of the upper respiratory tract, nasal and ocular discharge, foamy conjunctivitis, sneezing, tracheal rales and swollen infraorbital sinuses (Buys & du Preez, Citation1980; Jones et al., Citation1987, Citation1988; Buys et al., Citation1989; Cook, Citation2000a). aMPV infections were first described in South Africa during 1978, and are currently found in poultry in Europe, Asia, South America and the USA (Buys & du Preez, Citation1980; Giraud et al., Citation1986; McDougall & Cook, Citation1986; Otsuki et al., Citation1996; Dani et al., Citation1999). The aMPVs have been placed into four subgroups (A to D) based on antigenic and sequence analysis (Cook, Citation2000a). While the majority of aMPVs isolated in Europe, Asia, and South America belong to the subtype A or B, subtype C aMPVs were first reported in the USA (Edson, Citation1997; Seal, Citation1998; Cook, Citation2000b) and subsequently isolated from farmed ducks in France in 1999 (Toquin et al., Citation2006) and in a live bird market in Korea in 2005 (Lee et al., Citation2007). In 2000, it was reported that aMPV viruses isolated in France in 1985 belong to subtype D (Bayon-Auboyer et al., Citation2000).
The presence of aMPV infection in USA was first reported during 1996 in Colorado (Cook et al., Citation1999). The virus was classified as subtype C due to the low sequence identity to subtype A and B viruses. After the initial identification of aMPV in Colorado, the presence of the virus was reported in North Dakota, South Dakota and Minnesota (Chiang et al., Citation2000; Goyal et al., Citation2000; Panigrahy et al., Citation2000). To date, most aMPV subtype C infections occur in Minnesota, with approximately 37% of turkey farms being affected annually (Goyal et al., Citation2003). The sudden emergence and sporadic occurrence of aMPV disease in the USA has lead to speculation that wild birds may be involved in viral spread. Wild birds are an important reservoir for other avian viruses including avian influenza and Newcastle disease viruses (Rosenberger et al., Citation1974; Slemons et al., Citation1974). In an attempt to examine the possible involvement of wild birds as a reservoir, research until now has focused on virus recovery from wild birds captured in Minnesota. Through this work, aMPV has been detected in sentinel birds placed on farms with aMPV infections, wild birds captured on aMPV-infected turkey farms, or birds sampled from other areas in the state (Shin et al., Citation2000, Citation2002b; Bennett et al., Citation2002). More recent research has identified the presence of aMPV in domestic turkeys in Wisconsin, North Dakota, South Dakota and Iowa, and wild birds in Minnesota and Canada (Bennett et al., Citation2004, Citation2005). The ability of aMPV subtype C viruses to infect and replicate in ducks has also been reported, suggesting isolates from poultry can infect ducks (Shin et al., Citation2001). What is currently not known is whether the existence of aMPV in these wild birds is directly due to the proximity to infected poultry farms or whether wild birds throughout the USA have evidence of aMPV infection.
To better address the role of wild birds in aMPV epidemiology, it is important to determine the presence and distribution of aMPV in wild bird populations outside endemic areas. To date aMPV infections have only been reported in a few states, with Minnesota being the most consistently infected. The current study focused on determining whether wild bird species have evidence of aMPV infection in states that have not reported aMPV infection. Our initial objective was to identify species of wild birds with antibodies to aMPV through serological testing. Once species of birds with aMPV antibodies were identified, our second objective was to detect the presence of virus in oral swabs from these species. Focusing on wild bird species with a higher prevalence of aMPV antibodies should increase chances for virus detection. Any identified virus would be characterized by sequence analysis.
Materials and Methods
Serum samples
Serum samples from multiple wild bird species from Georgia, South Carolina and Arkansas were obtained from archived serum samples collected in 2000 by the Southeastern Cooperative Wildlife Disease Study, located in Athens, Georgia, USA. Additional serum samples were collected from Canada geese in Ohio state during spring 2002. Seven hundred and thirty-two serum samples, representing 15 species of wild birds, were included in this study ().
Table 1. Serum samples screened for the presence of aMPV antibodies by bELISA, virus neutralization, and western blot assays
Enzyme-linked immunosorbent assay
Serum samples were screened utilizing a blocking enzyme linked immunosorbent assay (bELISA) as previously described (Turpin et al., Citation2003). Briefly, microtitre plates were coated with sucrose-purified aMPV subtype C Colorado isolate (aMPV/CO), isolated in 1997 from domestic turkeys (Edson, Citation1997). The plates were blocked with 1% polyvinylpyrrolidone (Sigma Biochemicals) and serum samples were tested at a 1:5 dilution. A horseradish peroxidase-conjugated polyclonal antibody to aMPV/CO was used for detection. Antibodies were detected using phenylenediamine dihydrochloride substrate (Sigma), the reaction was stopped with the addition of 2 M sulphuric acid and the optical density at 490 nm was determined. Serum samples with optical density three standard deviations below the optical density of negative controls were considered positive. The cutoff value of three standard deviations (97.7%) was chosen to increase the stringency of the test and to help eliminate potential false positives. The bELISA is specific for aMPV subtype C viruses and has low cross-reactivity with other aMPV subtypes (Turpin et al., Citation2003).
Virus neutralization
The presence of neutralizing antibodies in serum samples was determined in a virus neutralization (VN) assay. A starting dilution of 1:10 was chosen to minimize serum toxicity, followed by two-fold dilutions. Diluted serum samples were incubated with 100 median tissue culture infectious doses of aMPV/CO for 30 min at 37°C. After incubation, the mixture of serum and virus was added to monolayers of Vero cells. At 7 days post inoculation, cells were observed for the presence of cytopathic effect. Serum samples that were able to inhibit virus induced cytopathic effect were considered positive for neutralizing antibodies.
Western blot
Sucrose-purified aMPV/CO antigen was separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis using a 10% gel under reducing conditions, transferred to a nitrocellulose membrane and blocked with polyvinylpyrrolidone. Wild bird serum samples were tested at a 1:50 dilution and detected with an anti-bird antibody (Bethyl Laboratories). Positive and negative turkey and polyclonal rabbit serum samples were included as controls and detected using antibodies from Southern Biotech. Western blots were developed using ECL chemiluminescence reagents (Amersham Pharmacia Biotech).
Swab samples
Choanal swab samples were collected individually from Canada geese and American coots captured on Lake Thurmond, Georgia, USA and Canada geese captured in Ohio. The swabs were placed in 1.5 ml phosphate-buffered saline with antibiotics (1000 u/ml penicillin, 10 µg/ml gentamicin, and 5 µg/ml amphotericin B) (Sigma) and held at 4°C until facilities were available for storage at −70°C.
Virus detection
Oral swabs from wild birds were passed three times in chicken embryo fibroblast (CEF) cell cultures prior to virus detection by reverse transcriptase-polymerase chain reaction (RT-PCR). CEF cell cultures were inoculated with 200 µl swab material. After a 30 min incubation period, the media was replaced and cells were incubated for 1 week. Inoculated CEF cells were subjected to freeze–thawing at 7 days post inoculation and cultures were used for additional cell culture passes. Original swab samples and/or cell culture passes, referred to as wild bird samples, were subsequently used for RNA extractions.
Reverse transcriptase-polymerase chain reaction
RNA was extracted from wild bird samples with TRIzol® reagent (Invitrogen), following the manufacturer's protocol, resuspended in 30 µl RNase-free water, and stored at –70°C. RT-PCR was used to detect the presence of aMPV nucleic acid in the samples. Primers were designed to amplify a portion of the matrix (M) gene conserved in the subtype A, B and C aMPVs. These primers were used for the initial screening of samples for the presence of aMPV (). RT-PCR was performed with the One-Step RT-PCR kit (Qiagen, Valencia, California, USA) following the manufacturer's instructions. Five microlitres of sample RNA was added to the RT-PCR mixture with 10 u ribonuclease inhibitor (Invitrogen) and 0.6 µM each primer in a final volume of 25 µl. An additional PCR reaction was included to amplify low levels of viral nucleic acid not detected with the initial RT-PCR reaction (Bayon-Auboyer et al., Citation1999). For the second PCR reaction, 5 µl of the first reaction was added to a Taq PCR Master Mix (Qiagen) along with 0.5 µM each primer in a total volume of 25 µl.
Table 2. Primers used for RT-PCR detection of aMPV genes
Samples that were positive for the M gene were subsequently analysed using primers specific for the attachment protein (G) and the short hydrophobic (SH) gene. All primers used in this study are presented in . RNA extracted from aMPV/CO viral stocks was used as a positive control, while uninfected CEF cells and no template reactions served as a negative control. RT-PCR products were visualized on agarose gels.
Sequencing
RT-PCR products were sequenced using Taq polymerase, fluorescently labelled dideoxyneucleotides and an automated sequencer (Sanger et al., Citation1977; Smith et al., Citation1986; Sangster et al., Citation2003). At least four RT-PCR products were sequenced to generate a consensus sequence for each virus identified. Nucleotide sequence editing and alignments of sequences were completed using DNASTAR (Madison, Wisconsin, USA). Alignments were performed using the CLUSTALW method (Thompson et al., Citation1994). To determine the relationship among the aMPV isolates, phylogenetic analysis using maximum parsimony and bootstrapping was performed (Swofford, Citation2000). Analysis of the M, F, SH and G genes included all available isolates from wild birds, representative turkey isolates belonging to subtype A, B, C and D, representative viruses from the two subgroups of hMPV and respiratory syncytial virus (RSV). Isolates from GenBank used in the alignments for the generation of the M gene phylogenetic tree included subtype C isolates AF187151, AF298635, AF26675, AY26675, AF26673, AF26674 and AF269171; subtype C isolates from Korea EF199771 and EF199772; subtype A X58639, and subtype B U37586. Owing to the limited sequence available for the SH genes, only AJ457961, EF199771, EF199772 (subtype C), S40185 (subtype A) and J492378 (subtype B) aMPVs were included. G gene analysis included isolates AF457967, EF199771, EF199772 (subtype C), S40185 (subtype A), L34031 (subtype B), and both subtype D sequences AJ251085 and AJ288946. Also included in the phylogenetic analysis for the M, F, SH and G genes were the hMPVs, NC004148 (subtype A), AY297748 (subtype B) and RSV NC001803.
Results
Serology
A total of 732 serum samples collected from wild birds in Georgia, South Carolina, Arkansas and Ohio were tested individually in the bELISA. Antibodies to aMPV were detected in five species of wild birds collected in these states (). Species in which aMPV antibodies were identified include the American crow, American coot, Canada goose, cattle egret and rock pigeon. The geese in Ohio had the highest percentage of positive serum samples determined by bELISA (49%), followed by Canada geese (25%) and American coots (18%) from Arkansas, Georgia and South Carolina (). Lower percentage positive sera were detected in crows (12%), egrets (5%), and rock pigeons (0.5%). No increased prevalence was found based on sampling locations.
To confirm the specificity of bELISA results, positive serum samples were tested individually by western blot and VN assays. Most of the bELISA-positive serum samples from the crows, egrets and rock pigeons contained detectable antibodies to aMPV by western blot analysis (). Owing to the large number of positive serum samples from the coots and geese, representative serum samples were analysed. Western blotting detected antibodies specific for aMPV in all of the representative geese and coots serum samples (). Representative serum samples found to be negative by the bELISA were tested in western blot and did not recognize any viral proteins. While all five species had detectable antibodies to aMPV using the bELISA, neutralizing antibodies to aMPV were only detected in Canada geese and American coots ().
Detection of aMPV by RT-PCR
Although antibodies to aMPV were detected in five species of wild birds tested in the present study, oral swabs were only collected from Canada geese and American coots due to the ease of sampling large numbers of birds and high antibody prevalence in these species. Of the 181 oral swabs collected from the American coots in Georgia, 11 samples resulted in the amplification of the correct size band with RT-PCR primers to the M gene. From the 525 oral swabs collected from the Canada geese, aMPV was detected by the RT-PCR for the M gene in four samples obtained from geese in Ohio and in two samples obtained from geese in Georgia ().
Table 3. Avian species tested for the presence of avian metapneumovirus nucleic acids by RT-PCR
Sequence analysis
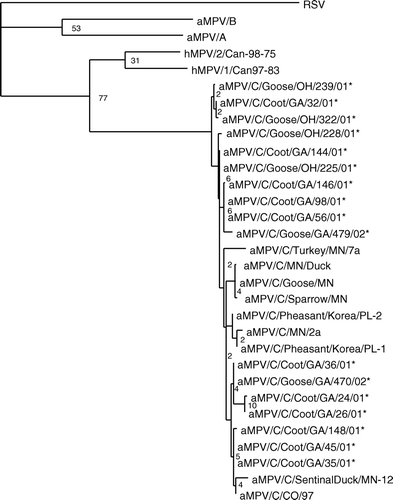
Once potential aMPV-positive samples were identified with degenerate primers to the matrix gene of aMPV subtypes A, B, and C, subtype C-specific primers were used to amplify the entire M gene. Primers to the full-length M gene amplified an 849-nucleotide product, including the entire open reading frame encoding a 254 amino acid product. Alignment of the sequences confirmed the viruses detected in the goose and coot swabs were aMPVs closely related to the subtype C aMPVs found in the USA (). Sequences from the 11 coot viruses and six goose viruses have 96% to 99% nucleotide identity with each other and other subtype C viruses, and shared 61% to 62% identity with subtype A, 64% to 65% with subtype B, and 73% to 76% with the hMPVs. There was only 36% identity with RSV. While the viruses isolated in Minnesota from wild birds captured on or near turkey farms in 2000 (Shin et al., Citation2002b) clustered closely together, the wild bird viruses isolated in 2001 and 2002 in Georgia and Ohio were found throughout the group C viruses. The newly described viruses did not separate based on species or sampling location. As previously reported, the subtype C viruses, including the newly identified viruses, appear to share closer sequence identity with the hMPVs than subgroup A or B aMPVs (Cook, Citation2000b).
Figure 1. Phylogenetic relationship of the newly detected aMPVs from wild birds based on 849 nucleotides from the matrix gene. The phylogenetic tree was constructed with wild bird isolates, representative isolates from subtype A, B and C aMPVs, hMPV and RSV. Following alignments, rooted phylograms were generated by maximum parsimony and 1000 bootstraps. *Viruses identified in the present study.
In addition to sequencing the entire M gene, amplification and sequencing of the G and SH genes were also attempted. The G and SH genes are the least conserved of all the MPV genes. The G gene has been previously sequenced for subtype C viruses with differences in gene lengths of 783, 1321, and 1798 being reported (Alvarez et al., Citation2003; Toquin et al., Citation2003; Govindarajan et al., Citation2004). Differences in gene length have contributed to inclusion of other genes and complex secondary structure masking the entire gene (Govindarajan et al., Citation2004). The short hydrophobic protein is a type 2 glycoprotein with unknown function. The SH gene is found directly 3′ to the glycoprotein genes and is reported to be 628 nucleotides long including non-translated sequences (Toquin et al., Citation2003; Yunus et al., Citation2003; Govindarajan & Samal, Citation2005). Overlapping primers were designed to amplify the G, SH and G–SH intergenic regions. Portions of the SH, G and SH–G intergenic regions were amplified from all 17 wild bird viruses detected by M-gene RT-PCR (data not shown). Amplification of full-length SH and a large portion of the G genes from wild birds was only detected in three swab samples, one from a coot in Georgia and two from geese in Ohio. Using primers that amplified G, SH and their intergenic region resulted in the amplification of a 1472-nucleotide product. This includes the 528 nucleotides encoding SH and 783 nucleotides encoding G. This is in agreement with sequences reported by Toquin et al. (Citation2003) and Yunus et al. (Citation2003) but is shorter than the sequences reported by Govindarajan et al. (Citation2004) and Bennett et al. (Citation2005).
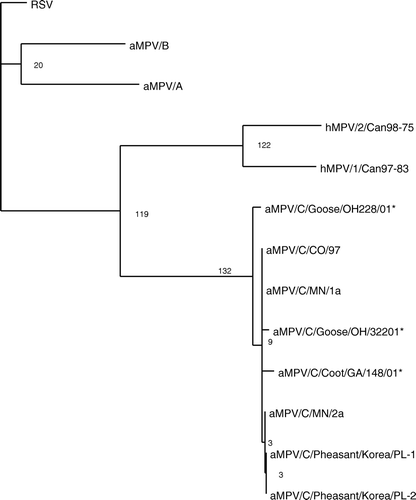
The G and SH genes had the lowest sequence identity among the genes of the aMPVs. Analysis of these genes identified in wild birds revealed that the viruses are most closely related to subtype C viruses. Using phylogenetic analysis of the G gene, the wild bird isolates cluster with the subtype C aMPVs (). The G gene of the newly identified viruses had 93% to 97% nucleotide identity with the subtype C viruses; this includes the Colorado, Minnesota and Korea isolates. The wild bird G sequences had low nucleotide identity with the subtype A (27%), B (27%), and D (28%) aMPVs and RSV (29%). Similar results were found with the hydrophobic gene (). The SH gene from wild bird viruses had high identity to the subtype C virus sequences (97%) and low identity to subtype A and B viruses (27%) and RSV (26%). The wild bird SH genes had the closest identity with the hMVPs (31%). This is in agreement of previous reports of the G and SH genes of other subtype C viruses (Toquin et al., Citation2003; Yunus et al., Citation2003). The sequence analysis of the M, F, SH and G genes of the wild bird viruses identified in the present study indicates that the aMPVs detected should be classified as subtype C viruses.
Figure 2. Phylogenetic relationship of the newly detected aMPVs from wild birds based on 783 nucleotides of the attachment gene (G) of aMPV. A phylogenetic tree was constructed using the wild bird isolate, all subtype C isolates, representative subtype A, B and D aMPVs, hMPV and RSV. Following alignments, rooted phylograms were generated by maximum parsimony and 1000 bootstraps. *Viruses identified in the present study.
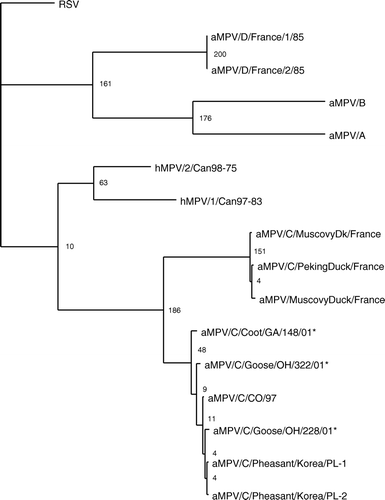
Figure 3. Phylogenetic relationship of the newly detected aMPVs from wild birds based on 538 nucleotides of the SH gene. A phylogenetic tree was constructed using representative A, B and C aMPVs, hMPVs and RSV. Following alignments, rooted phylograms were generated using maximum parsimony and 1000 bootstrap repetitions. *Viruses identified in the present study.
Discussion
The sudden and sporadic appearance of MPV infections in avian species has led many to speculate on the origin and reservoir for these viruses. Even more intriguing is the close nucleotide and amino acid similarities of hMPVs with subtype C viruses (van den Hoogen et al., Citation2001). While the hMPVs can be found throughout the world, the subtype C aMPVs have only been reported in the USA (Cook et al., Citation1999), France (Toquin et al., Citation2006) and Korea (Lee et al., Citation2007). Subtype A and B aMPVs have been reported throughout Asia, South America and Europe since the 1980s (Cook, Citation2000a). The appearance of aMPV in turkeys in Colorado in 1996 was the first report of any aMPV in the USA (Cook et al., Citation1999). Although the original source of the virus is unknown, wild birds have been implicated in the introduction of aMPV in the USA. aMPV has been isolated from a few wild birds sampled in Minnesota and one sampling in Canada. These isolates were closely related to the aMPVs that were recovered from domestic turkeys in the area (Shin et al., Citation2000, Citation2002a; Bennett et al., Citation2002, Citation2004). This supports the hypothesis that wild birds may be involved in the local ecology of aMPV. However, these data do not address whether wild birds first encountered the virus on aMPV-infected turkey farms or whether wild birds were responsible for the introduction of virus into domestic poultry. A key component to the wild bird transmission hypothesis is the idea that wild birds are able to carry the virus from one area to another. This has been reported with other respiratory viruses that affect poultry, for example Newcastle disease virus and avian influenza virus (Rosenberger et al., Citation1974; Slemons et al., Citation1974). Therefore, it is critical to determine whether wild birds outside the aMPV-infected turkey areas harbour aMPV. To address this question our research has focused on determining whether wild birds outside Minnesota, specifically in Georgia, South Carolina, Arkansas and Ohio, had evidence of aMPV infection.
When aMPV has been isolated from wild birds in areas with endemic aMPV infections in domestic turkeys, the recovery rate has been very low (Shin et al., Citation2002a). To improve our chances of recovering aMPV from wild birds, we first identified populations of birds with anti-aMPV antibodies and then focused virus detection attempts on species with evidence of aMPV antibodies. This was accomplished by screening serum samples collected in 2000 from Georgia, South Carolina and Arkansas and in Ohio during 2002 using a bELISA. Antibodies to aMPV were identified in five species of wild birds; American crows, American coots, Canada geese, cattle egrets and rock pigeons. When serum samples from all five species were analysed by western blot assays, antibodies to aMPV were detected in the goose, coot, crow, rock pigeon and egret samples. This indicates that all five species had been exposed to aMPV and developed a measurable humoral immune response. Neutralizing antibodies were detected only in the goose and coot serum samples. This again confirms the presence of aMPV-specific antibodies in these two species, but does not rule out the possibility of aMPV-specific antibodies in the other species sampled. The VN assay is generally less sensitive than enzyme-linked immunosorbent assay due to its limitation of only detecting neutralizing antibodies. Neutralizing antibodies often occur in low levels and are not always generated in low-grade infections. The lack of detectable aMPV antibodies in the other species tested may reflect a lack of infection or may be the result of the small sample size for several species tested. The differences in the percentage of seropositive birds in the Canada geese sampled in Georgia compared with Ohio may be due to differences in age, sample size, location, or migratory patterns. Owing to the sampling dates, the geese from Ohio contained a higher percentage of juvenile birds than was seen in the geese sampled in Georgia. In addition, the geese in Georgia are resident birds and therefore may have limited exposure to other birds compared with the migratory geese sampled in Ohio. Although five species of wild birds did have detectable antibodies to aMPV, virus detection attempts focused on the Canada geese and American coots, due to higher levels of seropositive birds in these species and ease of sampling.
Oral swabs were collected in winter 2001 from American coots and in spring 2002 from the Canada geese. Sampling times were determined by the availability and ease of capture for each species. Although the bELISA could only detect antibodies directed against subtype C aMPV, degenerate primers to the M gene were designed to detect subtype A, B and C aMPVs to ensure any aMPV present would be detected. These primers were used for an initial screening of the wild bird samples, leading to the identification of 17 samples with amplification products specific to aMPV. This included 11 samples from coots in Georgia, four samples from geese in Ohio and two samples from geese in Georgia. After initial screening with degenerate primers to the M gene, subtype C-specific primers were tested and amplified the entire M gene. Sequence analysis of the M gene revealed that the aMPV sequences identified in wild birds have high nucleotide sequence identity (93% to 98%) to subtype C viruses, this indicates that the newly identified coot and goose viruses belong to subtype C aMPVs, as do all aMPVs isolated in the USA to date. Once samples were determined to be positive by the initial screening, the RNAs were then analysed by RT-PCR with primers specific to the G and SH genes. These genes were chosen due to the high levels of heterogeneity seen among the four subgroups of aMPV. Analysis of these genes could help to determine the relationship of aMPV isolates from wild birds to those collected from turkeys. Portions of the SH and G genes were detected in all 17 swabs in which the M gene was sequenced. Large portions of the SH and G could only be detected in three samples. Interestingly, in these samples PCR products were detected in both the original swabs and cell culture passaged material. The lack of amplification of full-length gene products in the other samples may be a result of degradation of the viral RNA during transport or storage, or sequence divergence. The sequence data from the SH and G genes of the three viruses detected in this study confirm that the viruses detected in wild birds in Georgia and Ohio belong to the subtype C aMPVs and are closely related to the aMPVs found in turkeys in Minnesota.
These data demonstrate the presence of aMPV and anti-aMPV antibodies in wild birds outside Minnesota, specifically Georgia, South Carolina, Arkansas and Ohio. This indicates that aMPV is able to replicate and induce antibody production in some species of wild birds. The presence of antibodies to aMPV in the wild birds sampled during 2000 demonstrates the presence of aMPV outside the endemic areas. Older serum samples and more extensive testing are necessary to determine the extent and duration of aMPV infection in wild bird populations. The high number of lakes and migratory birds in close proximity to turkey farms in Minnesota has been implicated in the continuing circulation of aMPV infection in this area (Shin et al., Citation2002b). Although the theory of repeated introductions of aMPV from wild birds has been suggested, sequence analysis of the F gene of aMPV isolates from Minnesota suggests that there has been only a single recent introduction of aMPV into turkey populations there (Dar et al., Citation2002). This suggests that following the original introduction of aMPV in Minnesota, lineage of viruses has spread from farm to farm and is not the result of repeated introductions of the virus from wild birds. The lack of infection of turkeys in the southern states, where aMPV can be found in wild birds, suggests an infrequent introduction of virus from wild birds into the poultry population. The presence of the virus among wild birds in Georgia, South Carolina, Arkansas and Ohio could eventually result in the introduction of aMPV into poultry flocks, providing additional justification for poultry companies to continue to maintain high biosecurity standards.
Acknowledgements
The authors would like to acknowledge Joyce Bennett and Joan Beck for all their assistance and the Southeastern Cooperative Wildlife Disease Study and the Ohio State University and for providing serum and swab samples, specifically the efforts of Britta Hansen, Casey Sanders, Robbie Edalgo, Rob Olson, Lynn Lewis-Weis, Lisa Balmert, Nate Mechlin, Daniel Corn, Clay George, Anna Yellin, Ann Baltzell, Heidi Knoblich, and Allison Bowman. This research was funded by USDA/ARS CRIS project 6612-32000-038D.
Additional information
Notes on contributors
E.A. Turpin†
†Current address: Pfizer Animal Health, Poultry Health Division, Research Triangle Park, NC 27709, USAReferences
- Alvarez , R. , Lwamba , H.M. , Kapczynski , D.R. , Njenga , M.K. and Seal , B.S. 2003 . Nucleotide and predicted amino acid sequence-based analysis of the avian metapneumovirus type C cell attachment glycoprotein gene: phylogenetic analysis and molecular epidemiology of U.S. pneumoviruses . Journal of Clinical Microbiology , 41 : 1730 – 1735 .
- Bastien , N. , Normand , S. , Taylor , T. , Ward , D. , Peret , T.C. , Boivin , G. , Anderson , L. J. and Li , Y. 2003 . Sequence analysis of the N, P, M and F genes of Canadian human metapneumovirus strains . Virus Research , 93 : 51 – 62 .
- Bayon-Auboyer , M.H. , Jestin , V. , Toquin , D. , Cherbonnel , M. and Eterradossi , N. 1999 . Comparison of F-, G- and N-based RT-PCR protocols with conventional virological procedures for the detection and typing of turkey rhinotracheitis virus . Archives of Virology , 144 : 1091 – 1109 .
- Bayon-Auboyer , M.H. , Arnauld , C. , Toquin , D. and Eterradossi , N. 2000 . Nucleotide sequences of the F, L and G protein genes of two non-A/non-B avian pneumoviruses (APV) reveal a novel APV subgroup . Journal of General Virology , 81 : 2723 – 2733 .
- Bennett , R.S. , McComb , B. , Shin , H.J. , Njenga , M.K. , Nagaraja , K.V. and Halvorson , D.A. 2002 . Detection of avian pneumovirus in wild Canada (Branta canadensis) and blue-winged teal (Anas discors) geese . Avian Diseases , 46 : 1025 – 1029 .
- Bennett , R.S. , Nezworski , J. , Velayudhan , B.T. , Nagaraja , K.V. , Zeman , D.H. , Dyer , N. , Graham , T. , Lauer , D.C. , Njenga , M.K. and Halvorson , D.A. 2004 . Evidence of avian pneumovirus spread beyond Minnesota among wild and domestic birds in central North America . Avian Diseases , 48 : 902 – 908 .
- Bennett , R.S. , LaRue , R. , Shaw , D. , Yu , Q. , Nagaraja , K.V. , Halvorson , D.A. and Njenga , M.K. 2005 . A wild goose metapneumovirus containing a large attachment glycoprotein is avirulent but immunoprotective in domestic turkeys . Journal of Virology , 79 : 14834 – 14842 .
- Buys , S.B. and du Preez , J.H. 1980 . A preliminary report on the isolation of a virus causing sinusitis in turkeys in South Africa and attempts to attenuate the virus . Turkeys , 28 : 36
- Buys , S.B. , du Preez , J.H. and Els , H.J. 1989 . Swollen head syndrome in chickens: a preliminary report on the isolation of a possible aetiological agent . Journal of South African Veterinary Association , 60 : 221 – 222 .
- Chiang , S. , Dar , A.M. , Goyal , S.M. , Sheikh , M.A. , Pedersen , J.C. , Panigrahy , B. , Senne , D. , Halvorson , D.A. , Nagaraja , K.V. and Kapur , V. 2000 . A modified enzyme-linked immunosorbent assay for the detection of avian pneumovirus antibodies . Journal of Veterinary Diagnostic Investigation , 12 : 381 – 384 .
- Cook , J.K. 2000a . Avian pneumovirus infections of turkeys and chickens . Veterinary Journal , 160 : 118 – 125 .
- Cook , J.K. 2000b . Avian rhinotracheitis . Revue Scientifique et Technique de L Office International des Epizooties , 19 : 602 – 613 .
- Cook , J.K.A. , Huggins , M.B. , Orbell , S.J. and Senne , D.A. 1999 . Preliminary antigenic characterization of an avian pneumovirus isolated from commercial turkeys in Colorado, USA . Avian Pathology , 28 : 607 – 617 .
- Dani , M.A. , Arns , C.W. and Durigon , E.L. 1999 . Molecular characterization of Brazilian avian pneumovirus isolates using reverse transcription-polymerase chain reaction, restriction endonuclease analysis and sequencing of a G fragment . Avian Pathology , 28 : 473 – 476 .
- Dar , A.M. , Munir , S. , Goyal , S.M. and Kapur , V. 2002 . A single subtype of avian pneumovirus circulates among Minnesota turkey flocks . Journal of Veterinary Diagnostic Investigation , 14 : 371 – 376 .
- Ebihara , T. , Endo , R. , Kikuta , H. , Ishiguro , N. , Yoshioka , M. , Ma , X. and Kobayashi , K. 2003 . Seroprevalence of human metapneumovirus in Japan . Journal of Medical Virology , 70 : 281 – 283 .
- Edson , R.K. 1997 . Committee on Transmissible Diseases of Poultry—Experience with Avian Pneumovirus , Richmond , VA : Pat Campbell Press and Spectrum Press .
- Esper , F. , Boucher , D. , Weibel , C. , Martinello , R.A. and Kahn , J.S. 2003 . Human metapneumovirus infection in the United States: clinical manifestations associated with a newly emerging respiratory infection in children . Pediatrics , 111 : 1407 – 1410 .
- Freymouth , F. , Vabret , A. , Legrand , L. , Eterradossi , N. , Lafay-Delaire , F. , Brouard , J. and Guillois , B. 2003 . Presence of the new human metapneumovirus in French children with bronchiolitis . The Pediatric Infectious Disease Journal , 22 : 92 – 94 .
- Giraud , P. , Bennejean , G. , Guittet , M. and Toquin , D. 1986 . Turkey rhinotracheitis in France: preliminary investigations on a ciliostatic virus . Veterinary Record , 119 : 606 – 607 .
- Govindarajan , D. and Samal , S.K. 2005 . Analysis of the complete genome sequence of avian metapneumovirus subgroup C indicates that it possesses the longest genome among metapneumoviruses . Virus Genes , 30 : 331 – 333 .
- Govindarajan , D. , Yunus , A.S. and Samal , S.K. 2004 . Complete sequence of the G glycoprotein gene of avian metapneumovirus subgroup C and identification of a divergent domain in the predicted protein . Journal of General Virology , 85 : 3671 – 3675 .
- Goyal , S.M. , Chiang , S.J. , Dar , A.M. , Nagaraja , K.V. , Shaw , D.P. , Halvorson , D.A. and Kapur , V. 2000 . Isolation of avian pneumovirus from an outbreak of respiratory illness in Minnesota turkeys . Journal of Veterinary Diagnostic Investigation , 12 : 166 – 168 .
- Goyal , S.M. , Lauer , D. , Friendshuh , K. and Halvorson , D.A. 2003 . Seroprevalence of avian pneumovirus in Minnesota turkeys . Avian Diseases , 47 : 700 – 706 .
- Jones , R.C. , Baxter-Jones , C. , Savage , C.E. , Kelly , D.F. and Wilding , G.P. 1987 . Experimental infection of chickens with a ciliostatic agent isolated from turkeys with rhinotracheitis . Veterinary Record , 120 : 301 – 312 .
- Jones , R.C. , Williams , R.A. , Baxter-Jones , C. , Savage , C.E. and Wilding , G.P. 1988 . Experimental infection of laying turkeys with rhinotracheitis virus: distribution of virus in the tissue and serological response . Avian Pathology , 17 : 841 – 850 .
- Lamb , R.A. and Kolakofsky , D. 1996 . “ Paramyxoviridae: the viruses and their replication ” . In Fields Virology , Edited by: Fields , B.N. , Knipe , P.M. and Howeley , P.M. 1177 – 1204 . Philadelphia , PA : Lippincott, Williams & Wilkins .
- Lee , E. , Song , M.S. , Shin , J.Y. , Lee , Y.M. , Kim , C.J. , Lee , Y.S. , Kim , H. and Choi , Y.K. 2007 . Genetic characterization of avian metapneumovirus subtype C isolated from pheasants in a live bird market . Virus Research , 128 : 18 – 25 .
- Ling , R. , Easton , A.J. and Pringle , C.R. 1992 . Sequence analysis of the 22K, SH and G genes of turkey rhinotracheitis virus and their intergenic regions reveals a gene order different from that of other pneumoviruses . Journal of General Virology , 73 : 1709 – 1715 .
- McDougall , J.S. and Cook , J. K. 1986 . Turkey rhinotracheitis: preliminary investigations . Veterinary Record , 118 : 206 – 207 .
- Nissen , M.D. , Siebert , D.J. , Mackay , I.M. , Sloots , T.P. and Withers , S.J. 2002 . Evidence of human metapneumovirus in Australian children . The Medical Journal of Australia , 176 : 188
- Otsuki , K. , Hirai , N. , Mitani , M. , Itani , M. , Shimohata , T. , Kunii , E. , Uramoto , K. , Kiyotake , M. , Kato , H. , Ellis , M.M. and Cook , J.K. 1996 . Demonstration of serum-neutralising antibody to turkey rhinotracheitis virus in serum from chicken flocks in Japan . Journal of Veterinary Medical Science , 58 : 869 – 874 .
- Panigrahy , B. , Senne , D.A. , Pedersen , J.C. , Gidlewski , T. and Edson , R.K. 2000 . Experimental and serologic observations on avian pneumovirus (APV/turkey/Colorado/97) infection in turkeys . Avian Diseases , 44 : 17 – 22 .
- Rosenberger , J.K. , Krauss , W.C. and Slemons , R.D. 1974 . Isolation of Newcastle disease and type-A influenza viruses from migratory waterfowl in the Atlantic flyway . Avian Diseases , 18 : 610 – 613 .
- Sanger , F. , Nicklen , S. and Coulson , A.R. 1977 . DNA sequencing with chain-terminating inhibitors . Proceedings of the National Academy of Sciences of U S A , 74 : 5463 – 5467 .
- Sangster , M.Y. , Riberdy , J.M. , Gonzalez , M. , Topham , D.J. , Baumgarth , N. and Doherty , P.C. 2003 . An early CD4+ T cell-dependent immunoglobulin A response to influenza infection in the absence of key cognate T–B interactions . Journal of Experimental Medicine , 198 : 1011 – 1021 .
- Seal , B.S. 1998 . Matrix protein gene nucleotide and predicted amino acid sequence demonstrate that the first US avian pneumovirus isolate is distinct from European strains . Virus Research , 58 : 45 – 52 .
- Shin , H.J. , Njenga , M.K. , McComb , B. , Halvorson , D.A. and Nagaraja , K.V. 2000 . Avian pneumovirus (APV) RNA from wild and sentinel birds in the United States has genetic homology with RNA from APV isolates from domestic turkeys . Journal of Clinical Microbiology , 38 : 4282 – 4284 .
- Shin , H.J. , Njenga , M.K. , Halvorson , D.A. , Shaw , D.P. and Nagaraja , K.V. 2001 . Susceptibility of ducks to avian pneumovirus of turkey origin . American Journal of Veterinary Research , 62 : 991 – 994 .
- Shin , H.J. , Cameron , K.T. , Jacobs , J.A. , Turpin , E.A. , Halvorson , D.A. , Goyal , S.M. , Nagaraja , K.V. , Kumar , M.C. , Lauer , D.C. , Seal , B.S. and Njenga , M.K. 2002a . Molecular epidemiology of subgroup C avian pneumoviruses isolated in the United States and comparison with subgroup A and B viruses . Journal of Clinical Microbiology , 40 : 1687 – 1693 .
- Shin , H.J. , Nagaraja , K.V. , McComb , B. , Halvorson , D.A. , Jirjis , F.F. , Shaw , D.P. , Seal , B.S. and Njenga , M.K. 2002b . Isolation of avian pneumovirus from mallard ducks that is genetically similar to viruses isolated from neighboring commercial turkeys . Virus Research , 83 : 207 – 212 .
- Slemons , R.D. , Johnson , D.C. , Osborn , J.S. and Hayes , F. 1974 . Type-A influenza viruses isolated from wild free-flying ducks in California . Avian Diseases , 18 : 119 – 124 .
- Smith , L.M. , Sanders , J.Z. , Kaiser , R.J. , Hughes , P. , Dodd , C. , Connell , C.R. , Heiner , C. , Kent , S.B. and Hood , L.E. 1986 . Fluorescence detection in automated DNA sequence analysis . Nature , 321 : 674 – 679 .
- Swofford , D.L 2000 . PAUP: Phylogenetic Analysis Using Parsimony Version 4 . Sunderland , MA : Sinauer Associates .
- Thompson , J.D. , Higgins , D.G. and Gibson , T.J. 1994 . CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice . Nucleic Acids Research , 22 : 4673 – 4680 .
- Toquin , D. , de Boisseson , C. , Beven , V. , Senne , D.A. and Eterradossi , N. 2003 . Subgroup C avian metapneumovirus (MPV) and the recently isolated human MPV exhibit a common organization but have extensive sequence divergence in their putative SH and G genes . Journal of General Virology , 84 : 2169 – 2178 .
- Toquin , D. , Guionie , O. , Jestin , V. , Zwingelstein , F. , Allee , C. and Eterradossi , N. 2006 . European and American subgroup C isolates of avian metapneumovirus belong to different genetic lineages . Virus Genes , 32 : 97 – 103 .
- Turpin , E.A. , Lauer , D.C. and Swayne , D.E. 2003 . Development and evaluation of a blocking enzyme-linked immunosorbent assay for detection of avian metapneumovirus type C-specific antibodies in multiple domestic avian species . Journal of Clinical Microbiology , 41 : 3579 – 3583 .
- van den Hoogen , B.G. , de Jong , J.C. , Groen , J. , Kuiken , T. , de Groot , R. , Fouchier , R.A. and Osterhaus , A.D. 2001 . A newly discovered human pneumovirus isolated from young children with respiratory tract disease . Nature Medicine , 7 : 719 – 724 .
- Yunus , A.S. , Govindarajan , D. , Huang , Z. and Samal , S.K. 2003 . Deduced amino acid sequence of the small hydrophobic protein of US avian pneumovirus has greater identity with that of human metapneumovirus than those of non-US avian pneumoviruses . Virus Research , 93 : 91 – 97 .