Abstract
Current knowledge about the expression of ABC transport proteins suggests that their expression is regulated by a variety of factors, including pathological conditions, and in particular inflammatory reactions to infection. As ABC transporters are major determinants of absorption, distribution and excretion of many antimicrobials, modulation of their activity may result in increased or decreased tissue levels of drugs, affecting the efficacy of treatment. As fluoroquinolones have been identified as modulators and substrates of a number of drug transporters, we evaluated the effect of danofloxacin mesylate and enrofloxacin treatment on the levels of expression of MDR1 and MRP2 mRNAs in the intestines and livers of broilers with experimentally induced colibacillosis. MDR1 mRNA expression was significantly decreased in infected animals and was partly restored over 5 days of treatment with orally administered danofloxacin mesylate or enrofloxacin. Changes in the level of expression of MRP2 mRNA were less prominent. The study suggests that the treatment of colibacillosis with fluoroquinolones, which resulted in a significant clinical improvement of the animals, also restored the expression of drug transporters. This is of clinical importance as these ABC transporters significantly contribute to the functionality of important biological barriers, protecting the bird and specific tissues from pathogens and bacterial toxins.
Introduction
Transmembrane efflux transporters of the ABC family are involved in the absorption, distribution and elimination of endogenous compounds, such as hormones and neurotransmitters, as well as of therapeutic agents and toxins (Dean & Annilo, Citation2005; Linton, Citation2007). Clinical experience and experimental work have indicated that exogenous substrates, including a number of drugs, modulate the expression and function of membrane transporters. In addition, metabolic disorders and infectious disease influence their activity (Yokooji et al., Citation2006; Naud et al., Citation2007).
Exposure to pathogens activates a cascade of defence mechanisms, generally described as the acute phase response to infection (Mireles et al., Citation2005). Part of this response involves the expression of cytokines, in particular tumour necrosis factor-α, interleukin-1β and interleukin-6, which are the major pro-inflammatory cytokines in all animal species, including chickens (Leshchinsky & Klasing, Citation2001). In mammals, the expression of pro-inflammatory cytokines is accompanied by a down-regulation of nuclear receptors, including farnesoid X receptor, pregnane X receptor and constitutive androstane receptor, which are involved in the transcriptional regulation of a number of biotransformation enzymes, as well as ABC transporters (Beigneux et al., Citation2002; Stienstra et al., Citation2004). A number of investigations have further defined the role of the individual cytokines in the transcriptional and post-transcriptional regulation of expression of the ABC transporters MDR1 and MRP2 (McRae et al., Citation2003; Siewert et al., Citation2004). MDR1 mRNA and P-glycoprotein (the product of the MDR1 gene) have been shown to be down-regulated by pro-inflammatory cytokines in a concentration-dependent manner. Interleukin-1β appears to act post-transcriptionally, while interleukin-6 affects P-glycoprotein expression at the transcriptional level (Sukhai et al., Citation2000, Citation2001).
Fluoroquinolones can be successfully used for treatment of colibacillosis in poultry (Charleston et al., Citation1998; Chansiripornchai & Sasipreeyajan, Citation2002). They are substrates for ABC transporters, and are able to modulate their expression (Gollapudi et al., Citation1995; Prime-Chapman et al., Citation2005). However, the interactions between fluoroquinolones and ABC transporters have only been studied in cell lines or in healthy laboratory animals (Cormet-Boyaka et al., Citation1998; Naruhashi et al., Citation2001; Rodriguez-Ibanez et al., Citation2003), and no studies have been conducted in birds. MDR1 and MRP2 are expressed in a variety of tissues, including the intestines. They may affect drug absorption (Giessmann et al., Citation2004) at tissue barriers, prevent the entry of drugs into tissues such as the central nervous system (Fromm, Citation2004), and in excretory organs, including the liver and the kidneys, they also contribute to elimination (Lagas et al., Citation2006; van de Water et al., Citation2007).
In the study described here, we investigated the effect of the therapeutic application of two different fluoroquinolones (danofloxacin mesylate and enrofloxacin) on the levels of expression of MDR1 and MRP2 mRNAs in the intestines and the liver of broilers with experimentally induced colibacillosis.
Materials and Methods
Drugs
Danofloxacin mesylate (Danocin 180, 18% sterile solution; Pfizer) and enrofloxacin (Baytril 5%, 5% sterile solution; Bayer) were dissolved in water and used for oral treatment.
Animals
Mixed-sex 3-week-old broiler chickens (Plymouth Rock, line 99 x Cornish, line K) were obtained from a commercial poultry farm. The weights of the chickens at the start of the experiment were between 0.6 and 0.7 kg. All birds were kept at 19°C to 20°C and had free access to standard commercial feed (without additives) and water. During the acclimatization period, the birds were examined for salmonellosis and colibacillosis. To this end, faecal samples from the birds were investigated for pathogenic serotypes of Escherichia coli and Salmonella spp. using standard microbiological assays.
Experimental infection
E. coli O78/K80 strain, isolated from a chicken with colibacillosis, was obtained from the National Scientific and Diagnostic Institute of Veterinary Medicine, Sofia, Bulgaria. The strain was stored on beads at −70°C prior to use. The day before infection, E. coli was inoculated onto trypticase soy blood agar (Becton Dickinson) and incubated at 37°C for 24 h, and then eight colonies were suspended in 9 ml Mueller–Hinton broth (Becton Dickinson) and the broth was incubated at 37°C for 18 h. A 0.2 ml volume of the overnight culture, containing 2.6 x 108 colony-forming units, was instilled into the trachea of each bird. The inoculum was also cultured on trypticase soy blood agar for enumeration and confirmation of the identity and viability of the bacteria. Clinical signs of infection were seen in most animals within 24 h of inoculation. Three birds died during this period with lesions typical of colibacillosis. Tissue samples collected from experimentally infected chickens at necropsy were cultured to confirm that the strain used to inoculate the birds caused the mortalities (Fernandez et al., Citation1998). The minimum inhibitory concentration of danofloxacin and enrofloxacin for the E. coli strain was 0.25 µg/ml.
Study design and treatment
Infected birds were randomly allocated to one of three groups. The first group (10 birds) was infected, but remained untreated and served as a positive control group. A second group of infected birds was treated with danofloxacin mesylate (10 birds), and a third group of infected birds was treated with enrofloxacin (eight birds). A fourth group of eight uninfected chickens served as uninfected controls. The birds from the first group were euthanized 24 h after infection, and tissue samples (duodenum, jejunum, ileum, caeca, colon and liver) were taken. Tissue samples were also collected from four uninfected broilers.
Treatment was provided in the drinking water, commenced 24 h after inoculation and continued for a period of 5 days. The medicated water was offered ad libitum, and fresh batches of medicated water were prepared every 12 h, with water intake monitored every 12 h. The concentrations of the drugs in the drinking water were checked with a microbiological assay, using E. coli ATCC 25922, immediately after preparation and at the end of each 12-h interval. The treatments aimed for a daily dose of danofloxacin mesylate of 6 mg/kg or a dose of enrofloxacin of 10 mg/kg, respectively. At the end of the experiment, water consumption and the concentration of fluoroquinolones in the water were used to calculate the mean dose ingested. The second group ingested 5 to 9.17 mg danofloxacin mesylate/kg, and the third group 10.43 to 13.95 mg enrofloxacin/kg. Twenty-four hours after the last treatment, the birds were euthanized and tissue samples (duodenum, jejunum, ileum, caeca, colon and liver) were collected. Tissue samples were also collected from the remaining four healthy animals, and served as control samples. All samples were stored at −70°C until analysis.
RNA isolation and cDNA synthesis
Total RNA was isolated using Trizol Reagent (Invitrogen Life Technologies) according to the manufacturer's instructions. RNA concentrations were determined by ultraviolet absorbance at 260 and 280 nm, and were stored for a short period at −70°C prior to cDNA synthesis.
Single-stranded cDNAs were synthesized from 1 µg total RNA using the iScript™ cDNA Synthesis Kit (Bio-Rad Laboratories). To a master mixture containing 5 x iScript Reaction Mix and iScript reverse transcriptase, 1 µg total RNA dissolved in sterile nuclease-free water was added. The reaction mixture (total volume 20 µl) was incubated for 5 min at 25°C, then for 45 min at 42°C, and then the enzyme was heat inactivated at 85°C for 5 min and the reaction mixture rapidly cooled to 4°C. The cDNA was diluted 1:10 in sterile RNase-free water and stored at −20°C until analysis by polymerase chain reaction (PCR).
Real-time polymerase chain reaction analysis
Primer pairs specific for MDR1 and MRP2 () and for the house-keeping genes for glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and β-actin were used for real-time PCR analysis. Primers were used at a concentration of 10 pmol/µl. Ten microlitres of diluted cDNA were mixed with 15 µl iQTM SYBR Green Supermix (Bio Rad Laboratories), forward and reverse primers and sterile water according to the manufacturer's instructions. Real-time PCR was performed using an iCycler iQ system and MyiQ System software version 1.0.410 (Bio Rad Laboratories). The stability of transcription of the housekeeping genes during experimental infection with E. coli O78/K80, treatment with danofloxacin mesylate and treatment with enrofloxacin was tested using the algorithm described by Radonic et al. (Citation2004). Gene expression was assessed using the algorithm described by Vandesompele et al. (Citation2002) and the geNorm software available online (http://medgen.ugent.be/∼jvdesomp/genorm/). Dilution series of target and reference nucleic acids were used to determine the fit coefficients of the relative standard curves (the efficiency of the PCRs). The standards were run at the same time as the samples. Each PCR included a negative control that did not contain a cDNA template. All samples were run in duplicate.
Table 1. Nucleotide sequences of PCR primers used to assay gene expression by real-time PCR
Statistical analysis
All data were analysed using the Statistica 6.1 program (Statistica for Windows; StatSoft). Data were compared using the Mann–Whitney test with P<0.05 considered significant. Comparative levels of transcription in different organs were assessed using the classification system of Vandesompele et al. (Citation2002), with low levels of expression defined as being equal to or lower than the MDR1 mRNA levels in the caecum (0.2 relative units of expression), moderate levels defined as those lying between high and low levels of expression, and high levels defined as equal to or higher than MDR1 mRNA levels in the ileum (1 relative unit of expression).
Results
All birds developed clinical signs of disease, including depression and weakness, within 24 h of inoculation with E. coli O78/K80. Gross lesions included a two-fold to three-fold increase in the size of the spleen, pericarditis and dilation of the blood vessels in the gastrointestinal tract. Mild airsacculitis was observed in a few chickens. The most prominent changes were found in the small and large intestine, and included haemorrhagic enterocolitis with a large number of erosions, which could be seen in all infected animals, particularly in the untreated infected group (positive controls).
MDR1 mRNA was detected at high levels in all samples of the small intestine in healthy broilers. While the levels of mRNA in the caeca were low, those in the colon and the liver were moderate (). After infection with E. coli O78/K80, there was a significant decrease in MDR1 mRNA levels in the duodenum, jejunum, caeca and liver within 24 h (a). After treatment with danofloxacin, MDR1 mRNA levels increased, but were still significantly lower in the duodenum and liver than in healthy controls. After enrofloxacin treatment, the MDR1 mRNA levels remained significantly lower in the duodenum and caeca (a).
Figure 1. Levels of MDR1 and MRP2 mRNA relative to those of GAPDH and β-actin mRNA in healthy broiler chickens (n = 8). Data presented as medians and ranges.
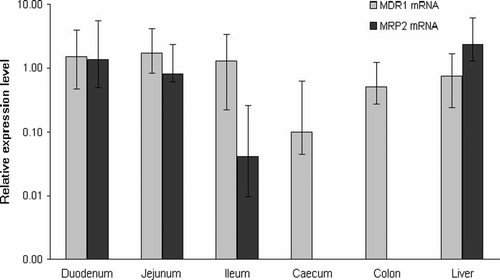
Figure 2. Levels of expression of (2a) MDR1 and (2b) MRP2 mRNA in chickens (n = 8) experimentally infected with E. coli relative to those of uninfected animals (n = 4). Tissue samples from both groups were obtained 24 h after inoculation of the experimentally infected birds. Data presented as medians and ranges. *P < 0.05 compared with uninfected controls.
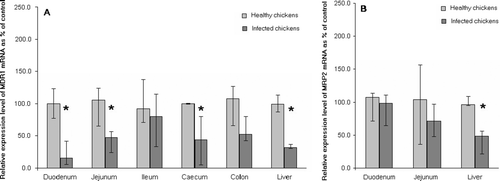
Figure 3. Levels of expression of (3a) MDR1 and (3b) MRP2 mRNA in control animals (n = 4) and in infected chickens (n = 8 per group). The infected chickens were treated with either danofloxacin mesylate or enrofloxacin for 5 consecutive days, starting 24 h after inoculation with E. coli. Tissue samples from the uninfected controls and the treated chickens were taken on day 6 after inoculation. Data presented as medians and ranges. *P < 0.05 compared with healthy controls.
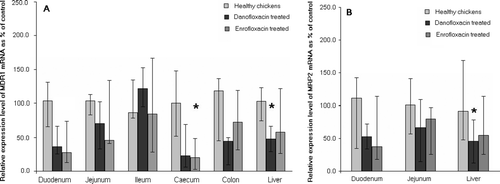
MRP2 mRNA was detected predominantly in the liver and small intestine, with high levels found in the duodenum, moderate levels in the jejunum and low levels in the ileum (). The levels in the caeca and colon were at the limit of detection (data not shown). MRP2 mRNA levels decreased significantly in the liver after infection (b). In the danofloxacin-treated birds, levels of MRP2 mRNA in the liver remained significantly lower than in uninfected birds, but there was no significant difference between the levels in birds treated with enrofloxacin and uninfected birds (b).
The levels of housekeeping gene mRNAs did not differ between the four experimental groups.
Discussion
There have been few studies examining the expression and function of ABC transporters in birds, and none have examined mRNA levels in different organs of chickens. The distribution of MDR1 and MRP2 mRNAs in the intestines and livers of healthy broilers had a distribution comparable with that seen in mammalian species (Stephens et al., Citation2002; Langmann et al., Citation2003; Maher et al., Citation2005; Thörn et al., Citation2005). Our observations confirm those of the two previous reports on MDR1 mRNA and P-glycoprotein in poultry (Edelman et al., Citation1999; Barnes, Citation2001), but there have not been any published studies on the expression and function of MRP2 in birds.
MDR1 and MRP2 were examined in these studies because of their role as defence transporters. The product of MDR1, P-glycoprotein, is an important component of tissue barriers, including that in the gut (Bertilsson et al., Citation2001; Borst & Elferink, Citation2002). The product of MRP2 plays a significant role in excretory organs, including the liver and the kidneys. In the liver, MRP2 (together with bile salt export pump) is involved in the efflux of bile acids and has a protective role against intracellular accumulation of bile acids and their conjugates, including conjugates to many drugs and toxins (Elias & Mills, Citation2007). Several diseases, including obstructive cholestasis, are associated with a down-regulation of hepatic ABC transporters during the acute phase response to infection (Geier et al., Citation2007). It is assumed that pro-inflammatory cytokines mediate this reduced expression and activity via hepatic nuclear receptors. In mice and rats, the expression of MRP2 mRNA is down-regulated in the liver and the intestines during the acute inflammatory response (Tang et al., Citation2000; Hartmann et al., Citation2002; Kalitsky-Szirtes et al., Citation2004). In obstructive cholestasis in humans, no changes in MRP2 mRNA levels were detected, even though MRP2 protein levels were markedly reduced, suggesting post-transcriptional regulation (Geier et al., Citation2005).
Injection of lipopolysaccharide has been shown to cause a decrease in the level of MRP2 and MDR1 in the livers of rats, but an increase in the level of other transporters belonging to the MRP family such as MRP1 (Cherrington et al., Citation2004). Dexamethasone was able to reverse these effects. On the basis of these results, the authors concluded that the adaptive response to lipopolysaccharide and to dexamethasone treatment is mediated through similar pathways, namely transcriptional regulation of hepatic transporters.
The work described in this paper found that infection of chickens with E. coli initially caused significant down-regulation of MDR1 mRNA in the liver and caeca, but the levels of this transporter in the small intestines also decreased, distinguishing the response in poultry from that seen in rodents to lipopolysaccharide. A decrease in MDR1 mRNA and a resultant decrease in P-glycoprotein expression in the intestines may increase permeability for toxins, including lipopolysaccharide, and accelerate the adverse effects of infection. MRP2 mRNA levels were significantly suppressed only in the liver after infection with E. coli. This decrease may affect excretory processes in the liver.
Following the 5-day treatment period with either of the fluoroquinolones, the levels of MDR1 mRNA in the small intestines returned towards those seen prior to infection, with the exception of those in the duodenum. This is most likely to have resulted from the antimicrobial effect of the fluoroquinolones and the consequent reduction in inflammation, rather than from a direct effect of these drugs on mRNA expression. Samples from the treated animals were obtained 6 days after infection, when the animals had improved clinically. Thus clinical improvement preceded the complete restoration of MDR1 mRNA levels.
The increase in the levels of MDR1 following therapy is likely to reflect a beneficial effect on the bird, as this up-regulation would improve the barrier function of the gastro-intestinal tract (Panwala et al., Citation1998; Schwab et al., Citation2003; Leslie et al., Citation2005). In the liver an increase in the levels of MRP2 is likely to be beneficial, as this transporter is essential for the efflux of glucuronides and other conjugates. Bilirubin can only be excreted as a glucuronide, and normal levels of MRP2 are required to protect hepatocytes from oxidative damage by free bilirubin. Thus, the persistence of down-regulation of MRP2 mRNA in the liver following the administration of danofloxacin mesylate requires further investigation.
In conclusion, our study provides evidence that the tissue distribution of these two ABC transporters in chickens is similar to that seen in mammals. The efflux transporters MRP2 and MDR1 are involved in a range of physiological functions, including the maintenance of the gut barrier, and the protection of hepatocytes. Infection with E. coli modulates the levels of mRNA from these genes in the liver and the intestines of chickens. Further investigation of the function of these ABC transporters is now needed to assess the clinical significance of these changes.
References
- Barnes , D.M. 2001 . Expression of P-glycoprotein in the chicken. Comparative Biochemistry and Physiology . Part A Molecular and Integrative Physiology , 130 : 301 – 310 .
- Beigneux , A.P. , Moser , A.H. , Shigenaga , J.K. , Grunfeld , C. and Feingold , K.R. 2002 . Reduction in cytochrome P-450 enzyme expression is associated with repression of CAR (constitutive androstane receptor) and PXR (pregnane X receptor) in mouse liver during the acute phase response . Biochemical and Biophysical Research Communications , 293 : 145 – 149 .
- Bertilsson , P.M. , Olsson , P. and Magnusson , K.-E. 2001 . Cytokines influence mRNA expression of cytochrome P450 3A4 and MDR1 in intestinal cells . Journal of Pharmaceutical Sciences , 90 : 638 – 646 .
- Borst , P. and Elferink , R.O. 2002 . Mammalian ABC transporters in health and disease . Annual Review of Biochemistry , 71 : 537 – 592 .
- Chansiripornchai , N. and Sasipreeyajan , J. 2002 . Efficacy of sarafloxacin in broilers after experimental infection with Escherichia coli . Veterinary Research Communications , 26 : 255 – 262 .
- Charleston , B. , Gate , J.J. , Aitken , I.A. , Stephan , B. and Froyman , R. 1998 . Comparison of the efficacies of three fluoroquinolone antimicrobial agents, given as continuous or pulsed-water medication, against Escherichia coli infection in chickens . Antimicrobial Agents and Chemotherapy , 42 : 83 – 87 .
- Cherrington , N.J. , Slitt , A.L. , Li , N. and Klaassen , C. 2004 . Lipopolysaccharide-mediated regulation of hepatic transporter mRNA levels in rats . Drug Metabolism and Disposition , 32 : 734 – 741 .
- Cormet-Boyaka , E. , Huneau , J.-F. , Morderelle , A. , Boyaka , P.N. , Carbon , C. , Rubinstein , E. and Tome , D. 1998 . Secretion of sparfloxacin from the human intestinal Caco-2 cell line is altered by P-Glycoprotein inhibitors . Antimicrobial Agents and Chemotherapy , 42 : 2607 – 2611 .
- Dean , M. and Annilo , T. 2005 . Evolution of the ATP-binding cassette (ABC) transporter superfamily in vertebrates . Annual Review of Genomics and Human Genetics , 6 : 123 – 142 .
- Edelmann , H.M. , Duchek , P. , Rosenthal , F.E. , Foger , N. , Glackin , C. , Kane , S.E. and Kuchler , K. 1999 . Cmdr1 a chicken P-glycoprotein, confers multidrug resistance and interacts with estradiols . Biological Chemistry , 380 : 231 – 241 .
- Elias , E. and Mills , C.O. 2007 . Coordinated defence and the liver . Clinical Medicine , 7 : 180 – 184 .
- Fernandez , A. , Lara , C. , Puyuelo , R. , Gomez , J. , Ramos , J. J. , Loste , A. , Marca , M.C. and Verde , M.T. 1998 . Efficacy of phosphomycin in the control of E. coli infection of broiler chickens . Research in Veterinary Science , 65 : 201 – 204 .
- Fromm , F.M. 2004 . Importance of Pglycoprotein at blood-tissue barriers . Trends in Pharmacological Sciences , 25 : 423 – 429 .
- Geier , A. , Dietrich , C.G. , Voigt , S. , Ananthanarayanan , M. , Lammert , F. , Schmitz , A. , Trauner , M. , Wasmuth , H.E. , Boraschi , D. , Balasubramaniyan , N. , Suchy , F.J. , Matern , S. and Gartung , C. 2005 . Cytokine-dependent regulation of hepatic organic anion transporter gene transactivators in mouse liver. American Journal of Physiology . Gastrointestinal and Liver Physiology , 289 : G831 – G841 .
- Geier , A. , Wagner , M. , Dietrich , C.G. and Trauner , M. 2007 . Principles of hepatic organic anion transporter regulation during cholestasis, inflammation and liver regeneration . Biochimica et Biophysica Acta , 1773 : 283 – 308 .
- Giessmann , T. , May , K. , Modess , C. , Wegner , D. , Hecker , U. , Zschiesche , M. , Dazert , P. , Grube , M. , Schroeder , E. , Warzok , R. , Cascorbi , I. , Kroemer , H.K. and Siegmund , W. 2004 . Carbamazepine regulates intestinal P-glycoprotein and multidrug resistance protein MRP2 and influences disposition of talinolol in humans . Clinical Pharmacology and Therapeutics , 76 : 192 – 200 .
- Gollapudi , S. , Thadepalli , F. , Kim , C.H. and Gupta , S. 1995 . Difloxacin reverses multidrug resistance in HL-60/AR cells that overexpress the multidrug resistance-related protein (MRP) gene . Oncology Research , 7 : 213 – 225 .
- Hartmann , G. , Cheung , A.K.Y. and Piquette-Miller , M. 2002 . Inflammatory cytokines, but not bile acids, regulate expression of murine hepatic anion transporters in endotoxemia . The Journal of Pharmacology and Experimental Therapeutics , 303 : 273 – 281 .
- Kalitsky-Szirtes , J. , Shayeganpour , A. , Brocks , D.R. and Piquette-Miller , M. 2004 . Suppression of drug-metabolizing enzymes and efflux transporters in the intestine of endotoxin-treated rats . Drug Metabolism and Disposition , 32 : 20 – 27 .
- Lagas , J.S. , Vlaming , M.L. , van Tellingen , O. , Wagenaar , E. , Jansen , R.S. , Rosing , H. , Beijnen , J.H. and Schinkel , A.H. 2006 . Multidrug resistance protein 2 is an important determinant of paclitaxel pharmacokinetics . Clinical Cancer Research , 12 : 6125 – 6132 .
- Langmann , T. , Mauerer , R. , Zahn , A. , Moehle , C. , Probst , M. , Stremmel , W. and Schmitz , G. 2003 . Real-time reverse transcription-PCR expression profiling of the complete human ATP-binding cassette transporter superfamily in various tissues . Clinical Chemistry , 49 : 230 – 238 .
- Leshchinsky , T.V. and Klasing , K.C. 2001 . Divergence of the inflammatory response in two types of chickens . Developmental and Comparative Immunology , 25 : 629 – 638 .
- Leslie , E.M. , Deeley , R.G. and Cole , S.P.C. 2005 . Multidrug resistance proteins: role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense . Toxicology and Applied Pharmacology , 204 : 216 – 237 .
- Linton , K.J. 2007 . Structure and function of ABC transporters . Physiology , 22 : 122 – 130 .
- Maher , J.M. , Slitt , A.L. , Cherrington , N.J. , Cheng , X. and Klaassen , C.D. 2005 . Tissue distribution and hepatic and renal ontogeny of the multidrug resistance-associated protein (MRP) family in mice . Drug Metabolism and Disposition , 33 : 947 – 955 .
- McRae , M.P. , Brouwer , K.L.R. and Kashuba , A.D.M. 2003 . Cytokine regulation of P-glycoprotein . Drug Metabolism Reviews , 35 : 19 – 33 .
- Mireles , A.J. , Kim , S.M. and Klasing , K.C. 2005 . An acute inflammatory response alters bone homeostasis, body composition, and the humoral immune response of broiler chickens . Poultry Science , 84 : 553 – 560 .
- Naruhashi , K. , Tamai , I. , Inoue , N. , Muraoka , H. , Sai , Y. , Suzuki , N. and Tsuji , A. 2001 . Active intestinal secretion of new quinolone antimicrobials and the partial contribution of P-glycoprotein . Journal of Pharmacy and Pharmacology , 53 : 699 – 709 .
- Naud , J. , Michaud , J. , Boisvert , C. , Desbiens , K. , Leblond , F.A. , Mitchell , A. , Jones , C. , Bonnardeaux , A. and Pichette , V. 2007 . Down-regulation of intestinal drug transporters in chronic renal failure in rats . The Journal of Pharmacology and Experimental Therapeutics , 320 : 978 – 985 .
- Panwala , C.M. , Jones , J.C. and Viney , J.L. 1998 . A novel model of inflammatory bowel disease: mice deficient for the multiple drug resistance gene, mdr1a, spontaneously develop colitis . The Journal of Immunology , 161 : 5733 – 5744 .
- Prime-Chapman , H. , Moore , V. and Hirst , B.H. 2005 . Antibiotic exposure does not influence MRP2 functional expression in Caco-2 cells . Journal of Drug Targeting , 13 : 1 – 6 .
- Radonic , A. , Thulke , S. , Mackay , I.M. , Landt , O. , Siegert , W. and Nitsche , A. 2004 . Guideline to reference gene selection for quantitative real-time PCR . Biochemical and Biophysical Research Communications , 313 : 856 – 862 .
- Rodriguez-Ibanez , M. , Nalda-Molina , R. , Montalar-Montero , M. , Bermejo , M.V. , Merino , V. and Garrigues , T.M. 2003 . Transintestinal secretion of ciprofloxacin, grepafloxacin and sparfloxacin: in vitro and in situ inhibition studies . European Journal of Pharmaceutics and Biopharmaceutics , 55 : 241 – 246 .
- Schwab , M. , Schaeffler , E. , Marx , C. , Fromm , M.F. , Kaskas , B. , Metzler , J. , Stange , E. , Herfarth , H. , Schoelmerich , J. , Gregor , M. , Walker , S. , Cascorbi , I. , Roots , I. , Brinkmann , U. , Zanger , U. and Eichelbaum , M. 2003 . Association between the C3435T MDR1 gene polymorphism and susceptibility for ulcerative colitis . Gastroenterology , 124 : 26 – 33 .
- Siewert , E. , Dietrich , C.G. , Lammert , F. , Heinrich , P.C. , Matern , S. , Gartung , C. and Geier , A. 2004 . Interleukin-6 regulates hepatic transporters during acute-phase response . Biochemical and Biophysical Research Communications , 322 : 232 – 238 .
- Stephens , R.H. , Tanianis-Hughes , J. , Higg , N.B. , Humphrey , M. and Warhurst , G. 2002 . Region-dependent modulation of intestinal permeability by drug efflux transporters: in vitro studies in mdra (−/ − ) mouse intestine . The Journal of Pharmacology and Experimental Therapeutics , 303 : 1095 – 10101 .
- Stienstra , R. , Lichtenauer-Kaligis , E. and Muller , M. 2004 . Stress- (and diet-) related regulation of hepatic nuclear receptors and its relevance for ABC-transporter functions . Drug Metabolism Reviews , 36 : 391 – 406 .
- Sukhai , M. , Yong , A. , Kalitsky , J. and Piquette-Miller , M. 2000 . Inflammation and Interleukin-6 mediate reductions in the hepatic expression and transcription of the mdr1a and mdr1b genes . Molecular Cell Biology Research Communications , 4 : 248 – 256 .
- Sukhai , M. , Yong , A. , Pak , A. and Piquette-Miller , M. 2001 . Decreased expression of P-glycoprotein in interleukin-1b and interleukin-6 treated rat hepatocytes . Inflammation Research , 50 : 362 – 370 .
- Tang , W. , Yi , C. , Kalitsky , J. and Piquette-Miller , M. 2000 . Endotoxin downregulates hepatic expression of P-glycoprotein and MRP2 in 2-acetylaminofluorene-treated rats . Molecular Cell Biology Research Communications , 4 : 90 – 97 .
- Thörn , M. , Finnström , N. , Lundgren , S. , Rane , A. and Lööf , L. 2005 . Cytochromes P450 and MDR1 mRNA expression along the human gastrointestinal tract . British Journal of Clinical Pharmacology , 60 : 54 – 60 .
- van de Water , F.M. , Boleij , J.M. , Peters , J.G. , Russel , F.G. and Masereeuw , R. 2007 . Characterization of P-glycoprotein and multidrug resistance proteins in rat kidney and intestinal cell lines . European Journal of Pharmaceutical Sciences , 30 : 36 – 44 .
- Vandesompele , J. , De Peter , K. , Pattyn , F. , Poppe , B. , Van Roy , N. , De Paepe , A. & Speleman , F. 2002 . Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes . Genome Biology, 3, research0034.1–0034.11 .
- Yokooji , T. , Murakami , T. , Yumoto , R. , Nagai , J. and Takano , M. 2006 . Function of multidrug resistance-associated protein 2 in acute hepatic failure rats . European Journal of Pharmacology , 546 : 152 – 160 .