Abstract
Highly pathogenic influenza virus (HPAIV) H5N1 has caused mortality and morbidity in many species of domestic and wild birds. The Houbara bustard (Chlamydotis undulata macqueenii) is a solitary bird that inhabits semi-desert regions. It is known to be susceptible to avianpox, avian paramyxovirus type 1, and low-pathogenicity avian influenza H9N2. We report an outbreak of H5N1 HPAIV in Houbara bustards, which were introduced into the Kingdom of Saudi Arabia for falconry purposes. Ninety-three per cent mortality (38 out of 41 birds) in the infected Houbara bustard flock and about 62.5% mortality (10 out of 16 birds) in falcons that came in contact with these birds were observed. Pooled cloacal and tracheal swabs from Houbara bustards as well as visceral organ homogenates collected in Houbara bustards and falcons were tested by real-time reverse transcriptase-polymerase chain reaction, and virus isolation was attempted in specific pathogen free hens’ eggs. The viruses isolated were characterized as HPAIV H5N1. Phylogenetic analysis of the haemagglutinating and Neuraminidase (NA) genes revealed that the viruses isolated from Houbara bustards and falcons were closely related to each other and to Kuwaiti H5N1 strains isolated in 2007. Interestingly, they were genetically distinguishable from the co-circulating A/H5N1 viruses in Kingdom of Saudi Arabia causing outbreaks in domestic birds. This case emphasizes the need for surveillance of this endangered species in its natural habitat.
Introduction
Avian influenza is an infectious disease of birds caused by negative-sense, segmented RNA viruses belonging to genus Influenza virus A of the family Orthomyxoviridae. The infections of chickens caused by highly pathogenic influenza virus (HPAIV) subtype H5N1 generally result in high mortality that approaches 100% (Swayne & Suarez, Citation2000). Avian influenza has been isolated from many species of wild and domestic birds (Alexander, Citation2000), but there are no reports of HPAIV in Houbara bustards (Chlamydotis undulata macqueenii) in the literature.
The Houbara bustard is a rare shy bird inhabiting flat arid plains, steppe habitats and semi-desert areas, with open or scattered scrubby vegetation (IFCDW, Citation2006). The Arabs have a fascination for this bird, as it is one of the traditional quarries for falconers since ancient times. The free-living Houbara bustards are trapped from countries such as Pakistan, Iran and Afghanistan and illegally exported to Middle East countries where they are used by falconers to train their falcons (Bailey et al., Citation2000). In recent decades, there has been a drastic decline in the population of Houbara bustards because of over-hunting and habitat degradation. Because of this alarming situation, this bird is classified as vulnerable in the 2007 International Union for the Conservation of Nature Red List of threatened species (IUCN, Citation2007) and is listed in Appendix I of the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES, Citation2007).
The present paper describes the isolation and characterization of H5N1 HPAIV from Houbara bustards recently introduced in desert camps for the training of falcons and hunting purposes. Within a few days of their arrival, the birds showed high mortality with nervous manifestations, this led to the suspicion of infection with avian influenza and/or avian paramyxovirus type 1.
Materials and Methods
Case history
A total of 41 adult Houbara bustards were introduced in the Kingdom of Saudi Arabia (KSA) and kept in a desert camp in the Samman area, northeast of Riyadh, for falconry purposes. Mortality began in Houbara bustards 2 days after their arrival. Later, it was reported to the avian influenza emergency room of the Ministry of Agriculture that six Houbara bustards along with two falcons had died. According to the anamnestic records collected at the time of the epidemiological investigation, the falcon owners reported that falcons became sick after eating or coming into contact with the carcasses of Houbara bustards from the infected group. At the time of the first expert mission performed by official veterinarians, an additional six falcons and 20 Houbara bustards showed nervous signs of disease.
Sample collection
Samples were collected from the two falcons that died first and from Houbara bustards that died in the subsequent days or from those showing clinical signs at the time of the visit. In detail, tissue samples from the brain, trachea, lungs, spleen and intestine as well as pooled tracheal and cloacal swabs (5 swabs/pool) were collected from six recently dead and five live Houbara bustards showing clinical signs (). Tissue samples from the brain, trachea and lungs were also collected from the two dead falcons (). All samples were collected in phosphate-buffered saline (pH 7 to 7.4) supplemented with antibiotics and were transported refrigerated to the Central Veterinary Diagnostic Laboratory (CVDL) in Riyadh, KSA.
Table 1. Type of samples processed and the results of virus isolation and RRT-PCR from different clinical samples collected in Houbara bustards and falcons
Virus isolation in specific pathogen free hens eggs
Samples were processed at the CVDL as described previously (Senne, Citation1998) for virus isolation in specific pathogen free (SPF) hens’ eggs (Venky's Ltd, Pune, India). All collected tracheal and cloacal swabs from the Houbara bustards as well as tissue samples from Houbara bustards and falcons were inoculated for virus isolation (). Following embryonic death, the amnio allantoic fluid (AAF) was harvested and tested for haemagglutinating (HA) activity and was typed according to standard methods (OIE, Citation2004) using subtype-specific antisera obtained from the Veterinary Laboratory Agency (VLA), Weybridge, UK. Neuraminidase inhibition tests were carried out in two distinct OIE Reference Laboratories (VLA, Weybridge, UK and Istituto Zooprofilattico Sperimentale delle Venezie, Padova, Italy).
Real-time reverse transcriptase-polymerase chain reaction
The same samples processed for virus isolation were also tested by real-time reverse transcriptase-polymerase chain reaction (RRT-PCR) (). RNA was extracted at the CVDL from clinical specimens and from AAF with the QIAamp® Viral RNA Mini Kit (Qiagen, Germany) following the manufacturer's protocol.
A two-step RRT-PCR was applied. For the RT reaction, c-DNA was synthesized using the Transcriptor First Strand cDNA Synthesis Kit (Roche Diagnostic, Germany) following the manufacturer's protocol. Each 20 µl reaction mixture contained 4 µl eluted RNA, 2 µl random hexamer primer (600 pmol/µl), 4 µl of 5× transcriptor RT reaction buffer, 2 µl deoxynucleotide mix (10 mM each), 0.5 µl protector RNAse inhibitor (40 u/µl), 0.5 µl transcriptor RT (20 u/µl) and 7 µl PCR-grade water. After incubation for 10 min at 25°C, RT was carried out for 30 min at 55°C, followed by RT inactivation for 5 min at 85°C. The cDNA was stored at −70°C until use.
A commercially available, ready-to-use, mix of primers and probes—LightMix® for the detection of influenza A virus M2 (TIB MOLBIOL GmbH, Germany)—was used for the real-time PCR screening of samples for influenza virus A type in the LightCycler® 2.0 real-time PCR machine (Roche Diagnostic). The A matrix-positive samples were further subtyped for H5N1 by LightMix® for the detection of (subtype Asia) H5N1 (TIB MOLBIOL GmbH). A 161 base pair fragment of the avian influenza A virus H5 gene was amplified with specific primers and detected with probes labelled with LightCycler® Red 640. Another 198 base pair fragment of the avian influenza A virus N1 gene was amplified with specific primers and detected with probes labelled with LightCycler® Red 705.
The H5 and N1 target DNA supplied with the kit at different concentrations was used as the positive control. A previously characterized Newcastle disease virus isolate was included in the RRT-PCR assay as the negative control virus along with no template control containing PCR-grade water.
Gene sequencing
Viral RNA was extracted at the OIE/FAO Reference Laboratory for avian influenza in Italy (IZSVe) from virus isolation-positive allantoic fluids submitted as pools from the CVDL. One pool was constituted of allantoic fluids collected from SPF eggs inoculated with swabs and tissues from Houbara bustards. The second pool was constituted of allantoic fluids collected from SPF eggs inoculated with the organs of the two falcons that died at the same outbreak site. The RNA was extracted using the Nucleospin RNA II Kit (Macherey-Nagel Düren, Germany). Amplification of the HA and Neurominidase (NA) gene segments were carried out by RT-PCR using gene-specific primers (available upon request). The purified (ExoSAP-IT; USB, Cleveland, Ohio, USA) PCR products were sequenced using an ABI PRISM BigDye Terminator V3.1 Cycle Sequencing kit (Applied Biosystems, Foster City, California, USA) and corresponding primers for each gene. The products of the reaction sequence were purified with an Autoseq™ G-50 Dye Terminator Removal kit (GE Healthcare, Little Chalfont, UK) and run on an ABI PRISM 3130xl Genetic Analyzer (Applied Biosystems). Genetic comparison was carried out using the ClustalW software and the N-J algorithm in the MEGA 3 programme. Bootstraps of 1000 replicates were conducted (Kumar et al., Citation2004).
Accession number
The GenBank accession numbers of the respective HA and NA gene segments of the A/houbara bustard/Saudi Arabia/6732-1/2007 strain are EU424135 and EU445682. For A/falcon/Saudi Arabia/6732-2./2007, and for Strains are EU650486 and EU650487.
Results
At the time of the clinical visit, diseased Houbara bustards with nervous manifestations of torticollis, paralysis of the leg and imbalance were observed. Swollen head, nasal discharge, and greenish diarrhoea were also observed. Feathers around the vent were soiled, and cyanotic shanks and loss of appetite were seen. The falcons showed nervous signs and diarrhoea. Within a span of 4 days, 32 more Houbara bustards died and mortality reached 93% (38 out of 41 birds). The three remaining live birds were killed humanely after H5N1 diagnosis was confirmed.
Generalized congestion, severe tracheitis, haemorrhages in the proventriculus and brain, splenomegaly and nephritis were observed in the Houbara bustards. Following the epidemiological investigation, it was found that 10 out of 16 falcons (62.5%) had also died. These falcons were in contact with the Houbara bustards at the outbreak sites or were fed with carcasses of Houbara bustards from the infected group.
Virus isolation and real-time PCR
For all the samples processed (), the mortality of embryos in inoculated SPF eggs was recorded within 48 h. The harvested AAF was tested for HA activity, and the HA agent was characterized by means of HI and neuraminidase inhibition tests. Based on the results, the isolates were identified as type A avian influenza viruses belonging to the H5N1 subtype. Real-time PCR results were in agreement with virus isolation and confirmed the identification of the virus in the clinical specimens and AAF of Houbara bustards and falcons.
Gene sequencing
Virus sequences for the HA and NA genomic segments were obtained from the two pooled AAF samples analysed. Isolates were identified as A/houbara bustard/Saudi Arabia/ 6732-1/2007 (H5N1) and A/falcon/Saudi Arabia/6732-2/2007(H5N1). For both Houbara bustard and falcon isolates, the deduced amino acid sequence at the cleavage site of the pre-cursor HA0 was PQGERRRKKR*GLF, which is characteristic of current H5N1 HPAIV.
The phylogenetic analysis of the haemagglutinin () and neuraminidase (data not shown) genes showed that A/houbara bustard/Saudi Arabia/ 6732-1/2007 and A/falcon/Saudi Arabia/6732-2/2007 isolates belong to the EMA-3 sublineage (Salzberg et al., Citation2007), named 2.2.3 according to the recently recommended nomenclature (WHO/OIE/FAO H5N1 Evolution Working Group, Citation2008) and located within the genetic cluster 2.2 of H5N1 ().
Figure 1. Unrooted phylogenetic tree for representative HA nucleotide sequences of clade 2.2 constructed by the neighbour-joining method. Black circles, sequences generated from the viruses described in this study. Selected A/H5N1 viral sequences are included for comparison. White circles, sequences generated from viruses isolated in poultry in the KSA. Bootstrap values >50% are shown at the nodes. Genetic sublineages included in lineage 2.2 are indicated on the right-hand side.
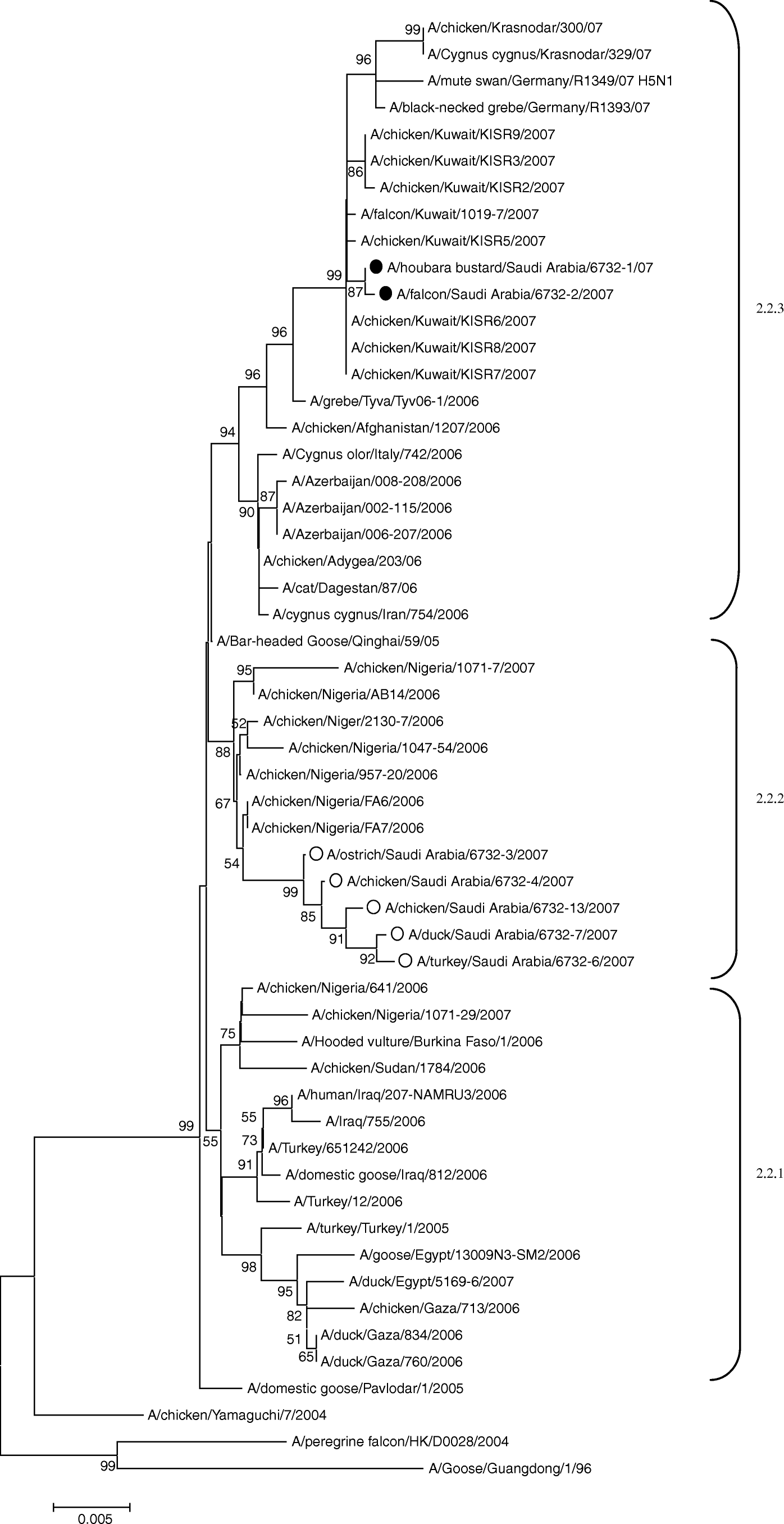
Comparison with sequences of viruses isolated in other species in KSA or in the Middle East showed that the HA and NA genes of the A/houbara bustard/Saudi Arabia/ 6732-1/2007 and A/falcon/Saudi Arabia/6732-2/2007 strains were closely related to each other (99.9% similarity at the nucleotide level for HA and NA gene segments). Amino acid sequence analysis revealed that the NA protein of the two isolates was identical, while only one amino acid difference was observed in the HA protein at position 110 (asparagine for A/houbara bustard/Saudi Arabia/6732-1/2007 and aspartic acid for A/falcon/Saudi Arabia/6732-2/2007). These isolates also showed a close relationship with the isolates from falcons and chickens from Kuwait in 2007 (homology ranging between 99.8% and 99.9% for HA and NA genes). In addition, close HA nucleotide homology (99.3% to 99.5%) was revealed with H5N1 isolates from wild birds in Germany (A/black-neckedgrebe/Germany/R1393/07 and A/mute swan/Germany/R1349/07).
Interestingly, the KSA isolates under study were genetically distinguishable from the KSA viruses isolated from domestic birds in the same period, which were distinctly grouped in the 2.2.2 sublineage () (Monne et al., Citation2008).
Discussion
Avian influenza virus is a global disease affecting many species of birds, but to our knowledge this is the first report of HPAI in this particular species. Avian paramyxovirus type 1 and avianpox infections are important causes of mortality in confiscated Houbara bustards (Bailey et al., Citation2002). Avian influenza virus has been reported only once in Houbara bustards, involving H9N2 viruses of low pathogenicity (Wernery et al., Citation2001). Our investigation has showed that adult Houbara bustards are vulnerable to the HPAIV H5N1 and suffer high mortality (up to 93%) in the infected flocks. The isolation of the same genotype of virus from in-contact falcons has substantiated the finding that birds of prey are at increased risk of infection with HPAIV because of feeding on avian carcasses and diseased avian prey (Temple, Citation1987; Kuiken et al., Citation2004). Since the viral genotypes co-circulating in the KSA in poultry were grouped in a distinct sublineage (Monne et al., Citation2008), direct links between the outbreaks in poultry and in the falconry sector in this country could be ruled out.
As falconry is a traditional sport of the Arab culture, the close contact of falcons to their trainer and owner has potential zoonotic significance. The close relationship observed between the Kuwaiti H5N1 viruses and KSA isolates from Houbara bustards and falcons may be indicative of an epidemiological link between these viruses as the Samman area shares a border with Kuwait. However, it was not possible to assess how these Houbara bustards contracted infection. Presumably, they became in contact with some infected birds during transit, as these birds are illegally transported in very poor conditions, including mingling of a variety of different bird species (Bailey et al, Citation2000). Interestingly, the Houbara bustard and falcon isolates showed high genetic homologies with A/H5N1 viruses circulating in the wild birds during the same year in Central Europe, indicating that the same genotype was actively circulating and widespread in 2007. Based on our findings, it appears necessary to include this species of bird in avian influenza surveillance programmes and to collect data on the prevalence of avian influenza infection in this endangered species. Legal and illegal trade of live birds, poultry and poultry products still remains a threat for the introduction of avian influenza infections in free areas or compartments, as demonstrated by this and previous reports (Tumpey et al., Citation2002; Van Borm et al., Citation2005; Beato et al., Citation2006).
Acknowledgements
The authors are grateful to the General Director, Veterinary Laboratory Administrations for permission to publish this information. Thanks are also due to Dr T.A. Bailey, Dubai Falcon Hospital, and to Dr U. Wernery, Central Veterinary Research Laboratory, Dubai, for providing research papers. The authors wish to thank Dr P. Nettleton and Dr I. Capua for the critical revision of this manuscript. The present work was also supported by the European Union project EPIZONE (Network of Excellence of Epizootic Disease Diagnosis and Control).
Additional information
Notes on contributors
Owais Ahmed Khan†
†Present address: 101 Wiley Laboratory, Pennsylvania State University, Orchard Road, University Park 16802, PA, USAReferences
- Alexander , D.J. 2000 . A review of avian influenza in different bird species . Veterinary Microbiology , 74 : 3 – 13 .
- Bailey , T.A. , Silvanose , C. , Naldo , J. , Combreau , O. , Launay , F. , Wernery , U. , Kinne , J. , Gough , R. and Manvell , R. 2000 . Health considerations of the rehabilitation of illegally traded houbara bustards Chlamydotis undulata macqueenii in the Middle East . Oryx , 34 ( 4 ) : 325 – 334 .
- Bailey , T.A. , Silvanose , C. , Manvell , R. , Gough , R.E. , Kinne , J. , Naldo , J. , Combreau , O. and Launay , F. 2002 . Medical dilemmas associated with rehabilitating confiscated houbara bustards (Chlamydotis undulata macqueenii) after avian pox and paramyxovirus type 1 infection . Journal of Wildlife Diseases , 38 ( 3 ) : 518 – 533 .
- Beato , M.S. , Terregino , C. , Cattoli , G. and Capua , I. 2006 . Isolation and characterization of an H10N7 avian influenza virus from poultry carcasses smuggled from China into Italy . Avian Pathology , 35 ( 5 ) : 400 – 403 .
- CITES . 2007 . The CITES Appendix . Available online at: http://www.cites.org/eng/app/index.shtml (accessed 2 June 2008) .
- IFCDW . 2006 . What is Houbara . Available online at: http://www.houbara.com/index1.php?page=abouth (accessed accessed 2 June 2008) .
- IUCN . 2007 . IUCN Red List of Threatened Species . Available online at: http://www.iucnredlist.org (accessed accessed 2 June 2008) .
- Kuiken , T. , Rimmelzwaan , G. , van Riel , D. , van Amerongen , G. , Baars , M. , Fouchier , R. and Osterhaus , A. 2004 . Avian H5N1 influenza in cats . Science , 306 : 241
- Kumar , S. , Tamura , K. and Nei , M. 2004 . MEGA 3: integrated software for molecular evolutionary genetics analysis and sequence alignment . Briefings in Bioinformatics , 5 ( 2 ) : 150 – 163 .
- Monne , I. , Fusaro , A. , Al-Blowi , M.H. , Ismail , M.M. , Khan , O.A. , Dauphin , G. , Tripodi , A. , Salviato , A. , Marangon , S. , Capua , I. and Cattoli , G. 2008 . Co-circulation of two sublineages of HPAI H5N1 virus in the Kingdom of Saudi Arabia with unique molecular signatures suggesting separate introductions into the commercial poultry and falconry sectors . Journal of General Virology , 89 : 2691 – 3691 .
- OIE . 2004 . Manual of Diagnostics for World Terrestrial Animals 5th edn . Available online at: http://www.oie.int/eng/norms/mmanula/a_00037.htm (accessed 4 May 2008) .
- Salzberg , S.L. , Kingsford , C. , Cattoli , G. , Spiro , D.J. , Janies , D.A. , Aly , M.M. , Brown , I.H. , Couacy-Hymann , E. , De Mia , G.M. , Dung do , H. , Guercio , A. , Joannis , T. , Maken Ali , A.S. , Osmani , A. , Padalino , I. , Saad , M.D. , Savic , V. , Sengamalay , N.A. , Yingst , S. , Zaborsky , J. , Zorman-Rojs , O. , Ghedin , E. and Capua , I. 2007 . Genome analysis linking recent European and African influenza (H5N1) viruses . Emerging Infectious Diseases , 13 : 713 – 718 .
- Senne , D.A. 1998 . “ Virus propagation in embryonating eggs ” . In A Laboratory Manual for the Isolation and Identification of Avian Pathogens , 4th edn , Edited by: Swayne , D.E. , Glisson , R. , Jackwood , M.W. , Pearson , J.E. and Reed , W.M. 235 – 240 . Kennett Square , PA : American Association of Avian Pathologists .
- Swayne , D.E. and Suarez , D.L. 2000 . Highly pathogenic avian influenza . Revue Scientifique et Technique Office International des Epizooties , 19 : 463 – 482 .
- Temple , S.A. 1987 . Do predators always capture substandard individuals disproportionately from prey populations? . Ecology , 68 : 669 – 674 .
- Tumpey , T.M. , Suarez , D.L. , Perkins , L.E. , Senne , D.A. , Lee , J. , Lee , Y.J. , Mo , I.P. , Sung , H.W. and Swayne , D.E. 2002 . Characterization of a highly pathogenic H5N1 avian influenza A virus isolated from duck meat . Journal of Virology , 76 : 6344 – 6355 .
- Van Borm , S. , Thomas , I. , Hanquet , G. , Lambrecht , B. , Boschmans , M. , Dupont , G. , Decaestecker , M. , Snacken , R. and van den Berg , T. 2005 . Highly pathogenic H5N1 influenza virus in smuggled Thai eagles, Belgium . Emerging infectious Diseases , 11 ( 5 ) : 702 – 705 .
- Wernery , U. , Molnar , L. , Manvell , R. , Kinne , J. and Joseph , S. 2001 . “ Influenza virus infection in houbara bustards (Chlamydotis undulata macqueenii) in the United Arab Emirates ” . In Proceedings of the 6th European Conference Association of Avian Veterinarians , 271 – 276 . Munich, Germany .
- WHO/OIE/FAO H5N1 Evolution Working Group . 2008 . Toward a unified nomenclature system for highly pathogenic avian influenza virus (H5N1) [conference summary] . Emerging Infectious Diseases , 14(7). Available online at: http://www.cdc.gov/EID/content/14/7/e1.htm (accessed 10 May 2008 10 May 2008) .