Abstract
In broiler breeder flocks in one broiler integration in Hungary, a new syndrome appeared in January 2005 with initially four successive post-peak flocks experiencing significant decreases in egg production. Clinically birds became depressed and there was a small increase in the mortality rate. Postmortem examinations revealed enlarged livers in up to 19% of birds dying, and enlarged spleens in some. Also observed were birds with either clotted blood or serosanguineous fluid in the abdomen and subcapsular haemorrhages of the liver. Histopathology and polymerase chain reaction excluded tumours and the presence of common tumour-associated viruses. Chronic bacterial infections (especially causing hepatitis, peritonitis and airsacculitis) were common but many enlarged livers had no obvious bacterial involvement. After a 9-month period during which a majority of flocks became affected, no newly affected flocks occurred. Investigations showed that all tested affected flocks were seropositive in the big liver and spleen (BLS) Agar Gel Immunodiffusion test. Subsequent flocks without post-peak egg-production drops were shown to be seronegative in the BLS AGID test, as were all the parent flocks contributing to the affected flocks. Liver samples and cloacal swabs were positive by polymerase chain reaction (aHEV helicase target), and calicivirus-like particles were demonstrated in bile samples from affected birds. These observations are similar to hepatitis-splenomegaly syndrome as described in North America and BLS syndrome as described in Australia. Histopathological features were a non-specific chronic hepatitis similar to those described in BLS and hepatitis-splenomegaly syndrome. Immunohistochemistry using a BLS-specific monoclonal antibody confirmed the presence of avian hepatitis E virus antigen in livers and spleen.
Introduction
Avian hepatitis E virus (aHEV) is a recently identified hepevirus infecting chickens that has been associated with big liver and spleen (BLS) disease in Australia (Payne, Citation2003) and hepatitis-splenomegaly syndrome (HSS) in North America (Shivaprasad, Citation2003). Decreased egg production associated with the presence of big livers and spleens among flock mortalities characterize both these syndromes. Mortality increases and decreased egg production may be subtle. In HSS there is often blood-stained fluid in the abdomen. Common histopathological changes in these syndromes include a non-specific hepatitis, although hepatic amyloidosis and vascular lesions have been described with HSS.
aHEV is genetically related to human hepatitis E virus but is distinct (Payne et al., Citation1999; Haqshenas et al., Citation2002). Although previous serological surveys have suggested a worldwide distribution of aHEV (Payne, Citation2003; Todd et al., Citation1993), clinical cases have been rarely reported outside North America and Australia. There is only one previous preliminary report of the disease in Europe occurring in Italy (Massi et al., Citation2005).
The present paper describes observations on broiler breeder flocks demonstrated to have aHEV infection in Hungary.
Materials and Methods
A 10-month field investigation into a novel clinical syndrome of an initially unknown cause was undertaken. The study had three distinct phases. Phase 1 was the detection of the problem during routine production and health monitoring (initially in four farms). The second phase was the realization that the problem was novel and possibly a variant of BLS after ruling out tumour aetiology. The third phase was the development of diagnostic tests to confirm and define the syndrome (so that future laboratory studies could be undertaken to reproduce the disease if required). A fourth phase to undertake longitudinal studies was planned but affected flocks stopped developing.
Epizootiological and clinical observations
Ross 308 parent stock flocks were reared and moved into production houses on 17 farms owned by one integrator in Hungary. These flocks were hatched in Hungary but derived from eggs produced by grandparent flocks in seven European countries. The average house size was 4000 birds (range 3000 to 6000) with 6 to 12 houses per farm. Farms were run as a single-age site (the maximum age range between houses was 3 weeks) on all-in and all-out principles. Not all farms in the integration were affected during the period of study. Also in the area were commercial layers, geese and broilers that were associated with the integration. Wild bird populations were moderate on farms but houses were well bird-proofed; the only birds that occasionally accessed sheds and feedbins were sparrows and swifts. Normal vaccination programmes included Marek's disease vaccine (serotypes 1 and 3), coccidiosis, Newcastle disease virus, infectious bronchitis virus including important local infectious bronchitis virus variants, avian metapneumovirus, infectious bursal disease, fowlpox, avian encephalomyelitis virus, chicken anaemia virus and killed Duck adenovirus A (EDS-76 strain) vaccines. The flocks were maintained free of Mycoplasma gallisepticum and Mycoplasma synoviae infections and were regularly tested to confirm freedom. Spiking (partial replacement or supplementation of males around 40 weeks) to maintain or restore fertility was practised on a shed by shed basis. Flocks were routinely culled at 60 to 65 weeks of age.
Egg production, fertility, hatchability, vaccination and laboratory records were examined and compared with breed standards. Management and environmental factors were assessed according to current modern practises.
Gross pathology
Once a week, all farm mortalities for the previous 2 days were surveyed by gross postmortem examination, a practice carried out for at least the past decade. Liver size was assessed by one experienced observer (G.S.) as either normal or enlarged. Loss of sharp edges of the lobes was the mildest change observed. Other features of enlarged livers included increased asymmetry between the lobes and the observation that the liver surface area was larger and affected livers bulged from the incised abdominal cavity. No data were collected on the incidence of enlarged spleens. presents a summary of the most significant lesions seen during routine examinations of flocks during Phase 1 of the investigation. Some keel bones were prepared by removing all the flesh by boiling and bleaching with hydrogen peroxide solution.
Table 1. Summary of postmortem findings for the first four flocks considered affected with a new syndrome of post-peak production drop and increased incidence of big livers
Histopathological examination
On six occasions, samples of formalin-fixed organs with gross pathological changes were selected for histopathological examination after haematoxylin and eosin staining—and where amyloid was suspected, Congo Red staining. Immunohistochemisty (IHC) was carried out on formalin-fixed paraffin-embedded tissues using the Dako EnVision kit (DAKO Corporation, Carpinteria, USA) with monoclonal antibody (mAb) 1H4 (7.8) (Ellis et al., Citation1995) at 1:1000 dilution for detection of BLS-associated antigen. Negative control sections were prepared using normal mouse serum in place of the mAb. The positive controls were sections from unassociated field cases with confirmed aHEV infection.
Serology
The AGID test was performed as described in Handlinger & Williams (Citation1988) using BLS reagents from the Veterinary Laboratory Agency, Addlestone, UK. The flock results were interpreted using the criteria that <5% reactors was a seronegative flock and >50% was a seropositive flock (P. Curtin, personal communication, 1992).
Electron microscopy
For electron microscopic analysis, bile fluid was diluted 1:10 in cold phosphate-buffered saline. The diluted suspension was centrifuged at a temperature of 4°C at 1300×g for 10 min; this process was repeated with the cleared supernatant. The supernatant was then ultracentrifuged with Beckman Airfuge for 15 min (91 124×g at 130 kPa) on carbon-coated Pioloform copper grids. Hydrophilicity of the carbon surface of the grids was achieved by UV irradiation and immersion in Alcian blue. Negative staining was performed using 1% aqueous uranyl acetate and 1% aqueous phosphotungstic acid. The samples were finally analysed in a Zeiss 906 at 80 kV.
Polymerase chain reaction
Tumour samples on FTA® Classic Cards (Whatman, Maidstone, UK) were subjected to polymerase chain reaction (PCR) examination for ALV-A&B, ALV-C and ALV-J subgroups (Smith et al., Citation1998), Marek's disease virus (Becker et al., Citation1992) and reticuloendotheliosis viruses (REV) (Davison et al., Citation1997).
Tissue samples from the livers, spleens and bile together with cloacal swabs were investigated for the presence of the nucleic acid of aHEV. RNA was isolated using a guanidinium thiocyanate method (Chomczynski & Sacchi, Citation1987). For this, 20 mg each tissue sample was minced into 400 µl of 0.1% diethylpyrocarbonate (DEPC)-treated water. Swab samples were processed in the same way, except that single swabs were soaked into 500 µl DEPC-treated water. Bile samples were investigated by mixing 20 µl bile with 180 µl DEPC-treated water. As a positive control, RNA was extracted from 20 µl material provided as antigen for the AGID test and processed in the way. For organ composite samples (liver, spleen and cloacal swab samples), 60 µl each tissue was pooled and then further processed.
For purification of the nucleic acid, sequentially equal volumes of 2 M sodium acetate, buffer-saturated phenol and chloroform/isoamylalcohol were added to 180 µl fluid and mixed by vigorous shaking for 10 sec before being removed. The sample was then centrifuged for 5 min at 14 000×g. The supernatant was transferred into a new Eppendorf tube and RNAwas ethanol precipitated. The pellet was dissolved in 20 ml TE (10 mM Tris-HCl and 0.1 mM of EDTA (pH 8.0)) buffer containing 0.1% DEPC. For cDNA preparation and reverse transcriptase (RT)-PCR, 3.5 µl extracted RNA was used and processed with the QIAGEN® OneStep RT-PCR kit (Qiagen, Hilden, Germany). For RT-PCR, the degenerate primers helicase F and helicase R were used together with the parameters described by Huang et al. (Citation2002).
Amplification products (25 µl) were analysed by agarose gel electrophoresis after ethidium bromide staining and visualized under UV light. Fragment sizes were determined with reference to a 100-base-pair ladder (Invitrogen GmbH, Lofer, Austria).
Results
The weekly routine necropsies revealed an increased incidence of large livers among the mortality of four flocks (farms) during late production. All affected flocks also had contemporaneous acute production drops of 5% to 25% eggs per hen day production. Neoplasia/tumours were ruled out (see below), and at this stage flocks with egg-production drops and increased big livers post mortem were considered “affected” flocks with a novel clinical syndrome.
Epizootiological and clinical observations
Affected flocks first appeared in January 2005. The age of onset of the syndrome in the first affected flocks is presented in . These flocks were all post peak egg production and post spiking of some sheds on the farm. Farm managers reported that in affected flocks there was an increase in time taken to consume feed and some hens became depressed and reluctant to move. Some flocks exhibited a partial moult. Reviewing data for all affected sheds revealed that egg production decreased in one of two patterns. The first pattern was a decrease of approximately 10% that persisted for about 6 weeks before coming back to near the breed standard. The second pattern was a sudden decrease in egg production of about 25% that recovered slightly after 2 weeks before remaining below target for the rest of the production period (). Fertility and hatchability were also decreased in affected flocks. Chronically affected flocks, especially in the second half of the production period, suffered greater decreased hatchability (as low as 30% to 40%) compared with unaffected flocks (expected 76% at 65 weeks). Fertility was 2% to 20%, on average, below expected. Early embryo deaths during incubation of eggs doubled in affected flocks compared with unaffected flocks. (Decreased fertility contributed to the decision of the manager to spike the flock.) Once birds in a shed developed an egg-production drop, the condition seemed to spread throughout the rest of the sheds on the farm over the following weeks.
Figure 1. Egg production and mortality from one house (flock) affected with decreased production and increased mortality from 43 weeks of age.
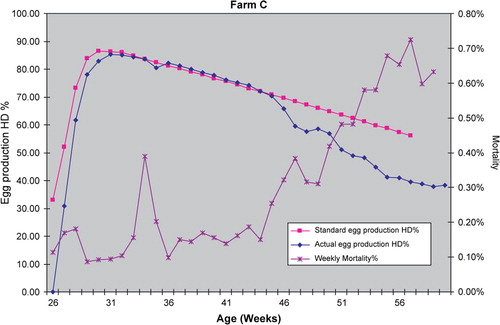
Mortality rarely increased to the stage where the farm managers considered it a feature, but examination of the records often revealed a slight increase in mortality. shows that affected flocks experienced increases in mortality up to nearly 1% per week, 4 to 10 weeks after the egg drop. Normal mortality would be considered <0.25% per week in well-managed flocks.
Investigations into other causes of egg-production decreases, including management factors (nutrition, feed allocation, environmental, etc.), infectious bronchitis virus infection and Mycoplasma infection (serology; data not shown), ruled out these factors as the primary cause of the decrease in egg production.
The number of enlarged livers and spleens in subsequent flocks decreased from 18% of mortality to below 5% of birds examined after 9 months, and the mortality rate also decreased. Clinical effects now appeared to be absent in subsequent flocks.
Pathology
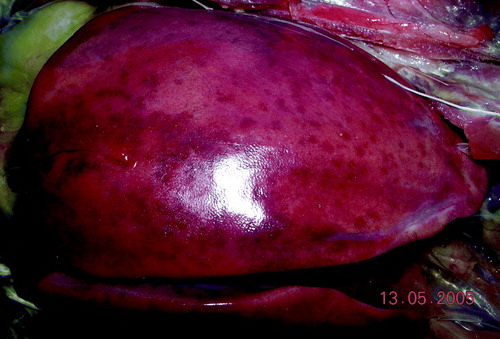
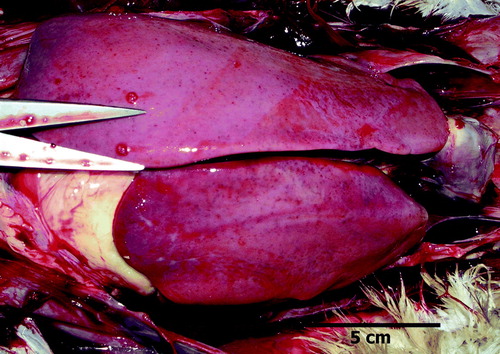
Examination of dead birds from affected flocks during and after the egg-production drop revealed a wide range of pathological conditions. presents a summary of the findings. Birds with large livers () were common, occurring in one affected flock in over 19% of the birds examined, and some of these were sampled for histopathology and bacteriology. Big livers were 5 to 15 times more commonly recorded in absolute terms in affected flocks compared with the control flock when the mortality rate is taken into account. Often the hepatic enlargement was more pronounced in the right lobe and the livers were firm and not friable (). Other enlarged livers were friable. Splenomegaly (the normal spleen in an adult broiler breeder is considered 0.05% to 0.1% of body weight (Handlinger & Williams, Citation1988) was also a frequent finding () but the incidence was not recorded in a systematic way. A variety of bacteria were isolated from the organs sampled, including Avibacterium gallinarum, Escherichia coli, and others. No consistent pattern in bacteriological findings was evident.
Figure 3. Liver with enlarged right lobe and yellow and green foci. Avibacterium gallinarum was isolated from this liver.
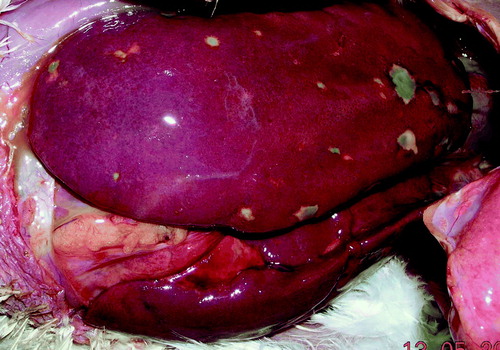
A proportion of birds had subcapsular haemorrhages in the liver or haemorrhages into the abdominal cavity ( and ), sometimes associated with rupture of the liver capsule. In these birds the spleen was often enlarged. This blood had clotted in some cases, but in others a serosanguineous fluid that did not clot with time was present in the abdomen.
Airsacculitis and peritonitis were common findings, but were also common findings in unaffected farms. Airsacculitis was not observed in one affected farm (Farm B; ).
The control farm had the highest incidence of peritonitis among dead birds, but when considered together with the lower mortality rate on the farm this did not represent an increased incidence of peritonitis compared with affected flocks.
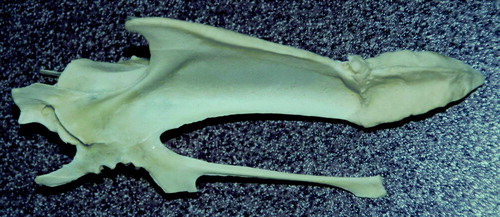
Up to 14% of birds post mortem had keel bones suspected of containing fractures ( and ). Extensive callus formation was observed in many cases. This had not been observed in the integration before the affected flocks were recognized and was also seen at a lower incidence in flocks considered unaffected (including the control farm in ), and indeed has continued to be seen in subsequent flocks after affected flocks had stopped occurring. Sometimes birds with broken keels also had blood-stained fluid in the abdomen that initially was assumed to be associated with trauma, but sometimes the two lesions were not contemporaneous.
Figure 6. Keel bone demonstrating callus. This condition appeared at the same time as the other clinical signs but persisted after affected flocks stopped occurring.
Histopathological examination excluded tumours in the liver (over 30 samples) except for one liver with a histocytic sarcoma and one with a scirrhous adenocarcinoma. Grossly, livers with these tumours were easily distinguishable from the other enlarged livers and spleens. The enlarged livers showed a range of histological lesions, including—in order of decreasing incidence congestion—moderate lymphoid hyperplasia, some diffuse lymphocytic infiltration, areas of diffuse and focal liver cell degeneration and necrosis, a few with areas of fibrinoid material, mild bile duct hyperplasia, some collections of pleomorphic myeloid cells and bacterial foci, and haemosiderin. The spleens (n=8) mostly showed congestion, a mild lymphoid depletion, and fewer with areas of fibrinoid material (in one case collected around the capillary sheaths). Scattered bacterial foci were often present. One spleen showed a plasmacytosis. The lesions were suggestive of a chronic splenitis.
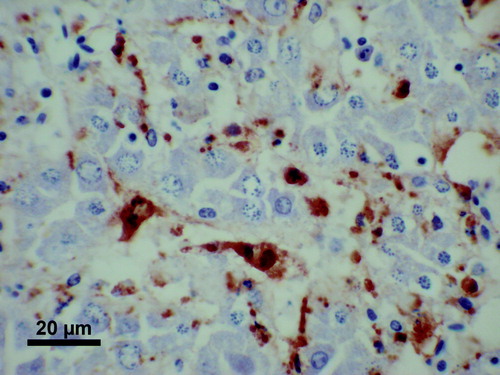
Immunohistochemistry using the 1H4 mAb on tissues already examined by routine histopathology demonstrated large amounts of antigen labelling in some livers (13/21 examined) and some spleens (1/3 examined) of birds that had died. The distribution of the antigen labelling in the liver appeared to be uniform throughout the sinusoidal lining cells (), with very little in areas of lymphoid or myeloid hyperplasia or in areas of chronic inflammatory change. In the spleen, the antigen labelling was often most intense in the peri-ellipsoidal zones, delineating the ellipsoids, but was not confined to these areas.
Serology
No evidence of REV infection was seen when two affected flocks were tested by enzyme-linked immunosorbent assay (data not shown). Testing of these samples in the BLS AGID revealed that 38/80 had precipitin lines of identity with the positive control. Further surveying of flocks (n=13) identified five farms having seropositive flocks corresponding to clinically affected flocks in a survey of all post-peak flocks in the integration (undertaken in Phase 3). Here sheds were either 0/10 positive or 6/10 to 10/10 positive, with all sheds on a farm being similarly classified (data not shown). A survey 1 year after the depletion of affected flocks demonstrated subsequent flocks (two houses per farm, n=10 farms) were seronegative in the AGID test (data not shown). This observation concurred with a disappearance of clinically affected flocks.
Electron microscopy
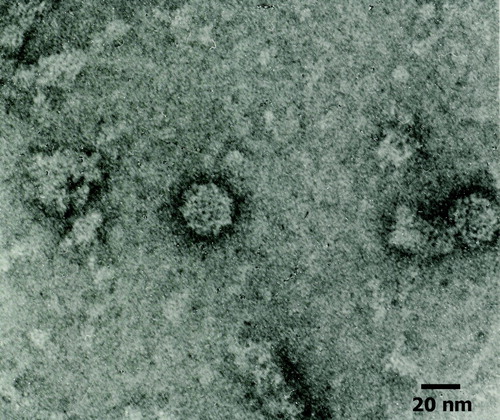
The virions in the bile (n=2) were icosahedral in shape and were non-enveloped. They measured 30 to 34 nm in diameter by negative stain electron microscopy, showing the typical cup-shaped depressions of capsomeres characteristic also for virions of the family Caliciviridae ().
Polymerase chain reaction
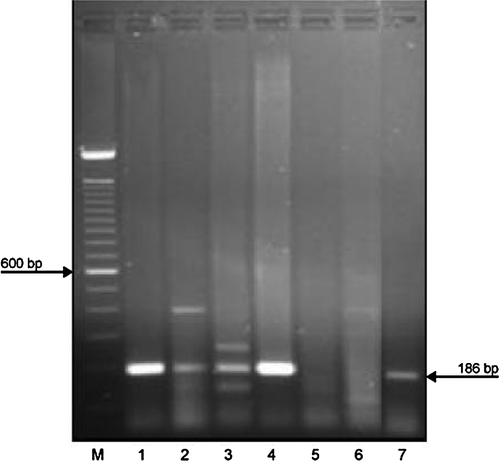
Tissue samples including blood from affected birds were positive by RT-PCR, producing a product of 186 base pairs. Cloacal swabs taken from birds in affected flocks were also RT-PCR-positive (). Bile samples where virions had been observed were also RT-PCR-positive. In total, 16 out of 51 RT-PCR samples were positive. The sequences obtained were related to reported sequences of aHEV viruses and differed significantly from the sequence of the BLS control material (M. Hess. personal communication, 2008).
Figure 9. Agarose gel electrophoresis of PCR products obtained from an organ mix (liver, spleen and cloacal swab material) from Birds 1 to 5 (lanes 1 to 5) of Farm 1. The pooled bile sample taken from Birds 1 to 3 was separated in lane 4. The RT-PCR product from the positive control (BLSV antigen, 187 base pairs) is in lane 7. M, DNA size marker, 100-base-pair (bp) ladder (Invitrogen GmbH, Lofer, Austria).
Sixteen suspect tumour liver samples inactivated on FTA cards (Whatman Plc, Maidstone, UK) were negative for Marek's disease virus, ALV-A, ALV-B, ALV-C, ALV-J and REV by PCR (data not shown).
Discussion
The present paper describes a clinical disease in broiler breeder flocks in Europe that were infected with aHEV. Drops in egg production after peak production, depression, partial moult, enlarged livers and spleens, modest increases in mortality, haemorrhage into the abdomen and fractured keel bones were the main features observed in these flocks when compared with the unaffected flocks. These observations are similar to those reported in North America for HSS, and in Australia and Italy for BLS. The data presented in probably underestimate the impact of aHEV infection because of averaging of all houses on a farm over a long time period. Birds from affected flocks often had peritonitis and chronic bacterial hepatitis, and some birds also had thoracic airsacculitis.
The drops in egg production had one of two patterns. Flocks recovering egg production after 1 month appeared to be those where body weight followed to the breed standard, whereas flocks that gained excessive body weight had more chronic egg-production deficits. This was similar to the experience in Australia with BLS-affected flocks (G. Lea, personal communication, 2007).
The enlarged livers and spleens prompted an investigation into the possibility that a tumour outbreak may have been occurring but no ALV, REV or Marek's disease viruses could be demonstrated. The diagnosis of one histocytic sarcoma raised the question of ALV-J infection (Arshad et al., Citation1997) but this was ruled out by PCR. Histologically no neoplastic features were identified as a flock problem. Enlarged right liver lobes and subscapular haemorrhages as described in experimental aHEV (HSS) infections by Sun et al. (Citation2004b) were observed (although their inocula could also contain other agents) (Billiam et al., 2005).
Histopathological changes were largely non-specific and were summarized as chronic hepatitis and chronic splenitis. Vascular lesions as described for HSS in field cases (Agunos et al., Citation2006) or experimental studies (Sun et al., Citation2004b) were not observed in our field cases. It is not known how long aHEV (or associated antigen) remains present in tissues or birds, but this study suggests that infection is persistent in some birds and seropositivity also persists on a flock basis. Amyloidosis is sometimes seen in HSS livers (Shivaprasad, Citation2003) but was not seen in the birds described in this paper (except for IHC antigen-negative but Congo red-positive (amyloid) material seen in the wall of an artery within the liver of one bird). The vasculitis in HSS was often initially confused with myeloid leucosis in North America (C. Riddell, personal communication, 2007).
The IHC results are interesting, with large amounts of BLS antigen being demonstrated in the big livers and spleens of some dead birds from affected farms. The antigen distribution was similar to that seen in experimental infections with BLS inocula with polyclonal anti-BLS antibody (Clarke et al., Citation1990) but did not appear to be associated with the more dramatic pathological features in the liver or spleen observed in this study.
The observations of Rampin et al. (Citation1989) that repeated vaccination with oil-emulsion vaccines caused amyloidosis and abdominal bleeding are interesting in the comparison with HSS. Clarke et al. (Citation1990) demonstrated that the BLS antigen in experimental infections appeared to be complexed with antibody in dendritic cells in the liver and speculated that the antigen may be being elaborated at another site in the hen. The abdominal bleeding and serosanguineous fluid seen in this study is more characteristic of HSS but vascular lesions were not confirmed histologically. Perhaps interaction with killed vaccination and aHEV infection should be investigated.
The positive controls and reagents used in this study were all derived from BLS material of Australian origin including the 1H4 mAb, although the PCR was designed by workers investigating HSS. The flocks were seropositive for BLS infection by AGID test. PCR of the helicase gene of aHEV was positive in tissue samples, bile samples and cloacal samples. The sequencing of these products confirmed the presence of aHEV (M. Hess, personal communication, 2008). Electron microscopy of bile from affected livers visualized particles consistent with calcivirus-like particles.
Future studies on field cases should collect more control material for histology and IHC studies, and undertake a longitudinal study to investigate antigenaemia and subsequent seroconversion (Crerar & Cross, Citation1994). Antibody-detecting enzyme-linked immunosorbent assays for human hepatitis E virus have been modified and used in chickens to demonstrate antibody (Haqshenas et al., Citation2002; Massi et al., Citation2005), but these need further validation.
The source of the infection is unknown but is being investigated. A survey of the many grandparent flocks producing these flocks confirmed all of them to be seronegative by the AGID test (data not shown), and the observation that other flocks derived from these grandparent flocks were placed all over Europe and were not affected by such production problems provides evidence against vertical transmission as the origin of the infection. It appeared that the infection had a local origin, and anecdotally a layer flock in the region had similar clinical signs 6 months before the first broiler breeder flock was affected. Spiking was practiced extensively during the period when affected flocks were seen and may have played a large role in introduction of the causative agent. Not all sheds on a farm were spiked, but once an affected flock was observed clinical signs rapidly appeared in other houses on the farm—including those that were not spiked. No clinical evidence of vertical transmission from affected flocks was seen with the broiler progeny.
Not all of the clinical observations recorded in these flocks may necessarily be due to aHEV infection. The healed broken keel bones are difficult to explain as a viral infection, and they were also were observed in the control flock at a lower incidence and have continued to occur in subsequent flocks. The high incidence of chronic bacterial hepatitis poses the possibility that aHEV infection predisposes (perhaps via immunosuppression) for bacterial infection.
Recent experimental infections with aHEV have concentrated on virus kinetics (Billam et al., Citation2005) rather than on gross pathological features. Further confirmation by experimental infections using an infectious clone of aHEV similar to the methodology of Huang et al. (Citation2005) is needed to prove causation, but variation in viral virulence and tropism, host genotype, timing and dose of infection and interaction with other management factors, pre-existing immunity and agents may explain the variation in clinical effects of infection between flocks and even individual birds. Viral genotypic features are currently being examined to see whether they can be correlated with phenotypic effects seen in the field.
The variety of gross and histopathological lesions attributed to aHEV infections range from inapparent (Sun et al., Citation2004a) to necrotic, haemorrhagic, hepatomegalic hepatitis associated with vasculitis and amyloidosis (Shivaprasad, Citation2003; Agunos et al., Citation2006) and raises questions as to whether other agents (e.g. Chicken Anaemia Virus) may also be involved, the effects of management factors (timing of infection, killed vaccination load, concurrent fatty liver, etc.), virus strain and bird genotype. It is now suggested that the minimum criterion for the diagnosis of field aHEV infection should be demonstration of specific viral RNA in samples from flocks. BLS and HSS can be diagnosed on clinical signs and pathological grounds with BLS being confirmed by positive serology in the AGID test (antigenaemia and then antibody; Crerar & Cross, Citation1994). HSS can be confirmed by the demonstration of histological vascular lesions. The effects of aHEV infection in broiler breeders requires further investigation based on field observations and experimental infections using currently available tools.
Acknowledgements
The authors would like to thank Peter Curtin for the gift of reagents and Anne Masters for the gift of mAb 1H4, Dr Jim Payne, Dr Grace MacKenzie for histopathological examination, Joyce Shannan and Michael Algar VLA (Lasswade) and staff at the Aviagen laboratory, VLA (Lasswade) and University of Veterinary MedicineVienna for technical assistance. Also a special thanks to Kirsten Gooding for help with the illustrations.
References
- Agunos , A.C. , Yoo , D. , Youssef , S.A. , Ran , D. , Binnington , B. and Hunter , D.B. 2006 . Avian hepatitis E virus in an outbreak of hepatitis-splenomegaly syndrome and fatty liver haemorrhage syndrome in two flaxseed-fed layer flocks in Ontario . Avian Pathology , 35 : 404 – 412 .
- Arshad , S.S. , Bland , A.P. , Hacker , S.M. and Payne , L.N. 1997 . A low incidence of histiocytic sarcomatosis associated with infection of chickens with the HPRS-103 strain of subgroup J avian leukosis virus . Avian Diseases , 41 : 947 – 956 .
- Becker , Y. , Asher , Y. , Tabor , E. , Davidson , I. , Malkinson , M. and Weisman , Y. 1992 . Polymerase chain reaction for differentiation between pathogenic and non-pathogenic serotype 1 Marek's disease viruses (MDV) and vaccine viruses of MDV-serotypes 2 and 3 . Journal of Virological Methods , 40 : 307 – 322 .
- Billam , P. , Huang , F.F. , Sun , Z.F. , Pierson , F.W. , Duncan , R.B. , Elvinger , F. , Guenette , D.K. , Toth , T.E. and Meng , X.J. 2005 . Systematic pathogenesis and replication of avian hepatitis E virus in specific-pathogen-free adult chickens . Journal of Virology , 79 : 3429 – 3437 .
- Chomczynski , P. and Sacchi , N. 1987 . Single-step method of RNA isolation by acid guanidium thiocyanate–phenol–chloroform extraction . Annals of Clinical Biochemistry , 162 : 156 – 159 .
- Clarke , J.K. , Allan , G.M. , Bryson , D.G. , Williams , W. , Todd , D. , Mackie , D.P. and McFerran , J.B. 1990 . Big liver and spleen disease of broiler breeders . Avian Pathology , 19 : 41 – 50 .
- Crerar , S.K. and Cross , G.M. 1994 . The experimental production of big liver and spleen disease in broiler breeder hens . Australian Veterinary Journal , 71 : 414 – 417 .
- Davidson , I. , Alphandary , R. , Novoseler , M. and Malkinson , M. 1997 . Replication of non-defective reticuloendotheliosis viruses in the avian embryo assayed by PCR and immunofluorescence . Avian Pathology , 26 : 579 – 593 .
- Ellis , T.M. , Payne , C.J. , Plant , S.L. and Gregory , A.R. 1995 . An antigen detection immunoassay for big liver and spleen disease agent . Veterinary Microbiology , 46 : 315 – 326 .
- Handlinger , J.H. and Williams , W. 1988 . An egg drop associated with splenomegaly in broiler breeders . Avian Diseases , 32 : 773 – 778 .
- Haqshenas , G. , Huang , F.F. , Fenaux , M. , Guenette , D.K. , Pierson , F.W. , Larsen , C.T. , Shivaprasad , H.L. , Toth , T.E. and Meng , X.J. 2002 . The putative capsid protein of the newly identified avian hepatitis E virus shares antigenic epitopes with that of swine and human hepatitis E viruses and chicken big liver and spleen disease virus . Journal of General Virology , 83 : 2201 – 2209 .
- Huang , F.F. , Haqshenas , G. , Shivaprasad , H.L. , Guenette , D.K. , Woolcock , P.R. , Larsen , C.T. , Pierson , F.W. , Elvinger , F. , Toth , T.E. and Meng , X.J. 2002 . Heterogeneity and seroprevalence of a newly identified avian hepatitis E virus from chickens in the United States . Journal of Clinical Microbiology , 40 : 4197 – 4202 .
- Huang , F.F. , Pierson , F.W. , Toth , T.E. and Meng , X.J. 2005 . Construction and characterization of infectious cDNA clones of a chicken strain of hepatitis E virus (HEV), avian HEV . Journal of General Virology , 86 : 2585 – 2593 .
- Massi , P. , Tosi , G. , Gelmetti , D. , Lavazza , A. , Lombardi , G. and Torcoli , G. 2005 . Big liver and spleen disease in broiler breeders in Italy . Italian Journal of Animal Science , 4 : 303 – 305 .
- Payne , C.J. 2003 . “ Big liver and spleen disease ” . In Diseases of Poultry , 11th edn , Edited by: Saif , Y.M. , Barnes , H.J. , Glisson , J.R. , Fadly , A.M. , McDougald , L.R. and Swayne , D.E. 1184 – 1186 . Ames : Iowa State Press .
- Payne , C.J. , Ellis , T.M. , Plant , S.L. , Gregory , A.R. and Wilcox , G.E. 1999 . Sequence data suggests big liver and spleen disease virus (BLSV) is genetically related to hepatitis E virus . Veterinary Microbiology , 68 : 119 – 125 .
- Rampin , T. , Sironi , G. and Gallazzi , D. 1989 . Amyloidoseepisoden bei Junghennen nach wiederholter Anwendung von antibakteriellen Ölemulsionvakzinen . Episodes of amyloidosis in young hens after repeated use of antibacterial oil emulsion vaccines] Deutsche Tierarztliche Wochenschrift , 96 : 168 – 172 .
- Shivaprasad , H.L. 2003 . “ Hepatitis splenomegaly syndrome ” . In Diseases of Poultry , 11th edn , Edited by: Saif , Y.M. , Barnes , H.J. , Glisson , J.R. , Fadly , A.M. , McDougald , L.R. and Swayne , D.E. 1186 – 1188 . Ames : Iowa State Press (on CD-ROM not in book) .
- Smith , L.M. , Brown , S.R. , Howes , K. , McLeod , S. , Arshad , S.S. , Barron , G.S. , Venugopal , K. , McKay , J.C. and Payne , L.N. 1998 . Development and application of polymerase chain reaction (PCR) tests for the detection of subgroup J avian leukosis virus . Virus Research , 54 : 87 – 98 .
- Sun , Z.F. , Larsen , C.T. , Dunlop , A. , Huang , F.F. , Pierson , F.W. , Toth , T.E. and Meng , X.J. 2004a . Genetic identification of avian hepatitis E virus (HEV) from healthy chicken flocks and characterization of the capsid gene of 14 avian HEV isolates from chickens with hepatitis-splenomegaly syndrome in different geographical regions of the United States . Journal of General Virology , 85 : 693 – 700 .
- Sun , Z.F. , Larsen , C.T. , Huang , F.F. , Billam , P. , Pierson , F.W. , Toth , T.E. and Meng , X.J. 2004b . Generation and infectivity titration of an infectious stock of avian hepatitis E virus (HEV) in chickens and cross-species infection of turkeys with avian HEV . Journal of Clinical Microbiology , 42 : 2658 – 2662 .
- Todd , D. , Mawhinney , K.A. , McAlinden , V.A. and Douglas , A.J. 1993 . Development of an enzyme-linked immunosorbent assay for the serological diagnosis of big liver and spleen disease . Avian Diseases , 37 : 811 – 816 .